Back to Journals » Drug Design, Development and Therapy » Volume 16
6-Methoxyflavone and Donepezil Behavioral Plus Neurochemical Correlates in Reversing Chronic Ethanol and Withdrawal Induced Cognitive Impairment
Authors Arif M, Rauf K , Rehman NU , Tokhi A , Ikram M, Sewell RD
Received 8 February 2022
Accepted for publication 9 May 2022
Published 28 May 2022 Volume 2022:16 Pages 1573—1593
DOI https://doi.org/10.2147/DDDT.S360677
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Mehreen Arif,1 Khalid Rauf,1 Naeem Ur Rehman,1 Ahmed Tokhi,1 Muhammad Ikram,1 Robert D Sewell2
1Department of Pharmacy, COMSATS University Islamabad, Abbottabad Campus, Abbottabad, Khyber Pakhtoonkhwa, 22060, Pakistan; 2Cardiff School of Pharmacy and Pharmaceutical Sciences, Cardiff University, Cardiff, CF10 3NB, UK
Correspondence: Khalid Rauf, Department of Pharmacy, COMSATS University Islamabad, Abbottabad Campus, Abbottabad, Khyber Pakhtoonkhwa, 22060, Pakistan, Tel +923459824468, Email [email protected]
Purpose: Chronic ethanol exposure causes neurotoxicity and long-term learning and memory impairment along with hippocampal and frontal cortical dysfunction. Flavonoids possess antioxidant and anti-inflammatory properties believed to be contributory factors in reversing cognitive decline. 6-Methoxyflavone (6-MOF), a flavonoid occurring naturally in medicinal plants, has been reported to instigate neuroprotection by reversing cisplatin-induced hyperalgesia and allodynia. Consequently, this study was designed to investigate 6-MOF activity in models of chronic ethanol-induced cognitive impairment along with neurochemical correlates.
Methods: Mice were given ethanol orally (2.0 g/kg daily) for 24 days plus either saline, 6-MOF (25– 75mg/kg) or donepezil (4mg/kg) and then ethanol was withdrawn for the next 6 days. Animals were subsequently assessed for their cognitive performance in several models on days 1, 12, and 24, during abstinence (Day-26) and on the 7th day of the washout period. Following behavioral assessment, post-mortem dopamine, noradrenaline and vitamin C concentrations were quantified in the frontal cortex, hippocampus and striatum, using HPLC with UV detection.
Results: Chronic ethanol treatment suppressed locomotor activity and impaired cognitive tasks, which included novel object recognition, performance in the Morris water maze as well as the Y-maze, socialization and nest-building behavior throughout the protocol and during withdrawal. These behavioral deficits were at least partially restored by the co-administration of 6-MOF or donepezil with ethanol as were ethanol-induced deficits in frontal cortical and hippocampal dopamine plus noradrenaline, together with striatal dopamine. 6-MOF co-administration with ethanol also modestly restored striatal vitamin C levels.
Conclusion: It is postulated that, apart from donepezil, 6-MOF may be useful not only in the treatment of ethanol withdrawal severity but also in the management of chronic ethanol withdrawal induced cognitive impairment.
Keywords: ethanol cognition, 6-methoxyflavone, dopamine, noradrenaline, hippocampus, frontal cortex
Introduction
Chronic alcoholism is invariably associated with cognitive abnormalities that give rise to a long-term impairment of learning and memory. Such outcomes have been coupled not only with shrinkage in brain volume but may also be detrimental to hippocampal and prefrontal cortical function.1 In addition, chronic alcohol exposure followed by abstinence, is injurious to grey matter by way of microstructural disruption of myelin and dysfunction in the prefrontal cortex instigating impaired retrieval and recall of fear memories.2 Studies have shown that chronic alcohol consumption modifies cholinergic and monoamine neurotransmission inducing a negative affective state, although in this respect, adaptation to hippocampal neuronal excitability is subject to a gender difference.3,4 Moreover, chronic ethanol induces further cognitive dysfunction as the result of cortical and hippocampal oxide-nitrosative stress, elevated cytokine levels, neuroinflammation and raised acetylcholinesterase (AChE) activity.5 In light of this, donepezil is an anticholinesterase drug reported to have a beneficial effect on cognitive functioning6 in alcoholic patients although further studies have been advocated to confirm any possible role in managing alcohol-related dementia. Donepezil has also been reported to possess neuroprotective properties7 and anti-apoptotic activity as well as an inhibitory action against alcohol-induced toxicity.8
Flavonoids are secondary plant metabolites with a broad spectrum of medicinal properties, and they have been employed as a fundamental constituent of pharmaceutical, cosmetic and nutraceutical preparations.9,10 Their diverse pharmacological properties include antioxidant,11 anti-inflammatory,12 anxiolytic13 and as neuroprotective,14 so they have been used in the treatment of several ailments. These polyphenolic phytochemical compounds have been reported to reverse neuronal damage, stroke and ischemia.15–18 Their antioxidant and anti-neuroinflammatory properties are considered to be major contributory factors in slowing rates of cognitive decline. Thus, it has been shown that the consumption of flavonoid-rich foods could be a valuable approach towards reducing cognitive impairment in older adults.19 Flavonoids modulate neuronal activity by interacting with γ-aminobutyric acid (GABA), dopamine, glycine and serotonin neurotransmitters.20 Even though there is evidence that flavonoids enhance cognitive function in both humans and animals, the underlying mechanism(s) have yet to be fully elucidated.10
6-Methoxyflavone (6-MOF, Figure 1), which can be found naturally in Anvillea garcini leaves, is a flavonoid21 capable of alleviating cisplatin-induced neuropathic-like pain.22 In addition, it has been shown to act as a flumazenil-insensitive positive allosteric modulator of GABA responses at human recombinant a1b2c2L and a2b2c2L GABAA receptors and of GABA at benzodiazepine sensitive mutant ρ1I307S/W328 M GABA receptors in Xenopus oocytes.23 It has also been reported that 6-MOF has immunomodulatory activity capable of suppressing NFAT-mediated T-cell activation.24
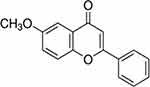 |
Figure 1 Molecular structure of 6-Methoxyflavone (C16H12O3). |
The present experiments were conducted, bearing in mind the effect of chronic ethanol on cognitive impairment and the pharmacological potential of flavonoids for improving cognition. Thus, the pharmacological effects of 6-MOF on chronic ethanol-induced cognitive deficits were investigated in a range of behavioral paradigms involving cognition in parallel with neurochemical studies. The tests included locomotor activity, spatial working memory in the Morris water maze as well as the Y-maze, novel object recognition, socialization and nest-building behavior while postmortem monoamine levels were determined in the prefrontal cortex, hippocampus and striatum.
Methods
Animals
Adult male BALB/c mice (n=6/group; 22–28 g) were acquired from the Veterinary Research Institute, Peshawar, Pakistan. Animals were kept under regular environmental conditions of temperature maintained at 22.0 ± 2.0 °C on a 12/12 h light/dark cycle with ad libitum food and water access. The ethical committee of COMSATS University Islamabad, Abbottabad campus approved all experimental procedures under the certificate number PHM.Eth/CS-M03-015-1106, which conformed to the guidelines of the Animals Scientific Procedure Act (1986) UK.
Drugs and Chemicals
6-MOF, (Purity 98% - Santa Cruz, USA); Dimethyl sulfoxide (DMSO), Tween 80, Normal Saline (Marions Laboratories Pakistan). 6-MOF was dissolved in a vehicle comprising DMSO: Tween 80: Normal saline (5:1:94).
Experimental Protocol
In experiments involving chronic ethanol-induced cognitive impairment, 2.0 g/kg (15% W/V) aqueous ethanol was given daily by the oral route (P.O.) for 24 consecutive days.25 6-MOF was given at previously established doses of 25, 50 and 75 mg/kg,22 and donepezil was administered at a dose of 4 mg/kg.26 Animals were divided into six groups: Group 1, received normal saline vehicle (10 mL/kg); Group 2 received ethanol (2.0 g/kg p.o); Group 3 received ethanol + donepezil (4.0 mg/kg P.O.), Group 4 received ethanol + 6-MOF (25 mg/kg P.O.); Group 5 received ethanol + 6-MOF (50 mg/kg P.O.); Group 6 received ethanol + 6-MOF (75 mg/kg P.O.). 6-MOF or donepezil was administered 15 min before ethanol administration for 24 successive days. After 24 days of co-administration (ethanol + test compounds), treatment was withdrawn for the next 6 days. Animals were assessed for their cognitive performances in several behavioral models on days 1, 12, and 24, during abstinence (Day-26) and on the 7th day of the washout period.25
Behavioral Activity Tests
Locomotor Activity Test (Open Field)
Locomotor activity was assessed in activity boxes (46 × 46 cm)27,28 internally divided into four quadrants measuring (23 × 23 cm) with floor line-markings.29 All animals were placed individually into the locomotor boxes, and activity was recorded by a video camera mounted 230 cm above the box. The number of lines crossed in 30 minutes was noted, and data were logged.30 During all experimental procedures, 70% ethanol was used to clean the apparatus thoroughly between recordings.31
Measurement of Spatial Learning and Memory in the Morris Water Maze
The Morris water maze was employed to evaluate spatial learning and memory. The test procedure was performed using a circular pool (120 cm in diameter and 60 cm in height) filled with water, which was rendered opaque with milk powder. The pool was divided into four notional quadrants, and spatial cues with geometrical shapes were attached to the walls of the pool. Phase −1 (training), mice were trained for 5 days to find the location of a platform (13 cm diameter and 34 cm high) that was hidden 1 cm below the water level. This platform was placed in the same target quadrant on each occasion during all training sessions. Phase 2 (Trial) 5 trials per day were given with an intervening time interval of 10 minutes. During each trial, mice were placed facing the wall in one of the four quadrants with a randomly selected starting point. Each animal was permitted to locate the hidden platform for 90 seconds and was then allowed to sit on the platform for 5 seconds. Animals failing to locate the hidden platform were gently placed on the platform for 20 seconds (Phase-3). On the test day, ie, probe trial day, the platform was removed from the quadrant and mice were allowed to explore the maze for 90 seconds while being recorded by the video camera. Cognitive function was evaluated by recording the time spent in the target quadrant, the number of entries in the target quadrant and the number of platform location crossings by each animal.32,33
Spontaneous Alternation Y-Maze
A Y-maze apparatus with three equal-length arms positioned at an angle of 120° (21 cm long × 8.5 cm wide × 40 cm height) was used. Each animal was positioned at the midpoint of the apparatus and permitted to freely explore all arms for 5 minutes. The time spent in each arm was recorded using the video camera and between each animal procedure, the apparatus was swabbed with 70% ethanol. In the analysis of spontaneous Y-maze activity, the number of alternations, number of entries in each arm and percent alternations were recorded. Alternations were measured as entries into each arm and percentage alternations were calculated using the following formula.34
Novel Object Recognition
A three-day protocol was followed using an open arena (60cm × 50cm × 40cm). On day-1 (Acclimatization phase), animals explored the experimental boxes for 10 minutes. On day-2 (The training phase), animals were exposed to two novel objects for 10 minutes. On the test day, one familiar object was replaced with a novel object. Animals explored the objects for 10 minutes. During the experimental protocol, 70% ethanol was used to clean the boxes and objects. The time spent with each object was recorded by the video camera.35
Socialization Test
Mice were habituated to the test environment for 30 minutes. A juvenile male was used as a presenter animal. A test involved a sampling phase in which a juvenile presenter was introduced to the test animal, allowed to interact for 5 minutes and then removed. After a 1 hr interval, in the recognition phase, the same juvenile male or a novel male juvenile was introduced into the test cage for a 5-minute interaction.36
Nest Building Behavior
A single animal was placed in each cage and provided with cotton nesting material (5 × 5 cm square) and then left overnight. Animals were not disturbed during the nest-building period. The height and width of each nest were measured and recorded, then each nest was scored according to the criteria of Kraeuter et al.37
Neurotransmitter and Vitamin C Quantification Using HPLC
Sample Preparation
After behavioral experimentation, all the animals were euthanized and different brain areas, ie, frontal cortex, striatum and hippocampus, were dissected on ice-chilled plates, weighed and then stored at −80°C. During sample preparation, a Teflon-glass homogenizer (Ultra-Turax®T-50) was used for tissue homogenization in 0.2% ice-cold perchloric acid at 5000 rpm. The sample was then cold centrifuged at 12,000 rpm/min (4°C) (DLAB Scientific), and the supernatant was filtered using a 0.45 mm filter (CNW technologies) before introduction into the HPLC autosampler.29
Chromatographic Conditions
Chromatographic analysis was performed utilizing a Waters Alliance 2690 separation module with PDA, UV detector, and autosampler (USA). A C18 stainless steel column (250× 4.6 mm) (Waters X Select® HSS Ireland) with a 5µm particle size was employed. The mobile phase comprised methanol and 20mM monobasic sodium phosphate (5:95, v/v); while detection was performed at 280 nm with isocratic elution. The elution rate was set at a flow rate of 0.5 mL/min while the column was maintained at a temperature of 35 ℃.29,38
Standard Preparation
For the preparation of the standard stock solutions, 1.0 mg of dopamine, noradrenaline and vitamin C were dissolved in 10 mL HPLC grade water. The stock solution of each neurotransmitter was then diluted to make 5 concentrations (100–500 ng/mL) used for the calibration curve. These samples were then placed in the HPLC autosampler, and a 20µL volume was withdrawn for injection into the system by the software (EmpowerTM). The calibration curve was then made by plotting the peak area of dopamine, noradrenaline and vitamin C (y) against the concentration of dopamine, noradrenaline and vitamin C (x), respectively, using linear regression analysis.29
Statistical Analysis
Data were presented as mean ± standard error and processed by Graph Pad Prism version 8 statistical software. One-way ANOVA followed by post hoc Dunnett’s test was applied. A value of p < 0.05 was taken as the threshold level of significance and data were considered significant if ***p < 0.001, **p < 0.01 and *p < 0.05.
Results
The Activity of Chronic Ethanol Treatment on Locomotor Activity (Open Field Test)
Ethanol (2.0g/kg/p.o) was given alone for 24 days followed by 6 days of ethanol abstinence, and locomotor activity was assessed on days-1, 12, 24, during abstinence (Day-26) and day-7 (post-withdrawal). There was significant hyperlocomotion observed in the ethanol-treated animals on day 12. However, there was a depression of locomotor activity during 6-days of ethanol abstinence and on day-7 (post-withdrawal) (Figure 2).
The Activity of 6-Methoxyflavone or Donepezil on Chronic Ethanol-Induced Suppression of Locomotor Activity (Open Field Test)
Ethanol (2.0 g/kg/P.O.) plus either 6-MOF (25, 50 and 75 mg/kg P.O.) or donepezil (4.0 mg/kg P.O.) were co-administered daily for 24 days followed by 6 days of ethanol abstinence. Animal locomotor activity was tested on days-1, 12 and 24, then during abstinence (Day-26) and on day-7 (post-withdrawal). There was a significant elevation of ethanol stimulated locomotion induced by 75 mg/kg of 6-MOF on day-12. During ethanol abstinence, the groups cotreated with all doses of 6-MOF up to day 24 expressed an increase in locomotor activity as did the group on the 7th protocol day (post-ethanol withdrawal). In contrast, the group co-administered donepezil with ethanol displayed a decrease in locomotion on protocol day-24 and during the abstinence period, locomotion was markedly increased (Figure 3).
The Activity of Chronic Ethanol Treatment on Novel Object Recognition
Ethanol (2.0g/kg/p.o) was administered for 24 days followed by 6 days of ethanol abstinence, and novel object recognition was assessed on days-1, 12 and 24, during abstinence (Day-26), and on day-7 (post-withdrawal). On each test day, a familiar object was replaced with a novel object and animals were tested for object recognition memory for 10 minutes. There was a significant decrease in exploration time with novel objects observed throughout treatment and upon withdrawal in ethanol-treated animals compared to that administered saline vehicle (Figure 4).
The Activity of 6-Methoxyflavone or Donepezil on the Chronic Ethanol-Induced Cognitive Deficit in the Novel Object Recognition Test
A significant increase in novel object exploration time was observed on protocol days-12, 24, abstinence (Day-26) and on the 7th-day (post-withdrawal) in the groups that received ethanol combined with all three 6-MOF doses (25, 50 and 75 mg/kg). In the chronic donepezil plus ethanol treatment group, there was also an increased novel object exploration time during the whole protocol in comparison with the ethanol alone treatment group (Figure 5).
The Activity of Chronic Ethanol Treatment on Morris Water Maze Performance
Ethanol (2.0g/kg P.O.) was administered for 24 days followed by 6 days of ethanol abstinence, and Morris water maze performance was assessed on days-1, 12 and 24, during abstinence (Day-26) and on day-7 (post-withdrawal). It produced a statistically significant decrease in the time spent in the target quadrant (Figure 6A), the number of entries in the target quadrant (Figure 6B) and the number of platform location crossings (Figure 6C) throughout the entire protocol.
The activity of 6-methoxyflavone or donepezil on the chronic ethanol-induced cognitive deficit observed in performance in the Morris water maze
Chronic ethanol treatment in combination with 6-MOF (50 and 75 mg/kg) caused an increase in the time spent in the target quadrant on protocol day 24, during abstinence (Day-26) and post-withdrawal. Likewise, donepezil had an augmenting action on the time spent in the target quadrant on day-24, during abstinence (Day-26) and post-withdrawal (Figure 7A). Regarding the number of entries in the target quadrant, all three concomitant doses of 6-MOF, as well as donepezil increased this parameter during the post-withdrawal period (Figure 7B). However, donepezil and only the two higher doses of 6-MOF increased the number of entries in the target quadrant during abstinence (Day-26).
Co-administration of 6-MOF with ethanol also augmented the number of platform location crossings on protocol day-12 (75mg/kg), at all doses on day-24, during abstinence (Day-26) and post-withdrawal. The chronic ethanol plus donepezil combination significantly increased the number of platform location crossings during abstinence (Day-26) and post-withdrawal (Figure 7C).
The Activity of Chronic Ethanol Treatment on Y-Maze Performance
Ethanol (2.0 g/kg/p.o) was given for 24 days followed by 6 days of ethanol abstinence and testing on Days 1, 12, and 24, abstinence (Day-26) and on post-withdrawal day 7. It produced statistically significant decrements in the total number of arm entries (Figure 8A), number of alternations (Figure 8B) and percentage alternations (Figure 8C) in Y-maze task performance throughout the entire protocol.
The Activity of 6-Methoxyflavone or Donepezil on the Chronic Ethanol-Induced Cognitive Deficit in Y-Maze Performance
Chronic ethanol treatment in combination with 6-MOF (50 and 75 mg/kg) caused an increase in the number of Y-maze arm entries on protocol days 12, 24, during abstinence (Day-26) and post-withdrawal. Likewise, donepezil had the same enhancing action on arm entry on day-24 and during post-withdrawal (Figure 9A). Regarding the number of alternations to chronic ethanol, all three concomitant doses of 6-MOF increased them throughout the whole protocol and donepezil had a similar action except during the post-withdrawal period (Figure 9B).
Co-administration of 6-MOF with ethanol also augmented the % alternations on protocol day-12 (75mg/kg), at all doses on day-24 and post-withdrawal, but only at the two higher doses during abstinence. The chronic ethanol plus donepezil combination significantly increased % alternations throughout except on day-24 (Figure 9C).
The Activity of Chronic Ethanol Treatment on Socialization Behavior
Mice chronically treated with ethanol were tested for their socialization behavior on days-1, 12, and 24, during abstinence (Day-26) and the 7th-day post-withdrawal. There was a significant decrease in exploration time in sniffing a novel juvenile animal throughout the whole course of the protocol (Figure 10).
The Activity of 6-Methoxyflavone or Donepezil on Chronic Ethanol-Induced Cognitive Deficit Expressed in Socialization Behavior
Chronic co-administration of 6-MOF with ethanol reversed the ethanol-induced cognitive deficit on socialization behavior. Hence, treatment with all three doses of 6-MOF significantly increased ethanol-induced exploration time in sniffing novel juvenile mice at all stages of the protocol similarly, chronic donepezil also had an identical pronounced socialization behavioral effect in combination with ethanol during the whole protocol (Figure 11).
The Activity of Chronic Ethanol Treatment on Nest-Building Behavior
Ethanol (2.0g/kg/p.o) was given for 24 days followed by 6 days of ethanol abstinence. A significant decrease in the height of nests built by ethanol-treated animals was observed in comparison with the saline-vehicle group all through the protocol (Figure 12A). Statistical analysis revealed that the width of the nests, indicating the amount of untouched material, was significantly increased in chronic ethanol-treated animals versus the saline-vehicle controls (Figure 12B). However, concerning the nest building score, the quality of nest building was impaired compared to the control animals (Figure 12C).
The Activity of 6-Methoxyflavone or Donepezil on the Chronic Ethanol-Induced Cognitive Deficit in Nest-Building Behavior
There was a considerable increase in the height of nests built by mice cotreated with ethanol plus all doses of 6-MOF or donepezil at each stage of the protocol (Figure 13A). Statistical analysis disclosed that the nest width, as an indication of the amount of untouched material, was decreased during the whole protocol by chronic ethanol with 6-MOF or donepezil concomitant dosing versus ethanol alone (Figure 13B). Also, as a consequence of evaluation by scoring, the quality of chronic ethanol-induced nest building was improved by combining it with 6-MOF or donepezil for the whole protocol duration (Figure 13C).
Quantification of Neurotransmitters and Vitamin C Using HPLC-UV
Action of Chronic Ethanol Treatment with 6-Methoxyflavone or Donepezil on Frontal Cortical Tissue Concentrations of Dopamine, Noradrenaline and Vitamin C
Chronic ethanol treatment induced a marked decrease in frontal cortical levels of dopamine, noradrenaline and vitamin C compared to the saline (vehicle) treated groups. However, combined treatment with 6-MOF (75 mg/kg) plus ethanol elevated the levels of dopamine and vitamin C, which were formerly suppressed by chronic ethanol. Additionally, all three doses of 6-MOF, as well as donepezil, manifestly reversed the repressive action of chronic ethanol on noradrenaline levels in the frontal cortex and the highest dose of 6-MOF also increased the vitamin C level (Table 1).
Action of Chronic Ethanol Treatment with 6-Methoxyflavone or Donepezil on Hippocampal Tissue Concentrations of Dopamine, Noradrenaline and Vitamin C
Chronic ethanol treatment produced a substantial decline in hippocampal levels of dopamine and noradrenaline. In contrast, chronic co-administered donepezil or 6-MOF at all doses increased the ethanol repressed hippocampal concentration of dopamine. Similarly, donepezil or the highest dose of 6-MOF in combination with chronic ethanol significantly elevated the ethanol attenuated hippocampal noradrenaline concentrations (Table 2).
Action of Chronic Ethanol Treatment with 6-Methoxyflavone or Donepezil on Striatal Tissue Concentrations of Dopamine, Noradrenaline and Vitamin C
Chronic ethanol treatment produced a marked decrease in striatal levels of dopamine, noradrenaline and vitamin C. Only donepezil or 6-MOF (75 mg/kg) caused a significant reversal of ethanol diminished striatal dopamine while noradrenaline remained unmodified by chronic coadministration of donepezil or any of the 6-MOF doses with chronic ethanol (Table 3).
Discussion
Chronic ethanol has been reported to alter the behavioral and cognitive aptitude of both animals and humans primarily by disrupting hippocampal-dependent learning and memory.39 This cognitive decline is largely attributable to the ability of ethanol to cross the blood–brain barrier inducing oxidative stress.40,41 Studies have shown that ethanol withdrawal produces cognitive dysfunction by perturbing frontal cortical, striatal and hippocampal functions.5,42,43 In the current study, an increase in spontaneous locomotor activity was observed on protocol days 12 and 24 of chronic ethanol administration. A similar treatment schedule has been shown to induce robust ethanol sensitization though in the present case, a further ethanol challenge was not presented to expose the expression of sensitization.44 However, a decrease in locomotor activity was noted during ethanol abstinence and on the 7th day, post-withdrawal compared to saline controls. During long-term ethanol withdrawal, it has been reported that the inhibitory action of GABA was diminished due to impaired GABAA receptor function leading to anxiety-like behavior and a downturn in locomotor activity.45 In contrast, our findings showed that 6-MOF reversed chronic ethanol-induced hyperlocomotion after 12 days of combined treatment. Concerning this, 6-MOF possesses an inherent GABAA agonist effect23 and it has also been reported that an increased release of GABA in the nucleus accumbens results in hyperlocomotion that is mediated by inhibition of dopaminergic pathways in the ventral tegmental area and nucleus accumbens.46
During abstinence and post-withdrawal from chronic ethanol plus 6-MOF or donepezil, there was augmented locomotor activity compared to chronic ethanol treatment alone. Thus, an enhanced GABA-ergic function or anticholinesterase activity may well have been a factor in the counteraction of ethanol withdrawal hypolocomotion. Additionally, long-term ethanol ingestion results in altered activity of dopaminergic and noradrenergic pathways, which affect the phosphorylation and trafficking of tyrosine kinase receptor B (TrkB) via cyclic AMP stimulation. Studies in humans and animals have suggested that there is a convincing link between memory formation in the hippocampus and dopaminergic neuromodulation.47 It is also relevant to mention that in our study, both 6-MOF and donepezil increased ethanol suppressed noradrenaline levels in the hippocampus. Moreover, postsynaptic activation of 5-HT1A receptors has been shown to elicit an increase in extracellular noradrenaline levels in the hippocampus causing psychomotor stimulation and hyperlocomotion.48 Additionally, donepezil is an AChE inhibitor with a neuroprotective effect and it has been shown to improve cognition in mild, moderate and severe Alzheimer’s disease by both cholinergic and non-cholinergic mechanisms.49,50 Thus, increased hippocampal noradrenaline levels can provide a plausible mechanism underlying the enhancement of ethanol withdrawal locomotor activity by 6-MOF and donepezil.
Chronic ethanol consumption results in anterograde amnesia mitigating the acquisition of new information through an impaired processing capability accompanied by the debility to forget rapidly.51 This feature is commonly found when there are lesions in the hippocampus and ethanol tends to decrease hippocampal synaptic plasticity promoting an inability to store information before it is consolidated in long-term memory.51,52
Previous findings employing the novel object recognition test have been heavily influenced by lesions in both the hippocampus and cortex.53 Hence, in rats and primates, the hippocampus is associated with the perirhinal and prefrontal cortical areas, which play a role in the recognition of memory tasks by activating the executive center.53,54 In our study, chronic ethanol administration in mice compromised object recognition memory and this accords with a similar outcome in rats.51 Furthermore, we demonstrated that sustained ethanol consumption decreased the time spent with a novel object not only during the period of treatment but also during withdrawal. Taking this into account, the medial prefrontal cortex is an important area that is responsible for a range of functions, not least of which include episodic and contextual memory formation.55
It has been demonstrated that ethanol-induced impairment of spatial memory may be ascribed to a reduction in the release of hippocampal glutamate56 in addition to attenuating BDNF levels.57 In this context, hippocampal glutamate release is modulated by BDNF-TrkB signaling58 and this has been shown to critically impact long-term potentiation and long-term memory formation.59 It is also noteworthy that BDNF is essential for maintaining noradrenergic tone in catecholaminergic neurons.59 Furthermore, ethanol consumption not only decreases levels of nerve growth factor but also inhibits NGF-mediated cell survival.60 Flavones are TrkB receptor agonists an example being 7, 8-dihydroxyflavone which improves object recognition memory in mice.61 Additionally, flavonoids affect the regulation of NGF leading to cellular phosphorylation of hippocampal proteins aiding memory formation.62 It is a distinct possibility, therefore, that 6-MOF similarly increases novel object exploration time via modulation of BDNF-TrkB receptors and stimulation of NGF release.
Monoamines in the frontal cortex and hippocampus are implicated in mnemonic processes, such as learning, memory consolidation, formation and retrieval,63 while ethanol consumption disrupts dopaminergic and noradrenergic pathways.47 Our experiments disclosed that donepezil or 6-MOF treatment with chronic ethanol escalated frontal cortical levels of noradrenaline, while only 6-MOF increased hippocampal dopamine. Both of these consequences may be instrumental towards the increased time spent with a novel object and the overall enhancement of object recognition memory. On top of this, chronic ethanol causes oxidative stress40 by lipid peroxidation41,64 and contrary to this, vitamin C has antioxidant properties. Consequently, vitamin C conserves oxidative mechanisms improving cognition and memory65,66 in addition to reversing an ethanol-generated apoptotic neuronal loss, neuroinflammation and oxidative stress.67 In our study, 6-MOF at the highest co-administered dose did evoke a moderate but significant reversal of chronic ethanol attenuated frontal cortical vitamin C level, which may have had a contribution to offsetting some oxidative stress.
The Morris water maze is a frequently used behavioral test to assess hippocampal-centered spatial reference and working memory.68 It has been documented that lesions in the dorsal hippocampus and striatum,69 along with the cerebral cortex, basal forebrain and cerebellum impair Morris’s water maze performance.70 Concerning this, it is notable that chronic ethanol consumption causes lesions in the hippocampus and cerebral cortex possibly by increasing both AChE activity and oxidative stress.71 The Y-maze paradigm is used for assessing spatial and short-term working memory and learning. The model evaluates spontaneous alternation behavior, which involves a functional association between the prefrontal cortex and hippocampus. Lesions in these key areas result in a deterioration of Y-maze performance72 and prolonged consumption of ethanol also produces prefrontal cortical dysfunction.73 Our findings exposed a substantially decreased performance by chronic ethanol-treated animals in the Morris water maze and Y-maze. A broad range of neurotransmitters, including dopamine, acetylcholine, noradrenaline, serotonin and glutamate as a monoamine co-transmitter, are involved in memory formation.74,75 However, the spatial choice of animals in the Morris water maze is altered due to changes in GABAergic, cholinergic and monoaminergic (particularly serotonergic and noradrenergic) neurotransmission76 while Y-maze performance is exacerbated by dopaminergic D1 blockade in the medial frontal cortex.77 Ethanol consumption has been reported to disrupt dopaminergic as well as noradrenergic transmission in addition to causing oxidative stress.47 Dopamine D1-like receptors, when activated in the hippocampus, enhance memory formation and monoamine oxidase-B (MAO-B) inhibitors have been reported to raise extracellular dopamine levels in this brain region.78 Intriguingly, O-methylated flavonoids inhibit MAO-B,79 which may contribute to increased dopamine levels in the hippocampus and the positive impact of 6-MOF on spatial memory in the Y-maze. Correspondingly, augmented brain noradrenaline levels improve cognition,80 and in our study, chronic ethanol plus 6-MOF produced a specific frontal cortical noradrenaline upsurge likely to improve Y-maze performance. Spatial learning, memory and neurogenesis in the hippocampus are compromised by depletion of hippocampal noradrenaline levels.81 Regarding this assertion, in our study 6-MOF increased ethanol suppressed hippocampal noradrenaline levels, which would have been likely to improve MWM performance.
Vitamin C has an antioxidant effect that tends to oppose memory-related impairment in several ailments and it improves memory chiefly by conserving the hippocampal antioxidant mechanism.65 We found that chronic ethanol administration diminished vitamin C levels in all three brain areas examined and in the chronic ethanol/6MOF combination group, only a marginal, though significant reversal, was observed in the frontal cortex. This may have had some bearing on the antioxidant status of the frontal cortex in ameliorating Y-maze performance. Oxidative stress and the generation of reactive oxygen species is one of the underlying mechanisms by which chronic ethanol induces impairment of spatial working memory.82 It has been shown that the prefrontal cortex and hippocampus are primarily involved in the modulation of spatial learning and memory and oxidative stress in these key brain areas results in spatial memory impairment.83 Vitamin C, a natural antioxidant, has been documented to facilitate hippocampal antioxidant enzyme activity thereby improving cognitive function.84 We found that 6-MOF treatment curtailed ethanol repressed frontal cortical vitamin C levels and this may have contributed to improved Morris water maze performance.
The socialization test has been used commonly for the quantification of short-term memory and is primarily centered on the innate preference of rodents to explore unknown conspecifics more strongly than familiar ones.85,86 Socialization is critical for survival, social collaboration, reproduction and adaptation of social behavior.86 The dorsal hippocampal CA1 region and frontal cortical area are involved in the sensory system and social interaction behaviors, whereas the amygdala and somatosensory areas appear to be more concerned with behavioral regulation.87 Chronic ethanol exposure modifies neuronal function in the hippocampus, medial prefrontal cortex and dentate gyrus.1 This neuronal activity would tend to impair socialization as confirmed by our findings that there was a decrease in social interaction (time spent sniffing novel juvenile mice) by chronic ethanol-treated animals. Flavonoids have been reported to ameliorate cognitive function in humans,88 and the outcome of treatment chronically with ethanol plus 6-MOF partially reversed ethanol impaired socialization behavior in our study. In this context, noradrenaline acting on β-adrenoceptors and dopamine operating through D1/D5 receptors, both play an essential role in social recognition memory.86 This was corroborated by our result that chronic ethanol plus 6-MOF treatment raised the levels of noradrenaline and dopamine in the frontal cortex, while the dopamine concentration was boosted in the hippocampus, thereby improving socialization.
Nest building behavior in mice has been utilized as an assay for affective states as well as sensorimotor function and these entities are modified even during acute ethanol withdrawal.89 The hippocampus plays an important role in nest building and hippocampal lesions lead to impairment of this behavior.90–92 In addition, to acute ethanol withdrawal, abstinence from chronic ethanol predictably modulates nest-building behavior93 and this is substantiated by our results. Nest building entails orofacial and forelimb measures, which are dopamine-dependent94 and it has been previously demonstrated that flavonoids improve deficits in nest building, social interactive behaviors and cognitive function.95 In our study, a treatment combination of ethanol with either donepezil or 6-MOF ameliorated ethanol impaired nest height, width and overall score. Both dopaminergic and noradrenergic pathways play an important role in nest-building and deficiencies in these systems are inclined to exacerbate the behavior.94 Thus, dopaminergic dysfunction and decreased dopamine levels in the striatum result in impaired nest-building activity; however, when dopamine levels are restored, nest-building is reinstated.94 Our outcome of chronic ethanol treatment with 6-MOF generated increased levels of noradrenaline and dopamine in the frontal cortex and dopamine in the hippocampus and striatum may well be underlying nesting mechanisms.
Conclusion
We have shown that chronic ethanol treatment suppressed locomotor activity in addition to impairing cognitive tasks, which included novel object recognition, performance in the Morris water maze and Y-maze, socialization and nest-building behavior throughout a 24-day protocol and during subsequent withdrawal. These behavioral deficits were at least partially restored by co-administration of ethanol plus 6-MOF or donepezil as were ethanol-induced deficits in frontal cortical dopamine and noradrenaline, hippocampal or striatal dopamine and frontal cortical vitamin C by 6-MOF cotreatment.
Flavonoids are not only AChE inhibitors but are also thought to be neuroprotective through protein kinase and lipid kinase signaling cascades, preserving neuronal Ca2+ homeostasis, binding ATP sites as well as BDNF-TrkB receptors and regulating NGF.96 6-MOF is a flavone flavonoid and after oral administration, it is well absorbed from the intestine and can cross the blood–brain barrier to impart neuroprotective activity.97 Accordingly, it may be postulated that it has conferred neuroprotection during ethanol-induced cognitive decline by one or more of its recognized mechanisms. It might be proposed as a consequence that 6-MOF would be useful not only in the treatment of ethanol withdrawal severity but also in the management of ethanol memory impairment. Taking this into account, it is worth remembering that flavonoids are generally considered to be safe.98
Limitations of the Study
This study has involved behavioral and biochemical techniques and more specific investigative work at the molecular level is needed to explore the underlying mechanism(s) of 6-MOF on cognitive decline.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Avchalumov Y, Oliver RJ, Trenet W, et al. Chronic ethanol exposure differentially alters neuronal function in the medial prefrontal cortex and dentate gyrus. Neuropharmacol. 2021;185:108438. doi:10.1016/j.neuropharm.2020.108438
2. Somkuwar SS, Villalpando EG, Quach LW, et al. Abstinence from ethanol dependence produces concomitant cortical gray matter abnormalities, microstructural deficits and cognitive dysfunction. Eur Neuropsychopharmacol. 2021;42:22–34. doi:10.1016/j.euroneuro.2020.11.010
3. Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Progress Neurobiol. 1998;56(4):385–431. doi:10.1016/S0301-0082(98)00032-X
4. Bach EC, Morgan JW, Ewin SE, Barth SH, Raab-Graham KF, Weiner JL. Chronic ethanol exposures leads to a negative affective state in female rats that is accompanied by a paradoxical decrease in ventral hippocampus excitability. Frontiers Neurosci. 2021;15:458. doi:10.3389/fnins.2021.669075
5. Uniyal A, Kotiyal A, Gadepalli A, Ummadisetty O, Tiwari V. Epigallocatechin-3-gallate improves chronic alcohol induced cognitive dysfunction in rats by interfering with the neuro-inflammatory, cell death and oxido-nitrosative stress pathways; 2021.
6. Zhang X, Lian S, Zhang Y, Zhao Q. efficacy and safety of donepezil for mild cognitive impairment: a systematic review and meta-analysis. Clinic Neurol Neurosurg. 2022;213:107134. doi:10.1016/j.clineuro.2022.107134
7. Li Y, Sun X, Zhuang J, Wang J, Yang C. Donepezil ameliorates oxygen‑glucose deprivation/reoxygenation‑induced cardiac microvascular endothelial cell dysfunction through PARP1/NF‑κB signaling. Mol Medi Rep. 2022;25(4):1–9.
8. Getachew B, Hudson T, Heinbockel T, Csoka AB, Tizabi Y. Protective effects of donepezil against alcohol-induced toxicity in cell culture: role of caspase-3. Neurotox Res. 2018;34(3):757–762. doi:10.1007/s12640-018-9913-3
9. Panche A, Diwan A, Chandra S. Flavonoids: an overview. J Nutr Sci. 2016;5:e47.
10. Rendeiro C, Rhodes JS, Spencer JP. The mechanisms of action of flavonoids in the brain: direct versus indirect effects. Neurochemi Int. 2015;89:126–139.
11. Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutri Biochem. 2002;13(10):572–584. doi:10.1016/S0955-2863(02)00208-5
12. Dong W, Wei T, Guang-Ming Y, Bao-Chang C. Anti-inflammatory, antioxidant and cytotoxic activities of flavonoids from Oxytropis falcata Bunge. J Nat Medi. 2010;8(6):461–465.
13. Ognibene E, Bovicelli P, Adriani W, Saso L, Laviola G. Behavioral effects of 6-bromoflavanone and 5-methoxy-6, 8-dibromoflavanone as anxiolytic compounds. Prog Neuro Psychopharmacol Biol Psychiatry. 2008;32(1):128–134. doi:10.1016/j.pnpbp.2007.07.023
14. Cho N, Lee KY, Huh J, et al. Cognitive-enhancing effects of Rhus verniciflua bark extract and its active flavonoids with neuroprotective and anti-inflammatory activities. Food Chemi Toxicol. 2013;58:355–361. doi:10.1016/j.fct.2013.05.007
15. Spencer JP. Food for thought: the role of dietary flavonoids in enhancing human memory, learning and neuro-cognitive performance: symposium on ‘diet and mental health’. Proc Nutri Soci. 2008;67(2):238–252. doi:10.1017/S0029665108007088
16. Pu F, Mishima K, Irie K, et al. Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J Pharmacol Sci. 2007;104:0707310004.
17. Cho J-S, Han C-K, Lee Y-S, Jin C-B. Neuroprotective and antioxidant effects of the butanol fraction prepared from opuntia ficus-indica var. Saboten Biomol Thera. 2007;15(4):205–211. doi:10.4062/biomolther.2007.15.4.205
18. Hwang S-L, Shih P-H, Yen G-C. Neuroprotective effects of citrus flavonoids. J Agri Food Chemi. 2012;60(4):877–885. doi:10.1021/jf204452y
19. Devore EE, Kang JH, Breteler MM, Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Annals Neurol. 2012;72(1):135–143. doi:10.1002/ana.23594
20. Johnston GA. Flavonoid nutraceuticals and ionotropic receptors for the inhibitory neurotransmitter GABA. Neurochem Int. 2015;89:120–125. doi:10.1016/j.neuint.2015.07.013
21. Ulubelen A, Mabry T, Aynehchi Y. Flavonoids of Anvillea garcini. J Nat Prod. 1979;42(6):624–626. doi:10.1021/np50006a007
22. Shahid M, Subhan F, Ahmad N, Sewell RD. The flavonoid 6-methoxyflavone allays cisplatin-induced neuropathic allodynia and hypoalgesia. Biomedi Pharmacothera. 2017;95:1725–1733. doi:10.1016/j.biopha.2017.09.108
23. Hall BJ, Karim N, Chebib M, Johnston GA, Hanrahan JR. Modulation of ionotropic GABA receptors by 6-methoxyflavanone and 6-methoxyflavone. Neurochemi Res. 2014;39(6):1068–1078. doi:10.1007/s11064-013-1157-2
24. So J-S, Kim G-C, Song M, et al. 6-Methoxyflavone inhibits NFAT translocation into the nucleus and suppresses T cell activation. J Immunol. 2014;193(6):2772–2783. doi:10.4049/jimmunol.1400285
25. Raghavendra V, Kulkarni SK. Possible antioxidant mechanism in melatonin reversal of aging and chronic ethanol-induced amnesia in plus-maze and passive avoidance memory tasks. Free Radical Biol Medi. 2001;30(6):595–602. doi:10.1016/S0891-5849(00)00447-0
26. Umukoro S, Adewole F, Eduviere A, Aderibigbe A, Onwuchekwa C. Free radical scavenging effect of donepezil as the possible contribution to its memory enhancing activity in mice. Drug Res. 2014;64(05):236–239.
27. Wang D-H, Li W, Liu X-F, Zhang J-M, Wang S-M. Chinese medicine formula “Jian-Pi-Zhi-Dong Decoction” attenuates Tourette Syndrome via downregulating the expression of dopamine transporter in mice. Evidence Based Comp Alter Medi. 2013;2013. doi:10.1155/2013/385685
28. Napier TC, Istre ED. Methamphetamine induced sensitization includes a functional upregulation of ventral pallidal 5 HT2A/2C receptors. Synapse. 2008;62(1):14–21. doi:10.1002/syn.20460
29. Rehman NU, Abbas M, Al-Rashida M, et al. Effect of 4-Fluoro-N-(4-Sulfamoylbenzyl) benzene sulfonamide on acquisition and expression of nicotine-induced behavioral sensitization and striatal adenosine levels. Drug Design Develop Thera. 2020;14:3777. doi:10.2147/DDDT.S270025
30. Egashira N, Kubota N, Goto Y, et al. The antipsychotic trifluoperazine reduces marble-burying behavior in mice via D2 and 5-HT2A receptors: implications for obsessive–compulsive disorder. Pharmacol Biochem Behav. 2018;165:9–13. doi:10.1016/j.pbb.2017.12.006
31. Golub HM, Zhou QG, Zucker H, et al. Chronic alcohol exposure is associated with decreased neurogenesis, aberrant integration of newborn neurons, and cognitive dysfunction in female mice. Clin Exp Res. 2015;39(10):1967–1977. doi:10.1111/acer.12843
32. Weitzner DS, Engler-Chiurazzi EB, Kotilinek LA, Ashe KH, Reed MN. Morris water maze test: optimization for mouse strain and testing environment. Jove J Visualized Exp. 2015;2015(100):e52706.
33. Hendrickx JO, De Moudt S, Calus E, De Deyn PP, Van Dam D, De Meyer GR. Age-related cognitive decline in spatial learning and memory of C57BL/6J mice. Behav Brain Res. 2022;418:113649. doi:10.1016/j.bbr.2021.113649
34. Prieur EA, Jadavji NM. Assessing spatial working memory using the spontaneous alternation Y-maze test in aged male mice. Bio Pro. 2019;9:3.
35. Lueptow LM. Novel object recognition test for the investigation of learning and memory in mice. J Visualized Exp. 2017;2017(126):e55718.
36. Chiang M-C, Huang AJ, Wintzer ME, Ohshima T, McHugh TJ. A role for CA3 in social recognition memory. Behav Brain Res. 2018;354:22–30. doi:10.1016/j.bbr.2018.01.019
37. Kraeuter A-K, Guest PC, Sarnyai Z. The nest building test in mice for assessment of general well-being. In: Pre-Clinical Models. Springer; 2019:87–91.
38. Hou X, Huang W, Tong Y, Tian M. Hollow dummy template imprinted boronate-modified polymers for extraction of norepinephrine, epinephrine and dopamine prior to quantitation by HPLC. Microchimica Acta. 2019;186(11):1–9. doi:10.1007/s00604-019-3801-2
39. Rubio J, Yucra S, Gasco M, Gonzales GF. Dose–response effect of black maca (Lepidium meyenii) in mice with memory impairment induced by ethanol. Toxicol Mecha Meth. 2011;21(8):628–634. doi:10.3109/15376516.2011.583294
40. Pinto LS, Gualberto FA, Pereira SR, Barros PA, Franco GC, Ribeiro AM. Dietary restriction protects against chronic-ethanol-induced changes in exploratory behavior in Wistar rats. Brain Res. 2006;1078(1):171–181. doi:10.1016/j.brainres.2005.12.092
41. Mansouri A, Demeilliers C, Amsellem S, Pessayre D, Fromenty B. Acute ethanol administration oxidatively damages and depletes mitochondrial DNA in mouse liver, brain, heart, and skeletal muscles: protective effects of antioxidants. J Pharmacol Exp Thera. 2001;298(2):737–743.
42. Steigerwald ES, Miller MW. Performance by adult rats in sensory‐mediated radial arm maze tasks is not impaired and may be transiently enhanced by chronic exposure to ethanol. Clin Exp Res. 1997;21(9):1553–1559. doi:10.1111/j.1530-0277.1997.tb04489.x
43. Lukoyanov NV, Madeira MD, Paula–Barbosa MM. Behavioral and neuroanatomical consequences of chronic ethanol intake and withdrawal. Physiol and Behavior. 1999;66(2):337–346. doi:10.1016/S0031-9384(98)00301-1
44. Ferreira SEM, Soares LM, Lira CR, et al. Ethanol-induced locomotor sensitization: neuronal activation in the nucleus accumbens and medial prefrontal cortex. Neurosci Letters. 2021;749:135745. doi:10.1016/j.neulet.2021.135745
45. Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28(8):837–850. doi:10.1016/j.neubiorev.2004.11.001
46. Ibos KE, Bodnár É, Bagosi Z, et al. Kisspeptin-8 induces anxiety-like behavior and hypolocomotion by activating the HPA axis and increasing GABA release in the nucleus accumbens in rats. Biomedi. 2021;9(2):112.
47. Heinz A, Beck A, Wrase J, et al. Neurotransmitter systems in alcohol dependence. Pharmacopsy. 2009;42(S 01):S95–S101. doi:10.1055/s-0029-1214395
48. Suwabe A, Kubota M, Niwa M, Kobayashi K, Kanba S. Effect of a 5-HT1A receptor agonist, flesinoxan, on the extracellular noradrenaline level in the hippocampus and on the locomotor activity of rats. Brain Res. 2000;858(2):393–401. doi:10.1016/S0006-8993(00)01941-7
49. Ongnok B, Khuanjing T, Chunchai T, et al. Donepezil provides neuroprotective effects against brain injury and Alzheimer’s pathology under conditions of cardiac ischemia/reperfusion injury. Biochimica et Biophysica Acta. 2021;1867(1):165975. doi:10.1016/j.bbadis.2020.165975
50. Ongnok B, Khuanjing T, Chunchai T, et al. Donepezil protects against doxorubicin-induced chemobrain in rats via attenuation of inflammation and oxidative stress without interfering with doxorubicin efficacy. Neurotherapeutics. 2021;18(3):2107–2125. doi:10.1007/s13311-021-01092-9
51. Garcı́a-Moreno LM, Conejo NM, Capilla A, Garcı́a-Sánchez O, Senderek K, Arias JL. Chronic ethanol intake and object recognition in young and adult rats. Progress Neuro Psychopharmacol Biol Psy. 2002;26(5):831–837. doi:10.1016/S0278-5846(01)00327-X
52. Morrisett R, Swartzwelder HS. Attenuation of hippocampal long-term potentiation by ethanol: a patch-clamp analysis of glutamatergic and GABAergic mechanisms. J Neurosci. 1993;13(5):2264–2272. doi:10.1523/JNEUROSCI.13-05-02264.1993
53. Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cog Proc. 2012;13(2):93–110. doi:10.1007/s10339-011-0430-z
54. Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31(29):10721–10731. doi:10.1523/JNEUROSCI.6413-10.2011
55. Spanswick SC, Dyck RH. Object/context specific memory deficits following medial frontal cortex damage in mice. PLoS One. 2012;7(8):e43698. doi:10.1371/journal.pone.0043698
56. Chastain G. Alcohol, neurotransmitter systems, and behavior. J General Psychol. 2006;133(4):329–335. doi:10.3200/GENP.133.4.329-335
57. Sakai R, Ukai W, Sohma H, et al. Attenuation of brain derived neurotrophic factor (BDNF) by ethanol and cytoprotective effect of exogenous BDNF against ethanol damage in neuronal cells. J Neural Trans. 2005;112(8):1005–1013. doi:10.1007/s00702-004-0246-4
58. Pereira DB, Rebola N, Rodrigues RJ, Cunha RA, Carvalho AP, Duarte CB. Trkb receptors modulation of glutamate release is limited to a subset of nerve terminals in the adult rat hippocampus. J Neurosci Res. 2006;83(5):832–844. doi:10.1002/jnr.20784
59. Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Frontiers Mol Neurosci. 2010;3:1.
60. Seabold GK, Luo J, Miller MW. Effect of ethanol on neurotrophin-mediated cell survival and receptor expression in cultures of cortical neurons. Develop Brain Res. 1998;108(1–2):139–145. doi:10.1016/S0165-3806(98)00043-1
61. Perez-Rando M, Castillo-Gomez E, Bueno-Fernandez C, Nacher J. The TrkB agonist 7, 8-dihydroxyflavone changes the structural dynamics of neocortical pyramidal neurons and improves object recognition in mice. Brain Struct Funct. 2018;223(5):2393–2408. doi:10.1007/s00429-018-1637-x
62. Kim HG, Oh MS. Memory-enhancing effect of Mori Fructus via induction of nerve growth factor. Brit J Nutri. 2013;110(1):86–94. doi:10.1017/S0007114512004710
63. Hamidkhaniha S, Bashiri H, Omidi A, et al. Effect of pretreatment with intracerebroventricular injection of minocycline on morphine‐induced memory impairment in passive avoidance test: role of P‐CREB and c‐Fos expression in the dorsal hippocampus and basolateral amygdala regions. Clin and Exp Pharmacol Physiol. 2019;46(8):711–722. doi:10.1111/1440-1681.13090
64. Assunção M, Santos-Marques M, De Freitas V, et al. Red wine antioxidants protect hippocampal neurons against ethanol-induced damage: a biochemical, morphological and behavioral study. Neurosci. 2007;146(4):1581–1592. doi:10.1016/j.neuroscience.2007.03.040
65. Alqudah MA, Alzoubi KH, Ma’abrih G, Khabour OF. Vitamin C prevents memory impairment induced by waterpipe smoke: role of oxidative stress. Inhalation Toxicol. 2018;30(4–5):141–148. doi:10.1080/08958378.2018.1474977
66. Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23(5):209–216. doi:10.1016/S0166-2236(99)01543-X
67. Ahmad A, Shah A, Badshah H, et al. Neuroprotection by vitamin C against ethanol-induced neuroinflammation associated neurodegeneration in developing rat brain. CNS Neurological Disorders Drug Tar. 2016;15(3):360–370. doi:10.2174/1871527315666151110130139
68. Othman MZ, Hassan Z, Has ATC. Morris water maze: a versatile and pertinent tool for assessing spatial learning and memory. Exp Animals. 2022;1:21–0120.
69. Miyoshi E, Wietzikoski EC, Bortolanza M, et al. Both the dorsal hippocampus and the dorsolateral striatum are needed for rat navigation in the Morris water maze. Behav Brain Res. 2012;226(1):171–178. doi:10.1016/j.bbr.2011.09.011
70. D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36(1):60–90. doi:10.1016/s0165-0173(01)00067-4
71. Tiwari V, Kuhad A, Chopra K. Suppression of neuro-inflammatory signaling cascade by tocotrienol can prevent chronic alcohol-induced cognitive dysfunction in rats. Behav Brain Res. 2009;203(2):296–303. doi:10.1016/j.bbr.2009.05.016
72. Pickering C, Alsiö J, Morud J, Ericson M, Robbins TW, Söderpalm B. Ethanol impairment of spontaneous alternation behaviour and associated changes in medial prefrontal glutamatergic gene expression precede putative markers of dependence. Pharmacol Biochem Behav. 2015;132:63–70. doi:10.1016/j.pbb.2015.02.021
73. Götesson J, Ericson M, Söderpalm B, Pickering C. Repeated ethanol but not phencyclidine impairs spontaneous alternation behaviour in the Y Maze. Basic Clin Pharmacol Toxicol. 2012;110(4):347–352. doi:10.1111/j.1742-7843.2011.00819.x
74. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutri. 2000;130(4):1007S–1015S. doi:10.1093/jn/130.4.1007S
75. Kalita J, Kumar V, Misra UK, Bora HK. Memory and learning dysfunction following copper toxicity: biochemical and immunohistochemical basis. Mol Neurobiol. 2018;55(5):3800–3811. doi:10.1007/s12035-017-0619-y
76. Faingold C, N’gouemo P, Riaz A. Ethanol and neurotransmitter interactions—from molecular to integrative effects. Prog Neurobiol. 1998;55(5):509–535.
77. Kozlov A, Druzin MY, Kurzina N, Malinina E. The role of D1-dependent dopaminergic mechanisms of the frontal cortex in delayed responding in rats. Neurosci Behav Physiol. 2001;31(4):405–411. doi:10.1023/A:1010488612338
78. Ayabe T, Ano Y, Ohya R, Kitaoka S, Furuyashiki T. The lacto-tetrapeptide Gly–Thr–Trp–Tyr, β-lactolin, improves spatial memory functions via dopamine release and D1 receptor activation in the hippocampus. Nutrients. 2019;11(10):2469.
79. Chaurasiya ND, Midiwo J, Pandey P, et al. Selective Interactions of O-methylated flavonoid natural products with human monoamine oxidase-A and-B. Molecules. 2020;25(22):5358. doi:10.3390/molecules25225358
80. O’Callaghan C, Hezemans FH, Ye R, et al. Locus coeruleus integrity and the effect of atomoxetine on response inhibition in Parkinson’s disease. MedRxiv. 2020;144:2513–2526.
81. Coradazzi M, Gulino R, Fieramosca F, Falzacappa LV, Riggi M, Leanza G. Selective noradrenaline depletion impairs working memory and hippocampal neurogenesis. Neurobiol Aging. 2016;48:93–102. doi:10.1016/j.neurobiolaging.2016.08.012
82. Vaghef L, Farajdokht F, Erfani M, et al. Cerebrolysin attenuates ethanol-induced spatial memory impairments through inhibition of hippocampal oxidative stress and apoptotic cell death in rats. Alcohol. 2019;79:127–135. doi:10.1016/j.alcohol.2019.03.005
83. Hatami S, Hatami H, Sheikhzade F, Dehghan G. Chronic administration of crystal meth in parallel with oxidative stress impaired spatial memory in prefrontal cortex of mail rats. J Sci. 2016;14(2):126.
84. Davis CK, Vemuganti R. Antioxidant therapies in traumatic brain injury. Neurochem Int. 2022;152:105255. doi:10.1016/j.neuint.2021.105255
85. Manrique HM, Miquel M, Aragon CM. Brain catalase mediates potentiation of social recognition memory produced by ethanol in mice. Drug Alc Depend. 2005;79(3):343–350. doi:10.1016/j.drugalcdep.2005.02.007
86. Zinn CG, Clairis N, Cavalcante LES, Furini CRG, de Carvalho Myskiw J, Izquierdo I. Major neurotransmitter systems in dorsal hippocampus and basolateral amygdala control social recognition memory. Proc Nat Acad Sci. 2016;113(33):E4914–E4919.
87. Marquardt K, Brigman JL. The impact of prenatal alcohol exposure on social, cognitive and affective behavioral domains: insights from rodent models. Alcohol. 2016;51:1–15. doi:10.1016/j.alcohol.2015.12.002
88. Baroni L, Sarni AR, Zuliani C. Plant foods rich in antioxidants and human cognition: a systematic review. Antioxidants. 2021;10(5):714. doi:10.3390/antiox10050714
89. Greenberg GD, Phillips TJ, Crabbe JC. Effects of acute alcohol withdrawal on nest building in mice selectively bred for alcohol withdrawal severity. Physiol Behav. 2016;165:257–266. doi:10.1016/j.physbeh.2016.08.006
90. Deacon RM, Croucher A, Rawlins JNP. Hippocampal cytotoxic lesion effects on species-typical behaviours in mice. Behav Brain Res. 2002;132(2):203–213. doi:10.1016/S0166-4328(01)00401-6
91. Jedynak P, Jaholkowski P, Wozniak G, Sandi C, Kaczmarek L, Filipkowski RK. Lack of cyclin D2 impairing adult brain neurogenesis alters hippocampal-dependent behavioral tasks without reducing learning ability. Behav Brain Res. 2012;227(1):159–166. doi:10.1016/j.bbr.2011.11.007
92. Kondratiuk I, Devijver H, Lechat B, Van Leuven F, Kaczmarek L, Filipkowski RK. Glycogen synthase kinase-3beta affects size of dentate gyrus and species-typical behavioral tasks in transgenic and knockout mice. Behav Brain Res. 2013;248:46–50. doi:10.1016/j.bbr.2013.03.045
93. Greenberg GD, Huang L, Spence S, et al. Nest building is a novel method for indexing severity of alcohol withdrawal in mice. Behav Brain Res. 2016;302:182–190. doi:10.1016/j.bbr.2016.01.023
94. Sager TN, Kirchhoff J, Mørk A, et al. Nest building performance following MPTP toxicity in mice. Behav Brain Res. 2010;208(2):444–449. doi:10.1016/j.bbr.2009.12.014
95. Gildawie KR, Galli RL, Shukitt-Hale B, Carey AN. Protective effects of foods containing flavonoids on age-related cognitive decline. Current Nutri Rep. 2018;7(2):39–48. doi:10.1007/s13668-018-0227-0
96. Spencer JP. The interactions of flavonoids within neuronal signalling pathways. Genes Nutri. 2007;2(3):257–273. doi:10.1007/s12263-007-0056-z
97. Chen W-F, Shih Y-H, Liu H-C, et al. 6-methoxyflavone suppresses neuroinflammation in lipopolysaccharide- stimulated microglia through the inhibition of TLR4/MyD88/p38 MAPK/NF-κB dependent pathways and the activation of HO-1/NQO-1 signaling. Phytomedicine. 2022;99:154025. doi:10.1016/j.phymed.2022.154025
98. Galati G. O’brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radical Biol Medi. 2004;37(3):287–303. doi:10.1016/j.freeradbiomed.2004.04.034
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.