Back to Journals » Clinical Interventions in Aging » Volume 19
A Comprehensive Patient Blood Management Program During Cardiopulmonary Bypass in Patients Over 60 Years of Age
Authors Zhang Q, Yan W, Gao S, Diao X, Liu G, Wang J, Ji B
Received 10 October 2023
Accepted for publication 14 February 2024
Published 7 March 2024 Volume 2024:19 Pages 401—410
DOI https://doi.org/10.2147/CIA.S443908
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Qiaoni Zhang,1 Weidong Yan,2 Sizhe Gao,3 Xiaolin Diao,4 Gang Liu,1 Jing Wang,1 Bingyang Ji1
1Department of Cardiopulmonary Bypass, Fuwai Hospital, Chinese Academy of Medical Science and Peking Union Medical College, National Clinical Research Center for Cardiovascular Disease, Beijing, People’s Republic of China; 2Department of Anesthesiology, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Department of Pain, Beijing Jishuitan Hospital, Capital Medical University, Beijing, People’s Republic of China; 4Department of Information Center, Fuwai Hospital, Chinese Academy of Medical science and Peking Union Medical College, National Clinical Research Center for Cardiovascular Disease, Beijing, People’s Republic of China
Correspondence: Bingyang Ji, Department of Cardiopulmonary Bypass, Fuwai Hospital, No. 167 Beilishi Road, Xicheng District, Beijing, 100037, People’s Republic of China, Email [email protected]
Purpose: There is currently no consensus on the most appropriate blood transfusion strategy for older adults undergoing cardiovascular surgery. We aimed to investigate the potential benefits of the patient blood management (PBM) program specifically for advanced age patients, and to evaluate the relationship of age and PBM in cardiovascular surgery.
Patients and Methods: We collected data from patients over 60 years old who underwent on-pump cardiovascular surgery. We compared transfusion and clinical outcomes between the pre-PBM and post-PBM groups using a propensity score matching method. Then, we conducted a subgroup analysis within the original cohort, specifically focusing on patients aged of 75 and above with multivariable adjusted models.
Results: Data of 9703 older adults were analyzed. Red blood cell (RBC) transfusion rates during cardiopulmonary bypass (CPB) (31.6% vs 13.1%, P< 0.001), during the operation (50.8% vs 39.0%, P< 0.001) and after the operation (5.6% vs 3.1%, P< 0.001) were significantly reduced, and mortality and the risk of some adverse events were also reduced after the PBM. Subgroup analysis showed that there was no interaction between age and PBM, and advanced age (over age 75) did not modify the effect of PBM program in reducing RBC transfusion (Pinteraction=0.245), on mortality (Pinteration=0.829) and on certain complications.
Conclusion: The comprehensive PBM program could reduce RBC transfusion without adverse outcomes in older patients undergoing CPB. Even patients over age 75 may benefit from a more stringent transfusion indication. Comprehensive blood conservation measures should be applied to optimize the blood management for older patients.
Keywords: cardiopulmonary bypass, cardiovascular surgery, older adults, patient blood management, red blood cell transfusion
Introduction
Older adults exhibit unique physiological characteristics, including reduced functional reserve, diminished compensatory capacity, and increased vulnerability to complications.1 Within this population, including those undergoing cardiovascular surgery, there is limited tolerance to anemia. Anemia has been linked to unfavorable outcomes such as mortality, acute kidney injury, and respiratory insufficiency.2–4 However, it is important to note that blood transfusion itself can pose risks for postoperative adverse events.5,6 Consequently, it becomes crucial to strike a balance between managing anemia and determining an appropriate transfusion threshold specifically for older adults.
Several studies have provided contrasting findings regarding the optimal blood transfusion strategy for older surgical patients. Some studies have indicated that a liberal transfusion strategy, with a hemoglobin threshold between 85–100 g/L, yields more favorable outcomes.7–9 On the other hand, other studies have suggested a restrictive blood transfusion approach, with a hemoglobin threshold between 65–75 g/L. This latter strategy has not been associated with an increase in the risk of adverse outcomes while reducing the utilization of blood products.10,11 Consequently, a consensus regarding the most appropriate blood transfusion strategy for older patients has yet to be reached. Further research and evidence are required to guide clinical practice and inform the decision-making process in this population.
In our previous study, we have demonstrated remarkable results of a comprehensive patient blood management (PBM) program during the period of cardiopulmonary bypass (CPB) for cardiovascular surgery patients.12 This study focused on older patients aged 60 years and above, with further subgroups analyses for those aged 60 to 74 and those over age 75. The primary objective was to determine whether the implementation of the PBM program provided benefits to advanced age patients. Additionally, we aimed to assess the relationship between age, the PBM program, and clinical outcomes in the context of cardiovascular surgery.
Material and Methods
Study Design and Patients
This retrospective study was based on the electronic medical records of our hospital. We collected patients aged more than 60 years old who underwent cardiovascular surgery with CPB between January 1, 2015 and December 31, 2020. The exclusion criteria were as follows: (1) patients who underwent heart transplantation, (2) patients who underwent left ventricular assist device (LVAD) implantation and (3) patients who underwent emergency surgery. Our quality management of the comprehensive PBM program was started from 2018. So, we divided patients into two groups: the pre-PBM group (January 1, 2015 - December 31, 2017) and the post-PBM group (January 1, 2018 - December 31, 2020).
We compared the baseline characteristics, blood transfusion rates, and clinical outcomes between these two groups. Furthermore, we conducted subgroup analyses within the original cohort, stratifying patients into two groups based on the age of 75. The aim was to assess whether there was an interaction effect between age and the PBM program, specifically examining whether advanced age modified the effects of the PBM program.
We used the STROBE reporting guidelines in designing this study.13
The Comprehensive PBM Program and Quality Management Methods
The comprehensive PBM program during CPB is composed of following five measures:
- The restrictive red blood cell (RBC) transfusion strategy:a. Hemoglobin <70 g/L during CPB.b. Hemoglobin <80 g/L after ultrafiltration or reinfusion of residual pump blood and washed RBCs.c. Hemoglobin <90 g/L after ultrafiltration or reinfusion of residual pump blood and washed RBCs for patients aged more than 70 years or who underwent aortic operations.
- Conventional ultrafiltration: If it is estimated that the remaining blood in the reservoir exceeds 1000mL after the conclusion of CPB, we then utilize conventional ultrafiltration before weaning of CPB.
- Routine use of cell salvage intraoperatively.
- Residual pump blood ultrafiltration before reinfusion: It is primarily to address volume overload issues. After the conclusion of CPB, residual pump blood was ultrafiltrated with hemoconcentrator until the level reached 0mL in the reservoir, then it was reinfused via aortic cannula as required by the anesthetists according to the patients’ volume status and cardiac function.14
- The modified minimal extracorporeal circulation (MECC) system. It was composed of a shortened circuit, roller pump, reservoir, oxygenator with arterial filter, vacuum-assist venous drainage (VAVD) device and microplegia. It was used in patients with small body size (weight less than 60 kg), preoperative anemia and less estimated operating time (less than three hours).
The detailed protocols of each measure and quality management methods are described in detail in our previous study12 as well as in the Supplemental Material of this study.
Outcomes
The primary outcomes for this study were RBC transfusion, 30-day mortality and major complications. Definitions of specific complication events are described in the Supplemental Material. Secondary outcomes included mechanical ventilation time, chest drainage, length of stay in the hospital and ICU lengths of stay, and results of some laboratory tests.
Statistical Analysis
Continuous variables with missing values of less than 5% were imputed using the median value. For categorical variables with missing values of less than 5%, the lowest risk category was assigned (eg, patients with missing smoking information were considered non-smokers). Patients were excluded if their variables had more than 5% missing values. Descriptive statistics for continuous variables were presented as mean ± standard deviation if the data followed a normal distribution, or as medians (25th percentile, 75th percentile) if the data were not normally distributed. Categorical variables were described as the number and percentage of patients in each category.
To compare the pre-PBM and post-PBM groups, we employed a 1:1 propensity score matching (PSM) technique to adjust for baseline differences. The PSM was carried out using a logistic regression model, incorporating variables that showed statistical significance in univariable analysis (P<0.1). A caliper value of 0.02 was used to ensure close matches between participants. A standardized mean difference (SMD) of less than 0.1 indicated balance within the model. Subsequently, we compared the blood transfusion outcomes and clinical results between the matched groups. Continuous variables were analyzed using the Mann–Whitney U-test, while categorical variables were assessed using the chi-square test or Fisher’s exact test, as appropriate. All statistical tests were two-sided.
The methodology for subgroup analysis is as follows. To assess the potential effect modification of advanced age on the PBM program, we employed multivariable adjusted models in the original cohort. These models incorporated an age-by-PBM interaction term to examine any interaction effect. For continuous dependent variables, multivariable linear regression models were utilized, while logistic regression models were employed for categorical dependent variables. Pinteraction value was obtained by the aforementioned models. For all continuous dependent variables and all categorical dependent variables in this subgroup analysis, independent variables involved in these multivariable models were the same, including the variables which showed statistical significance in univariable analysis (P<0.1) in Table S1, incorporated age (aged 60–74/over age 75), PBM (pre-PBM/post-PBM), age-by-PBM interaction term, sex, EuroSCORE, EF, previous myocardial infarction, hyperlipidemia, chronic lung disease, renal dysfunction, previous cardiac surgery, hematocrit, platelets, creatinine, surgery type, CPB time.
The significance level was 0.05. All statistical analyses were performed using SPSS version 25.0 software (IBM Crop, Chicago, IL, USA).
Results
Patient Basic Characteristics
A total of 10,116 patients aged 60 years and older underwent cardiovascular surgery with CPB between 2015 and 2020. Among them, 9703 patients met the inclusion and exclusion criteria and were included in the analysis. The study cohort consisted of 5006 patients before the PBM program and 4697 patients after the program (Figure 1).
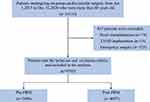 |
Figure 1 Flow chart of the study cohort. Abbreviations: LVAD, left ventricular assist device; PBM, patient blood management. |
After conducting propensity score matching, a total of 3611 pairs were created between the pre-PBM and post-PBM groups. This matching process resulted in balanced baseline and operative characteristics between the two groups (Table 1). The baseline characteristics of the pre-matching groups can be found in Table S1.
 |
Table 1 Basic Characteristics Between Two Groups After Matching |
Transfusion Results and Clinical Outcomes
Following the implementation of the PBM program, a significant decrease of RBC transfusion was observed during CPB (31.6% vs 13.1%, P<0.001), in the operation (50.8% vs 39.0%, P<0.001), and after the operation (in the ICU) (5.6% vs 3.1%, P<0.001) (Table 2).
 |
Table 2 Transfusion Results and Clinical Outcomes After Matching |
In comparison to patients before the PBM program, those who underwent the PBM exhibited several favorable outcomes, including a shorter length of hospital stay, reduced ventilation time, decreased chest drainage. Besides, the 30-day mortality reduced after PBM (1.0% vs 0.6%, P=0.048), the risk of combined complications, acute kidney injury (AKI), excessive chest drainage and re-sternotomy also reduced obviously after the PBM program (Table 2).
Subgroup Analysis
A total of 533 patients aged over 75 years were included in this study, consisting of 281 patients in the pre-PBM group and 252 patients in the post-PBM group. There was no interaction between age and PBM program, advanced age (over age 75) did not modify the effect of PBM program in reducing RBC transfusion rates during CPB (Pinteraction=0.245), during the operation (Pinteraction=0.329), and after the operation (Pinteraction=0.607) (Figure 2).
Likewise, advanced age did also not modify the PBM effect in mortality (aged 60–74, OR 0.64, 95% CI 0.35–1.16; over age 75, OR 0.33, 95% CI 0.07–1.64, Pinteration=0.829), combined complications (aged 60–74, OR 0.9, 95% CI 0.79–1.03; over age 75, OR 0.91, 95% CI 0.57–1.48, Pinteration=0.68) and other complications (Figure 3A). Other outcomes such as mechanical ventilation, chest drainage, length of stay in the hospital and ICU stays showed familiar result (Figure 3B).
Discussion
Our study demonstrated that the comprehensive PBM program during CPB effectively reduced RBC transfusion rates and lowered the risk of certain complications in older patients aged 60 and above. Additionally, we conducted subgroup analyses within this population, specifically focusing on patients aged 60–74 and those over the age of 75. The results indicated that the beneficial effects of the PBM program remained consistent regardless of increasing age. These findings underscore the potential benefits of implementing a comprehensive PBM program for older patients undergoing CPB in cardiovascular surgery.
Age has been consistently associated with an increased risk of mortality and complications. Research studies have indicated that every 10-year increase in age is associated with a 1.35-fold higher risk of 30-day mortality.15 Furthermore, age is recognized as an independent risk factor for the development of diseases, injuries, hospitalizations, prolonged hospital stays, and adverse drug reactions.16
The decision regarding blood transfusion requires a comprehensive evaluation of various factors, including anemia, transfusion-related complications, surgical considerations, medical techniques, and more. Numerous studies have highlighted the potential adverse reactions associated with red blood cell (RBC) transfusion.5,6 Most current PBM guidelines recommend a restrictive transfusion strategy with a hemoglobin threshold of 70–80 g/L perioperatively for cardiovascular patients.17–19 However, it is crucial to acknowledge that different patient groups may require different transfusion thresholds based on their individual physiological characteristics and clinical needs. The results of the TRICS-III trial20,21 demonstrated that a restrictive transfusion strategy could effectively reduce the risk of the primary composite endpoint in cardiac surgery patients over the age of 75, suggesting that these patients may benefit from less transfusion. Another study focusing on major surgeries also indicated that patients over the age of 65 should adopt a restrictive strategy to minimize unnecessary blood utilization.10
Our findings align with previous research, indicating that advanced age does not alter the effectiveness of the PBM program. Consequently, older patients should also receive the benefits of multiple blood conservation measures aimed at reducing blood utilization. This observation can be attributed to the complex health conditions often present in older patients, including multiple comorbidities, diminished organ function, and limited compensatory capacity. Such patients are at a higher risk of experiencing adverse reactions associated with blood transfusion, and any potential benefits of transfusion may be overshadowed by these adverse events. Additionally, older patients typically have a lower basal metabolic rate1 and may not require a high hemoglobin level to maintain adequate oxygen supply. Excessive transfusion in this population can lead to unfavorable outcomes due to transfusion-related adverse reactions. Therefore, physicians must carefully weigh the potential advantages and disadvantages of transfusion in older patients, considering the additional burden on their bodies and the potential for adverse consequences.
In contrast to the above-mentioned studies, another study has shown that the restrictive strategy in cardiac surgery patients over age of 60 led to increased risk of postoperative cardiogenic shock, compared with a liberal transfusion strategy.22 A meta-analysis involving cardiac surgeries and orthopedic surgeries showed that a liberal strategy was more beneficial for the older patients over age 65 on reducing short-term and long-term mortality.23
In our perspective, the conflicting results between these studies and our findings can be attributed to the focus solely on the transfusion strategy as the variable. Lowering the transfusion threshold alone may lead to lower postoperative hemoglobin levels or a predisposition to anemia, potentially impacting oxygen supply in the body. It is important to note that older patients have reduced tolerance to anemia and hypoxia,24 and the presence of anemia can affect patient prognosis. In our study, we implemented a comprehensive PBM program that combined multiple blood conservation measures alongside a lowered transfusion threshold. This approach aimed to maintain an appropriate postoperative hemoglobin level, ensuring adequate oxygen supply and preventing overt anemia. Through our comprehensive approach, we believe that older patients derived benefits from the PBM program, achieving the objective of reducing blood transfusions safely and effectively.
In cardiac surgery, CPB can lead to increased likelihood of blood transfusion due to factors such as hemodilution, blood destruction and activation of the coagulation system. As a result, this study places emphasis on blood management during the CPB period. However, it is important to note that blood management measures extend beyond this specific period and encompass preoperative, intraoperative, and postoperative phases as well. Comprehensive blood management strategies are necessary to optimize patient outcomes throughout the entire surgical process. First, preoperative management of anemia is important. AT our hospital, patients undergoing elective surgery with mild anemia (hemoglobin <110 g/L for women and <120 g/L for men) received iron treatment and nutrition supplementation, those whose hemoglobin was <90 g/L received erythropoietin two days before the scheduled operation, while those with a lever below 70 g/L were transfused. Furthermore, appropriate discontinuation of antiplatelet and anticoagulant medications prior to surgery is crucial for effective preoperative PBM. Antiplatelet drugs and aspirin should be halted 5–7 days before the scheduled surgery, while warfarin should be discontinued 2 days in advance, as its effects can be counteracted by vitamin K. This criterion remained consistent throughout the study period, so it was not included as a variable in our study. During the intraoperative phase, antifibrinolytic agents such as tranexamic acid and aminocaproic acid were routinely administered. For conventional on-pump cardiac surgeries, the total dosage of tranexamic acid ranged from 80–100 mg/kg, while for secondary cardiac surgeries, major aortic surgeries, and heart transplantation, the dosage ranged from 100–150 mg/kg. Our study found no statistical difference in the proportion of antifibrinolytic usage before and after the implementation of the PBM program (99.9% vs 99.7%). If necessary, blood products and derivatives such as prothrombin complex, fibrinogen and recombinant factor VII concentrate can be used intraoperatively. Postoperatively, human albumin can be used as a therapeutic measure to replenish fluids and reduce the need for blood transfusion when necessary. These considerations emphasize the importance of multidisciplinary communication and collaboration in implementing PBM protocols. It is essential for various clinical departments to work together, striving to achieve a consensus on optimal blood management practices.
The study has several limitations. Firstly, being a retrospective study conducted at a single center, inherent biases may exist, and the generalizability of the findings to other centers may be limited. Secondly, although we assessed the comprehensive effects of multiple blood conservation measures, the individual impact of each measure remains unclear. Thirdly, our study primarily focused on short-term outcomes, and the association between older age, PBM, and long-term clinical outcomes requires more in-depth investigation. Lastly, it is important to note that the surgical team might have changed dynamically over the six-year study period, potentially impacting the perioperative demand for blood transfusion. Unfortunately, we lacked specific quantifiable indicators to account for these changes, which could introduce a confounding factor that we were unable to mitigate. Despite these limitations, our study provides valuable insights into the impact of the PBM program on older patients undergoing cardiovascular surgery. Further research is needed to address the aforementioned limitations and validate our findings in larger, multicenter prospective studies.
Conclusion
The implementation of a comprehensive PBM program in older patients undergoing CPB can effectively reduce RBC transfusion requirements without adverse outcomes. Notably, advanced age does not alter the beneficial impact of the PBM program, indicating that older patients, including those over the age of 75, can benefit from a stricter transfusion indication. It is important to note that age alone should not be the sole determining factor for blood transfusion in older patients. Instead, a comprehensive approach involving multiple blood conservation measures should be adopted to optimize blood management in this patient population.
Abbreviations
AKI, Acute kidney injury; BMI, Body mass index; CABG, Coronary artery bypass grafting; CPB, Cardiopulmonary bypass; ECMO, Extracorporeal membrane oxygenation; EF, Ejection fraction; IABP, Intra-aortic balloon pump; ICU, Intensive care unit; LVAD, Left ventricular assist device; MECC, Mini-extracorporeal circulation system; PBM, Patient blood management; PSM, Propensity score matching; RBC, Red blood cell; SMD, Standardized mean difference.
Data Sharing Statement
The data underlying this article were provided by the Department of Information Center of our hospital under license. The data are not publicly available due to ethical restrictions.
Ethics Approval and Informed Consent
This study strictly adheres to the Helsinki Declaration, ensuring patient privacy and data confidentiality. We have established rigorous data management protocols, incorporating anonymization, authorized access, and thorough ethical review to guarantee full respect for patient rights. So, this study was approved by the Ethics Committee of Fuwai Hospital (NO.2020-1288, 31st December, 2020). Furthermore, the data were extracted from our hospital’s existing database, it was impractical to re-obtain all patients’ informed consent. Given that our study involves solely the analysis of anonymized data without any direct human intervention, we were exempted from the requirement for informed consent.
Consent for Publication
The details of any images, videos, recordings, etc. can be published.
Acknowledgments
The authors wish to thank Xu Wang for data curation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that there is no conflict of interest in this work.
References
1. Alvis BD, Hughes CG. Physiology considerations in geriatric patients. Anesthesiol Clin. 2015;33(3):447–456. doi:10.1016/j.anclin.2015.05.003
2. Rohrig G. Anemia in the frail, elderly patient. Clin Interv Aging. 2016;11:319–326. doi:10.2147/CIA.S90727
3. Pang WW, Schrier SL. Anemia in the elderly. Curr Opin Hematol. 2012;19(3):133–140. doi:10.1097/MOH.0b013e3283522471
4. Faria LB, Mejia OV, Miana LA, et al. Anemia in cardiac surgery - can something bad get worse? Braz J Cardiovasc Surg. 2021;36(2):165–171. doi:10.21470/1678-9741-2020-0304
5. Patel NN, Murphy GJ. Evidence-based red blood cell transfusion practices in cardiac surgery. Transfus Med Rev. 2017;31(4):230–235. doi:10.1016/j.tmrv.2017.06.001
6. Murphy GJ, Reeves BC, Rogers CA, Rizvi SIA, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116(22):2544–2552. doi:10.1161/CIRCULATIONAHA.107.698977
7. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after Hip surgery. N Engl J Med. 2011;365(26):2453–2462. doi:10.1056/NEJMoa1012452
8. Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165(6):964–971 e961. doi:10.1016/j.ahj.2013.03.001
9. Hovaguimian F, Myles PS. Restrictive versus liberal transfusion strategy in the perioperative and acute care settings: a context-specific systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2016;125(1):46–61. doi:10.1097/ALN.0000000000001162
10. Valero-Elizondo J, Spolverato G, Kim Y, et al. Sex- and age-based variation in transfusion practices among patients undergoing major surgery. Surgery. 2015;158(5):1372–1381. doi:10.1016/j.surg.2015.04.030
11. Fan YX, Liu FF, Jia M, et al. Comparison of restrictive and liberal transfusion strategy on postoperative delirium in aged patients following total Hip replacement: a preliminary study. Arch Gerontol Geriatr. 2014;59(1):181–185. doi:10.1016/j.archger.2014.03.009
12. Zhang QN, Zhao W, Gao SZ, et al. Quality management of a comprehensive blood conservation program during cardiopulmonary bypass. Ann Thorac Surg. 2022;114(1):142–150. doi:10.1016/j.athoracsur.2021.07.069
13. Altman DG, Egger M, Pocock SJ, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi:10.1016/S0140-6736(07)61602-X
14. Yan SJ, Zhao Y, Lou S, et al. Ultrafiltration and reinfusion of residual cardiopulmonary bypass pump blood: a prospective non-randomized controlled study. Artif Organs. 2019;43(7):641–646. doi:10.1111/aor.13412
15. Rooke GA. Cardiovascular aging and anesthetic implications. J Cardiothorac Vasc Anesth. 2003;17(4):512–523. doi:10.1016/S1053-0770(03)00161-7
16. Priebe HJ. The aged cardiovascular risk patient. Br J Anaesth. 2000;85(5):763–778. doi:10.1093/bja/85.5.763
17. Boer C, Meesters MI, Milojevic M, et al. 2017 EACTS/EACTA guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth. 2018;32(1):88–120. doi:10.1053/j.jvca.2017.06.026
18. Tibi P, Mcclure RS, Huang J, et al. STS/SCA/AmSECT/SABM update to the clinical practice guidelines on patient blood management. Ann Thorac Surg. 2021;112(3):981–1004. doi:10.1016/j.athoracsur.2021.03.033
19. Mueller MM, Van Remoortel H, Meybohm P, et al. Patient blood management: recommendations from the 2018 Frankfurt consensus conference. JAMA. 2019;321(10):983–997. doi:10.1001/jama.2019.0554
20. Mazer CD, Whitlock RP, Fergusson DA, et al. Restrictive or liberal red-cell transfusion for cardiac surgery. N Engl J Med. 2017;377(22):2133–2144. doi:10.1056/NEJMoa1711818
21. Mazer CD, Whitlock RP, Fergusson DA, et al. Six-month outcomes after restrictive or liberal transfusion for cardiac surgery. N Engl J Med. 2018;379(13):1224–1233. doi:10.1056/NEJMoa1808561
22. Nakamura RE, Vincent J-L, Fukushima JT, et al. A liberal strategy of red blood cell transfusion reduces cardiogenic shock in elderly patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2015;150(5):1314–1320. doi:10.1016/j.jtcvs.2015.07.051
23. Simon GI, Craswell A, Thom O, Fung YL. Outcomes of restrictive versus liberal transfusion strategies in older adults from nine randomised controlled trials: a systematic review and meta-analysis. Lancet Haematol. 2017;4(10):e465–e474. doi:10.1016/S2352-3026(17)30141-2
24. Simon GI, Craswell A, Thom O, Chew MS, Anstey CM, Fung YL. Impacts of aging on anemia tolerance, transfusion thresholds, and patient blood management. Transfus Med Rev. 2019;33(3):154–161. doi:10.1016/j.tmrv.2019.03.001
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.