Back to Journals » Open Access Emergency Medicine » Volume 16
A Case of Severe Rhabdomyolysis, Acute Myocardial Damage and Multi-Organ Dysfunction Syndrome in a Patient with Novel Coronavirus Pneumonia
Authors Yuan S, Huang Y, Xie P, Li P
Received 4 November 2023
Accepted for publication 24 January 2024
Published 1 February 2024 Volume 2024:16 Pages 19—28
DOI https://doi.org/10.2147/OAEM.S446994
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Hans-Christoph Pape
Shuaishuai Yuan, Yuting Huang, Pailing Xie, Peijun Li
Division of Cardiovascular Intensive Care (C-ICU), Cardiac and Vascular Center, The University of Hong Kong-Shenzhen Hospital, Guangdong, People’s Republic of China
Correspondence: Pailing Xie; Peijun Li, Email [email protected]; [email protected]
Abstract: In recent years, healthcare systems worldwide have faced the challenge of the severe COVID-19 pandemic. However, cases of severe rhabdomyolysis, acute myocardial damage, and multiple organ dysfunction syndrome (MODS) caused by COVID-19 are currently rare. This report presents a case of severe rhabdomyolysis, acute myocardial damage, and MODS caused by COVID-19. The patient was treated at The University of Hong Kong-Shenzhen Hospital. The purpose of this report is to aid clinicians in quickly identifying and treating similar cases, ultimately improving patient outcomes.
Keywords: COVID-19, pneumonia, rhabdomyolysis syndrome, acute myocardial damage, multiple organ dysfunction syndrome
Background
Since the emergence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in 2019, leading to COVID-19 disease, it has been linked to various complications, such as respiratory and thromboembolic events, and acute kidney injury (AKI).1,2 Rhabdomyolysis is a multifactorial disease caused by various congenital and acquired factors. Statin therapy is considered one of the most common acquired factors. The association between COVID-19 and rhabdomyolysis is not yet clear, and only a few case reports have described this link so far. It is known that viral infections can cause rhabdomyolysis,3 which is defined as damage to striated muscle cells. This damage causes intracellular substances, including myoglobin, to escape from creatine kinase into the bloodstream, resulting in acute kidney injury. This condition is life-threatening and has a high mortality rate.4 Based on current literature, it is possible to conclude that myocardial injury is a complication related to COVID-19 and may lead to myocarditis, myocardial infarction, Takotsubo syndrome, and other conditions. Myocardial injury is primarily caused by direct viral invasion or indirect downregulation of the angiotensin-converting enzyme 2 (ACE-2) receptor expression. This results in an immune-mediated overresponse, cytokine storm, and activation of the prethrombotic pathway through the interaction between viruses, myocardium, and endothelial cells.5 In addition to acute respiratory distress syndrome (ARDS), COVID-19 can induce an inflammatory cytokine storm, oxidative stress, and disseminated intravascular coagulation (DIC), which have been shown to cause organ damage and ultimately MODS in patients.6 This report presents a case of severe rhabdomyolysis, acute myocardial damage, and MODS in a COVID-19 patient to raise awareness among clinicians and prompt timely treatment measures.
Case Presented
A male migrant aged 53 presented with a fever and cough for one week. The patient started coughing on December 25th, 2022 and produced a small amount of yellow sticky sputum. This was followed by intermittent fever, with a maximum body temperature of 39.3°C, and dyspnea. The patient did not experience chills, vomiting, chest pain, or headache. Self-testing for new crown antigen was positive. The patient self-administered ibuprofen and other drugs, but their body temperature continued to rise. A chest CT scan conducted in the emergency department of the hospital revealed double lung viral pneumonia. Blood inflammation indicators also increased significantly. The patient was treated with moxifloxacin combined with cefoperazone sulbactam for anti-infection, as well as nebulized expectorant and other symptomatic treatments. During treatment, the patient experienced sudden cyanosis of the lips and shortness of breath at night. Their blood oxygen levels dropped to 80–85%, and coarse breath sounds were heard in both lungs. The patient had no history of smoking or drinking. He was admitted to our hospital with a positive nucleic acid test. The patient had undergone percutaneous coronary intervention (PCI) for coronary heart disease over 2 years ago and had a history of hypertension and diabetes for more than 20 years. The prescribed medication included oral aspirin, Lipitor, amlodipine, valsartan, and dapagliflozin. In March, the patient received the first dose of the inactivated Vero cell vaccine from the Beijing Institute of Biological Products Co., Ltd.
Upon admission, the patient’s physical examination showed a temperature of 37.5°C, a pulse rate of 104 beats per minute, a respiratory rate of 15 breaths per minute, and a blood pressure of 131mmHg/76mmHg. The patient was sedated and given analgesics and was assisted with breathing using a ventilator (SIMV (PS 10cmH2O, PEEP 5cmH2O, VT 420mL, F 15bpm, FiO2 50%)). The patient had slightly coarse breath sounds in both lungs, and crackles and sputum sounds were heard in both lower lungs. No bulge was observed in the precordial area, and the dullness boundary was normal. There was no tremor or pericardial friction. The heart rate was 104 beats per minute, with sinus rhythm and normal heart sounds. P2 was less than A2, and no pathological murmur was heard in the auscultation area of each valve. Additionally, no pericardial friction rub was detected. Please refer to Figure 1–2 for the admission CT and cardiac ultrasound.
 |
Figure 1 Chest CT screenshot upon admission. |
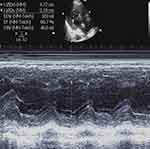 |
Figure 2 Echocardiographic screenshot of the heart. |
Regarding treatment, the patient received sedation and analgesia, as well as ventilator-assisted breathing with a lung-protective ventilation strategy to reduce ventilator-related lung injury. Additionally, antiviral and anti-infection medications have been administered, along with bedside bronchoscopy, cleaning of airway secretions, bronchoalveolar lavage, and other related pathogenic detection. Symptomatic treatment also included blood glucose control, antiplatelet therapy, blood lipid-lowering, blood pressure control, heart rate management, and improvement of the internal environment. During the treatment, the patient continued to test positive for nucleic acid and did not show significant improvement in arterial oxygen saturation. Intermittent treatment with prone ventilation, antivirals, hormones, and other therapies was administered. On the tenth day of hospitalization, the CT value of the new coronavirus nucleic acid remained low, and inflammatory factors such as white blood cells, C-reactive protein (CRP), and ferritin remained high. As a result, the treatment plan was adjusted. Please refer to Tables 1–2 for specific test results and treatment plans.
 |
Table 1 Patient Testing Information |
 |
Table 2 Patient Medication Information |
On the 13th day of hospitalization, the patient’s daytime test results showed abnormal indicators for blood routine, biochemical examination, interleukin-6, ferritin, procalcitonin, and CRP, as shown in Table 1. Atorvastatin was discontinued due to its potential influence on the results. Additionally, the patient reported having dark urine at night, similar to soy sauce. On the 14th day of hospitalization, the patient’s liver and kidney function deteriorated further. The patient developed oliguria, and critical values were reported for creatine kinase (CK), Troponin T (TnT), and myoglobin (MYO). As a result, the patient was immediately given continuous renal replacement therapy (CRRT) (CVVHDF mode, citrate anticoagulation) supportive treatment. Simultaneously, the patient received antibiotic treatment, anticoagulation therapy, antiviral medication, gamma globulin support, and other necessary treatments. Additionally, the patient underwent improved ECG, bedside cardiac ultrasound, and limb skeletal muscle ultrasound, as shown in Figure 3–4. The electrocardiogram and cardiac ultrasound did not reveal any abnormalities, while the limb skeletal muscle ultrasound showed increased echo thickening, uneven echo, a small amount of fluid in the muscle space, and suspected striated muscle damage. A muscle biopsy was not carried out due to personal reasons. Following treatment with anti-infection, anti-viral medication, inhibition of the inflammatory response, CRRT, and the prone position, the patient’s urine gradually returned to a clear colour. A tracheostomy was performed on the 18th day of hospitalization, and sedative and analgesic drugs were discontinued after the procedure. On the 20th day of hospitalization, the patient regained consciousness and scored E4VTM5 on the Glasgow Coma Scale (GCS). The patient’s troponin levels peaked on the same day. The electrocardiogram and cardiac ultrasound examinations showed improvement, and no pathological changes were found in the patient’s ECG monitoring, electrocardiogram, and cardiac ultrasound before and after the troponin peak.
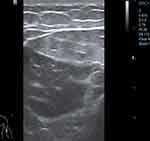 |
Figure 3 Ultrasound screenshot of upper limb muscles. |
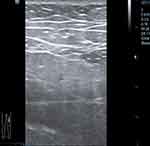 |
Figure 4 Ultrasound screenshot of lower limb muscles. |
On the 33rd day of admission, the patient suddenly lost consciousness with a GCS score of E1VTM3. The patient’s pupils were bilaterally equal in size with a diameter of 2.5mm and exhibited light reflexes, corneal reflexes, and cephalic-ocular reflexes. Faint frowning was visible in bilateral orbital compression, and the patient exhibited involuntary limb movements. The patient’s neck was soft, and no bilateral pathological signs were elicited. The study found that the cerebrospinal fluid tested negative for the new coronavirus nucleic acid. However, the cerebrospinal fluid oligoclonal zone analysis (OCB) showed a positive result for immunoglobulin G (CSF IgG), and the OCB showed that the cerebrospinal fluid had the same band as the blood (Figure 5). The lesion was determined to be outside the central nervous system (CNS), indicating a peripheral B cell immune response and damage to the blood-brain barrier. This is believed to be associated with systemic inflammation caused by COVID-19 infection. Due to the patient’s critical condition, an MRI brain evaluation was not performed. Instead, we continued with the current anti-infection and other treatments. As a result, the patient’s consciousness gradually recovered on the 45th day of hospitalization.
 |
Figure 5 Cerebrospinal Fluid Electrophoresis Results. |
The patient’s treatment plan was gradually adjusted based on the condition of their lungs and organs. This included respiratory function exercises, intermittent prone position, pulmonary physical therapy, intermittent CRRT, and vasoactive drug support. Individualized rehabilitation and psychological support were provided due to the patient’s long-term bed rest, muscle weakness, and skin damage. As a result, the patient was successfully weaned off the ventilator on the 52nd day and was given a tracheostomy tube connected to high-flow humidified oxygen therapy (Flow rate: 50 L/min, FiO2: 30%). On the 60th day of admission, the patient was discharged from the hospital after 78 days of recovery and treatment. The patient had a tracheostomy and was using artificial nasal inhalation at a rate of 3 L/min. At the time of discharge, all organs had been restored to full function and the patient’s pulse oxygen saturation was 98%, as shown in Figure 6.
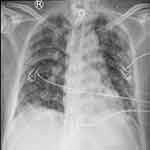 |
Figure 6 Chest X-ray at discharge. |
Discussion
Rhabdomyolysis is defined as CK levels above normal (>1000 IU/L) or five times the upper limit of normal.7 Patients with rhabdomyolysis may present with myalgia or weakness, red to brown urine, and markedly elevated levels of CK and MYO. Other manifestations include electrolyte abnormalities such as hyperkalemia, hyperphosphatemia, hyperuricemia, hypocalcemia, and metabolic acidosis, followed by complications such as AKI, compartment syndrome, and DIC.8 In a retrospective cohort study of 140 patients, the incidence of rhabdomyolysis was 16.7% in hospitalized patients with COVID-19, 47.1% in patients with COVID-19 and rhabdomyolysis, and 26.4% in patients with COVID-19 without rhabdomyolysis.9
Acute myocardial injury is defined as elevated troponin levels and all conditions leading to myocardial cell death.10 Studies have shown that the incidence of acute myocardial injury in hospitalized patients with COVID-19 ranges from 7% to 36%. Patients with COVID-19 have a four-fold higher risk of death from cardiovascular disease.11 In a study of hospitalized patients with COVID-19 from New York, USA, the mortality rate from myocardial injury was 18.5%.12 COVID-19 may have an impact on the cardiovascular system, resulting in acute coronary syndromes, myocarditis, and electrical heart disease.
All individuals are susceptible to COVID-19, but the severity of the disease and its impact on different bodily systems can vary greatly. Severe cases, particularly in certain populations, may result in ARDS and damage to multiple bodily systems, including the respiratory, cardiovascular, digestive, urinary, nervous, and immune systems. Severe cases, particularly in certain populations, may result in ARDS and damage to multiple bodily systems, including the respiratory, cardiovascular, digestive, urinary, nervous, and immune systems. The clinical features of 41 COVID-19 patients were analysed by the investigators.13 They found that 5 patients had acute myocardial injury and 3 had acute kidney injury, most of whom were critically ill. Chen14 conducted a statistical study of 99 COVID-19 patients and showed that 11% of patients experienced short-term exacerbations followed by death from MODS, including heart failure, acute kidney injury, and shock.
Research indicates that COVID-19 is associated with high mortality rates in cases of rhabdomyolysis, acute myocardial injury, and MODS. Therefore, it is crucial to determine the incidence of these conditions in COVID-19 patients to improve patient prognosis. In hospitalized patients with COVID-19, rhabdomyolysis, acute myocardial injury, and MODS are important and often underestimated complications. Mortality rates are higher when patients have concurrent rhabdomyolysis, acute myocardial injury, and MODS. It is crucial to monitor and manage these complications in COVID-19 patients. Retrospective cohorts have reported peak rhabdomyolysis CK levels of over 1000 units/L in COVID-19 patients.15 Few case reports have described severe rhabdomyolysis during the course of the disease, with peak CK levels reaching approximately 39580 units/L. This case report describes a unique instance of severe COVID-19-induced rhabdomyolysis, acute myocardial injury, and MODS to aid in timely detection and treatment.
The binding of the SARS-CoV-2 virus to ACE-2 is a crucial step in the pathophysiology of COVID-19 patients. SARS-CoV-2 binds to ACE-2 through the receptor-binding domain of its spike protein. Additionally, the serine protease TMPRSS2 may also play a significant role in facilitating SARS-CoV-2 invasion of cells.16,17 ACE-2 is widely distributed in bones and myocardium.18,19 The mechanism by which COVID-19 induces rhabdomyolysis, acute myocardial injury, and MODS remains unclear. The proposed mechanisms include both direct and indirect pathways. According to the first theory, the virus invades muscles directly through ACE-2, causing damage to skeletal and cardiac muscles as well as organs.9,20,21 However, the mechanism is not well understood. The second theory suggests that the host’s immune response, similar to a hyperinflammatory cytokine storm, may cause damage to skeletal muscle, myocardium, and organs.22 Studies have demonstrated the significant role of interleukins and tumor necrosis factor in the pathogenesis of the cytokine storm in COVID-19,23 which ultimately leads to fibrinolysis and fibrosis of skeletal and cardiac muscle, as well as an inflammatory response in organs.
Conclusion
The patient is a middle-aged male with a medical history of coronary heart disease, hypertension, and diabetes. Upon acute onset, he was admitted to the hospital due to respiratory failure. No other significant organ dysfunction was observed at the time of admission, except for the lungs. Considering the patient’s medical record, which indicates rhabdomyolysis, acute myocardial injury, and multi-organ function damage (including liver, kidney, and nervous system function), as well as the direct and indirect immune damage caused by the virus, there may be other contributing factors. Reason 1 is currently under investigation. Due to the patient’s pre-existing medical conditions, they have been taking statins orally for an extended period. Some studies suggest that statins may increase the risk of organ damage caused by rhabdomyolysis. However, despite the patient’s long-term use of oral statins, testing during treatment did not reveal any symptoms of organ damage or muscle aches. It is important to note that the effect of the new coronavirus on statin metabolism cannot be ruled out. 2. The patient’s medical history includes diabetes and chronic pathophysiological changes resulting from poor blood glucose control, as well as the new crown inflammatory storm. 3. The patient has undergone long-term treatment, including ventilator therapy and the use of analgesic and sedative drugs. 4. The patient has been bedridden for an extended period, which has resulted in limb restraint and compression due to the prone position, among other factors. During the treatment process, the patient’s new crown nucleic acid remained positive, and the CT value remained low while the inflammatory index continued to increase. This was due to the patient’s sustained high viral load and the inflammatory response in the body.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Consent for Publication
All authors agree to publication.
Ethical Approval of Studies and Informed Consent
This study was approved by The University of Hong Kong-Shenzhen Hospital Ethics Committee (No. [2023]025) and written informed consent was obtained from the patient. All authors have read the manuscript and approved its submission to the journal. This manuscript has the consent of the patient for the use of his data and for the publication of the data that appear in the article. All methods were carried out following the relevant guidelines and all methods were carried out under the Declaration of Helsinki.
Acknowledgments
The authors express their heartfelt thanks to all those who helped in the process of writing this paper. The authors also acknowledged The University of Hong Kong-Shenzhen Hospital for giving them such a good opportunity.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The general program of the National Natural Science Foundation of China (82170435).
Disclosure
All authors declare that they have no competing interests in this work.
References
1. Gavriatopoulou M, Korompoki E, Fotiou D, et al. Organ-specific manifestations of COVID-19 infection. Clin Exp Med. 2020;20(4):493–506. doi:10.1007/s10238-020-00648-x
2. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45(8):100618. doi:10.1016/j.cpcardiol.2020.100618
3. Torres PA, Helmstetter JA, Kaye AM, Kaye AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58–69.
4. Ali L, Mohammed I, Janjua I, et al. Acute Myocardial Injury and Rhabdomyolysis in COVID-19 Patients: incidence and Mortality. Cureus. 2021;13(10):1.
5. Del Prete A, Conway F, Della Rocca DG, et al. COVID-19, acute myocardial injury, and infarction. Card Electrophysiol Clin. 2022;14(1):29–39. doi:10.1016/j.ccep.2021.10.004
6. Zhao W, Li H, Li J, Xu B, Xu J. The mechanism of multiple organ dysfunction syndrome in patients with COVID-19. Med Virol. 2022;94(5):1886–1892. doi:10.1002/jmv.27627
7. Stahl K, Rastelli E, Schoser B. A systematic review on the definition of rhabdomyolysis. J Neurol. 2020;267(4):877–882. doi:10.1007/s00415-019-09185-4
8. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis -- an overview for clinicians. Crit Care. 2005;9(2):158–169. doi:10.1186/cc2978
9. Haroun MW, Dieiev V, Kang J, et al. Rhabdomyolysis in COVID-19 patients: a retrospective observational study. Cureus. 2021;13(1):e12552. doi:10.7759/cureus.12552
10. Sandoval Y, Jl J Jr, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19. J Am Coll Cardiol. 2020;76(10):1244–1258. doi:10.1016/j.jacc.2020.06.068
11. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi:10.1001/jamacardio.2020.0950
12. Russak AJ, Paranjpe I, Richter F, et al. Mount sinai COVID informatics center. prevalence and impact of myocardial injury in patients hospitalized with COVID-19 Infection. J Am Coll Cardiol. 2020;76(5):533–546. doi:10.1016/j.jacc.2020.06.007
13. Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J. Gen Intern Med. 2020;35(5):1545–1549. doi:10.1007/s11606-020-05762-w
14. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
15. Borku Uysal B, Ikitimur H, Yavuzer S, Islamoglu MS, Cengiz M. Case report: a COVID-19 patient presenting with mild rhabdomyolysis. Am J Trop Med Hyg. 2020;103(2):847–850. doi:10.4269/ajtmh.20-0583
16. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi:10.1038/s41586-020-2012-7
17. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry Depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi:10.1016/j.cell.2020.02.052
18. Zhang D, Zhang X, Ma R, et al. Ultra-fast and onsite interrogation Of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in waters via surface enhanced raman scattering (SERS). Water Res. 2021;200:117243. doi:10.1016/j.watres.2021.117243
19. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi:10.1002/path.1570
20. Fox SE, Lameira FS, Rinker EB, Vander Heide RS. Cardiac endotheliitis and multisystem inflammatory syndrome after COVID-19. Ann Intern Med. 2020;173(12):1025–1027. doi:10.7326/L20-0882
21. Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41(32):3038–3044. doi:10.1093/eurheartj/ehaa623
22. Fadila MF, Wool KJ. Rhabdomyolysis secondary to influenza a infection: a case report and review of the literature. N Am J Med Sci. 2015;7(3):122–124. doi:10.4103/1947-2714.153926
23. Chen R, Lan Z, Ye J, et al. Cytokine Storm: the Primary determinant for the pathophysiological evolution of covid-19 deterioration. Front Immunol. 2021;12:589095. doi:10.3389/fimmu.2021.589095
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
