Back to Journals » Journal of Blood Medicine » Volume 15
A Post-Authorization Safety Surveillance Study to Report Clinical Experience with Purified Factor IX Concentrate in Pediatric Patients with Hemophilia B
Authors Igrutinović Z , Hooimeijer HL, Kentouche K, Botha J, Turecek PL , Kokot-Kierepa M, Gazda HT
Received 21 July 2023
Accepted for publication 29 January 2024
Published 5 March 2024 Volume 2024:15 Pages 113—122
DOI https://doi.org/10.2147/JBM.S425617
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Zoran Igrutinović,1,2 Hélène Louise Hooimeijer,3 Karim Kentouche,4 Jaco Botha,5 Peter L Turecek,6 Marta Kokot-Kierepa,5 Hanna T Gazda7
1Clinic of Pediatrics, Department of Hemato-Oncology, University Clinical Center of Kragujevac, Kragujevac, Serbia; 2Faculty of Medical Sciences, Department of Pediatrics, University of Kragujevac, Kragujevac, Serbia; 3Pediatric Hematology, Beatrix Children’s Hospital, University Medical Center Groningen, Groningen, the Netherlands; 4Klinik für Kinder- und Jugendmedizin, Universitätsklinikum Jena, Jena, Deutschland; 5Takeda Pharmaceuticals International AG, Zürich, Switzerland; 6Baxalta Innovations GmbH, Part of Takeda, Vienna, Austria; 7Takeda Development Center Americas, Inc., Cambridge, MA, USA
Correspondence: Hanna T Gazda, Takeda Development Center Americas, Inc., 650 East Kendall Street, Cambridge, MA, 02142, USA, Tel +1 617 869-2632, Email [email protected]
Introduction: Purified factor IX (FIX) concentrate (IMMUNINE®, Takeda Manufacturing Austria AG, Vienna, Austria) is indicated for the treatment and prophylaxis of bleeding episodes in patients with congenital hemophilia B. Data on the use of purified FIX concentrate in patients ≤ 6 years old with congenital hemophilia B are limited.
Aim: Document real-world clinical experience with purified FIX concentrate in routine practice for pediatric patients with hemophilia B.
Methods: This prospective post-authorization safety surveillance study enrolled patients ≤ 6 years old with moderate or severe hemophilia B (baseline FIX ≤ 5%) who were prescribed purified FIX concentrate, as determined by the treating physician. The planned observation period for each patient was either 12 months or ≥ 50 exposure days, whichever occurred first. The primary endpoints were the occurrence of treatment-related adverse events (AEs) and serious AEs (SAEs), and inhibitor development.
Results: Thirteen male patients (mean ± standard deviation age, 3.80 ± 1.76 years) enrolled and received ≥ 1 treatment with purified FIX concentrate. Thirty-two AEs were reported in 6 patients; 4 were SAEs. No AEs were considered related to purified FIX concentrate. No patients developed inhibitory antibodies. Inhibitor testing was not conducted in 2 patients. Eighteen bleeding episodes were treated with purified FIX concentrate in 6 patients. Hemostatic efficacy was rated as either “excellent” or “good” in all patients with an available rating.
Conclusion: No treatment-related AEs were reported, and purified FIX concentrate was shown to be effective in treating and preventing bleeding episodes in pediatric patients ≤ 6 years old with hemophilia B.
Keywords: factor IX, hemophilia B, pediatrics, post-marketing product surveillance, surgery
Introduction
Congenital hemophilia B is a rare X-linked bleeding disorder caused by either a deficiency or absence of coagulation factor IX (FIX).1 The severity of hemophilia B is classified according to the level of residual FIX, with severe hemophilia B defined as a FIX level <1% and moderate hemophilia B defined as a FIX level 1–5%.1,2 Hemophilia B is characterized by bleeding episodes, particularly into large joints that can lead to painful and debilitating hemophilic arthropathy.1
The main treatment approach for patients with hemophilia B is replacement therapy with either plasma-derived or recombinant FIX concentrates.2 FIX concentrates can be further classified as either pure or containing factors II, VII, IX, and X, also known as prothrombin complex concentrates.2 The 2020 World Federation of Hemophilia (WFH) guidelines for the management of hemophilia recommend the use of pure FIX concentrates over prothrombin complex concentrates for the treatment of patients with hemophilia B, as pure FIX concentrates are associated with a reduced risk of thrombosis and disseminated intravascular coagulation compared with prothrombin complex concentrates.2 For patients with hemophilia B who are undergoing surgery, these guidelines also recommend the use of pure FIX concentrates rather than prothrombin complex concentrates.2
There are many plasma-derived and recombinant FIX concentrates available for the management of patients with hemophilia B, including extended half-life recombinant FIX concentrates which were developed to reduce the treatment burden associated with standard half-life products.2,3 Replacement therapies can be administered by either bolus injections or continuous infusions as an on-demand therapy for the treatment of bleeding episodes, or as prophylaxis for the prevention of bleeding episodes.3–5 These treatments can also be used for the perioperative management of patients who are undergoing surgery or invasive procedures.3 Studies have shown FIX concentrate prophylaxis to be associated with lower bleeding rates compared with on-demand treatment in patients with hemophilia B.6–9 Individualized prophylaxis is recommended by the 2020 WFH guidelines for the treatment of patients with hemophilia B with a severe phenotype, including patients with moderate hemophilia B who have a severe phenotype.2 For pediatric patients with severe hemophilia B, the WFH guidelines recommend the early initiation of prophylaxis, ideally before 3 years of age and prior to the onset of joint disease, with either standard or extended half-life FIX concentrates or other hemostatic agents.2
FIX concentrate (IMMUNINE®, Takeda Manufacturing Austria AG, Vienna, Austria) is purified from human plasma and contains only traces (≤0.02 IU) of factors II, VII, and X.10 FIX concentrate has also been shown to contain low levels of activated FIX, a product-related impurity in FIX products that has been associated with an increased risk of thrombogenicity.4,5 FIX concentrate is indicated for the treatment and prophylaxis of bleeding episodes in patients with congenital hemophilia B.10,11 However, indications may vary by country. Data on the use of purified FIX concentrate in patients ≤6 years old with hemophilia B are limited.11 Real-world studies have become increasingly significant for demonstrating treatment effectiveness and safety in routine clinical practice.12 This post-authorization safety surveillance (PASS) study was designed to document real-world clinical experience with purified FIX concentrate (IMMUNINE®) in routine practice for pediatric patients ≤6 years old with hemophilia B.
Methods
Study Design
This was a prospective, uncontrolled, open-label PASS study involving 14 study sites in 6 countries (Czech Republic, Germany, the Netherlands, Poland, Serbia, and Ukraine). The study surveillance period was from November 2009 to January 2014. The product formulation was the same across all six countries.
The study followed a cohort design and did not make any stipulations on treatment or observation schedule. Prophylaxis and on-demand dosing regimens were determined at the discretion of the treating physician, in accordance with prescribing information. The planned observation period for each patient was ~12 months or ≥50 exposure days (EDs), whichever occurred first (Supplementary Figure 1). A screening visit was required prior to enrollment and was scheduled to coincide with a routine visit to a hemophilia treatment center. A termination visit was planned to coincide with a routine visit at the end of the observation period. No stipulations were made on clinical or laboratory testing beyond what was required to determine patient eligibility.
The study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice E6, Title 21 of the US Code of Federal Regulations, the European Clinical Trial Directive, and applicable national and local regulatory requirements, including European regulations pertaining to PASS studies. This study was also conducted in accordance with the Declaration of Helsinki. Approval from Institutional Review Boards/Independent Ethics Committees, listed in Supporting Information, was obtained for the study protocol and informed consent form. All patients or their legally authorized representative signed an informed consent form prior to entering the study.
Patients
Patients with moderate or severe congenital hemophilia B (baseline FIX ≤5%) and ≤6 years old who had been prescribed purified FIX concentrate (IMMUNINE®) by their treating physician were eligible for inclusion. Patients were eligible regardless of whether or not they had previously received treatment with any FIX concentrates, including purified FIX concentrate (IMMUNINE®). Patients known to have disseminated intravascular coagulation or hyperfibrinolysis were not eligible for inclusion, as were those with FIX inhibitors or any other known clotting factor deficiency. Patients with a known hypersensitivity to the active substance, or any of its excipients, were also excluded from the study.
Endpoints
The primary endpoints were the incidence of adverse events (AEs) and serious AEs (SAEs) that were judged by the treating physician to be at least possibly related to purified FIX concentrate, and the occurrence of high-titer (>5 Bethesda Units [BU]), low-titer (1–5 BU) and transient inhibitor development during the course of treatment. Secondary endpoints included the hemostatic efficacy of prophylaxis and on-demand treatment as measured by the physicians’ overall assessment rating of poor, fair, good, or excellent (Supplementary Table 1), and the number of infusions required to achieve bleed resolution.
Statistical Analysis
Descriptive statistical data analyses were carried out for all safety, hemostatic efficacy, and immunogenicity parameters. All patients who received the study drug at any time during the observation period were included in the safety set. The intent to treat (ITT) set consisted of all enrolled patients who received at least 1 dose of study drug and provided any post-treatment data. The per protocol (PP) set was a subset of patients in the ITT set who had completed either 12 months or 50 EDs.
AEs were coded according to the Medical Dictionary for Drug Regulatory Activities version 17.0. The total number of infusions and dose of study drug required to achieve adequate hemostasis for each bleeding episode were reported. The rate of bleeding episodes was calculated (all bleeds and those secondary to trauma) for all patients. Overall hemostatic efficacy of treatment for all bleeding episodes was established based on individual assessment ratings provided by the treating physician. In general, missing data were not replaced except in calculations for age and body weight.
Results
Patients
In total, 13 patients were enrolled at 9 study sites (Poland, n = 7; Germany, n = 2; the Netherlands, n = 2; Czech Republic, n = 1; Serbia, n = 1). No patients were enrolled from Ukraine. All 13 patients received treatment with purified FIX concentrate and were included in the safety and ITT sets. Nine patients were included in the PP set (Figure 1).
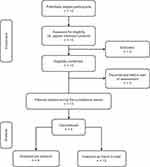 |
Figure 1 Patient disposition. |
All 13 patients were male, with a mean ± standard deviation (SD) age of 3.80 ± 1.76 years (Table 1). None of the 13 patients had a history of FIX inhibitors. All 13 patients had received treatment with purified FIX concentrate (IMMUNINE®) before enrollment. Six patients had also previously received treatment with another high-purity plasma-derived FIX. Nine patients had >50 EDs to FIX concentrate prior to enrollment.
 |
Table 1 Demographics and Baseline Characteristics (ITT Set) |
Safety
In total, 32 AEs were reported in 6 patients, of which 4 were considered to be SAEs (Table 2). The 4 SAEs were reported in 3 patients, of whom 2 patients experienced an accidental head injury and 1 patient experienced 2 cases of sepsis. At the time of the accidental head injury, one patient was receiving 48 IU/kg once weekly prophylaxis. This dosing regimen was not changed following the head injury, although the patient did receive a daily 48 IU/kg dose of purified FIX concentrate for 3 days immediately following this SAE. The second patient who experienced a head injury was receiving 30 IU/kg purified FIX concentrate prophylaxis. Following the head injury, the patient received a single 60 IU/kg dose after which the patient continued to receive 30 IU/kg prophylaxis. The dose of purified FIX concentrate was not changed in response to any of the other SAEs. None of the 32 AEs were considered related to purified FIX concentrate, and all events resolved during the surveillance period. No deaths occurred during the surveillance period.
 |
Table 2 Frequency of AEs and SAEs (Safety Set) |
Immunogenicity
Eleven of the 13 patients in the safety set underwent testing for FIX inhibitors. The Bethesda assay was used to test for FIX inhibitors in 6 patients and the Nijmegen assay was used for 2 patients. Both assays were used to test for FIX inhibitors in 2 patients. None of these 10 patients developed inhibitory antibodies (Supplementary Table 2). One patient underwent testing for FIX inhibitors twice and was reported as negative both times; however, the method used to test for FIX inhibitors was not reported. Inhibitor testing was not conducted in 2 patients. The observation period for 1 patient was 8 months. All other patients were observed for 12 months.
Bleeding Episodes and Treatment
In the ITT set, 18 bleeding episodes in 6 patients were treated with purified FIX concentrate. Of these, 10 bleeding episodes were classified as traumatic, 7 were classified as spontaneous, and 1 was undetermined. Thirteen bleeding episodes occurred in a joint, 1 occurred in muscle, and 4 were classified as “other”. All 18 bleeding episodes were assessed as either minor or moderate in severity. Of the 18 bleeding episodes treated with purified FIX concentrate, 14 episodes in 4 patients were included in the PP set.
Annualized bleeding rates are presented by treatment regimen in Supplementary Table 3 and by cause of bleeding episode in Supplementary Table 4. Table 3 presents the average number of infusions per bleeding episode and the average number of infusions during prophylaxis. The mean ± SD number of infusions per bleeding episode was 1.18 ± 0.57 in the ITT set and 1.03 ± 0.53 in the PP set. The mean ± SD number of infusions administered per year was 91.67 ± 25.15 for patients receiving prophylaxis (n = 11).
 |
Table 3 Average Number of Infusions |
Hemostatic Efficacy
In the ITT set, hemostatic efficacy ratings were reported in 7 patients over the surveillance period (Table 4 and Supplementary Table 5). All ratings were either “excellent” or “good”. For patient #2, a hemostatic efficacy rating of “good” was given by the treating physician at the termination assessment; however, no details of a bleeding episode were documented in this patient. There were 6 patients in whom no bleeding episodes were reported, and no hemostatic efficacy ratings were provided.
 |
Table 4 Hemostatic Efficacy Ratings by Physician for Bleeding Episodes Treated with the Study Drug Over the Observation Period |
Treatment Regimen
In the ITT set (N = 13), 11 patients received prophylaxis, 6 patients received on-demand treatment, and 2 patients received prophylaxis for surgery. EDs and study drug dose for each regimen are shown in Table 5. The average total dose of study drug per kilogram of body weight per bleeding episode (IU/kg) is presented in Supplementary Table 6. The mean ± SD dose per infusion per kilogram of body weight was 38.29 ± 8.86 IU/kg for patients receiving prophylaxis and 38.10 ± 12.47 IU/kg for patients receiving on-demand treatment.
 |
Table 5 Number of EDs to Study Drug and Average Dose per Infusion (ITT Set) |
Discussion
The aim of this PASS study was to document real-world clinical experience with purified FIX concentrate in routine practice for pediatric patients ≤6 years old with hemophilia B. Post-authorisation studies are used to obtain further information on the safety and effectiveness of a therapeutic agent in clinical practice and have been undertaken for other products available for the management of patients with hemophilia.13–15 In terms of study design, the approach used in this study is different to a safety assessment that was undertaken for another marketed plasma-derived highly purified FIX concentrate (Replenine®; Bio Products Laboratory, Elstree, UK), in which hospital notes were retrospectively reviewed for 114 patients with hemophilia B.15 The study identified 9 AEs, of which 4 were considered possibly related to the product, and no new FIX inhibitors developed.15
All 13 patients who enrolled in this PASS study were included in the safety and ITT sets. There were 9 patients who completed the study in line with the protocol. The mean patient age at enrollment was slightly above the treatment initiation age recommended by the 2020 WFH guidelines for the management of pediatric patients with severe hemophilia B.2 However, all enrolled patients had previously received treatment with purified FIX concentrate (IMMUNINE®), and some patients had also received previous treatment with another high-purity plasma-derived FIX.
None of the 32 AEs, including 4 SAEs, reported in this study were considered related to purified FIX concentrate, and all events resolved during the surveillance period. None of the 13 patients had a history of FIX inhibitors and there was no evidence of FIX inhibitor development in any of the 11 patients who received treatment with purified FIX concentrate and were assessed for the development of inhibitory antibodies.
Eighteen bleeding episodes in 6 patients were treated with the study drug. The mean ± SD dose per infusion per kilogram of body weight for patients receiving prophylaxis (n = 11) was 38.29 ± 8.86 IU/kg and for patients receiving on-demand treatment (n = 6) was 38.10 ± 12.47 IU/kg. Patients received prophylaxis either once or twice a week. At the time this PASS study was being conducted, the 2013 WFH guidelines made reference to the Malmö protocol (25–40 IU/kg twice per week) and the Utrecht protocol (15–30 IU/kg twice per week) regarding prophylaxis dosing regimens for patients with hemophilia B.16 For prophylaxis with standard half-life clotting factors in patients with hemophilia B, the 2020 WFH guidelines define high-dose prophylaxis as 40−60 IU FIX/kg twice per week (>4000 IU/kg per year), intermediate-dose prophylaxis as 20−40 IU FIX/kg twice per week (2000–4000 IU/kg per year), and low-dose prophylaxis as 10−15 IU FIX/kg 2 days per week (1000–1500 IU/kg per year).2
It should be acknowledged that patient recruitment was challenging, which resulted in a small number of patients enrolled into the study. The difficulties encountered in recruiting patients to this study are likely owing to the rarity of hemophilia B and the fact that this study focuses on a very specific age demographic. In addition, there were 4 patients who did not complete the study according to protocol, which may, in part, have been due to the young age of the patients. Despite a small patient population, this PASS study does provide real-world data on the use of purified FIX concentrate in routine clinical practice for the treatment of pediatric patients ≤6 years old who have hemophilia B.
Conclusions
Over the years, the therapeutic landscape for the management of patients with hemophilia B has expanded beyond plasma-derived replacement therapies to include recombinant FIX concentrates and, subsequently, extended half-life recombinant FIX products.3,17 Prophylaxis with extended half-life recombinant FIX concentrates has been shown to be effective in the treatment of bleeding episodes and associated with low annualized bleeding rates in patients with hemophilia B.18–20 The prolonged half-life of these products is also advantageous as they can reduce dosing frequency to once every 7–14 days.18–20
For pediatric patients with severe hemophilia B, the 2020 WFH guidelines recommend the early initiation of prophylaxis, ideally before 3 years of age and prior to the onset of joint disease, with either standard or extended half-life FIX concentrates or other hemostatic agents.2 Despite the preference for recombinant FIX products, plasma-derived FIX concentrates such as IMMUNINE® remain a viable and effective therapeutic option for the management of patients with hemophilia B, especially in countries where treatment options are more limited. In this PASS study, purified FIX concentrate was not associated with any treatment-related AEs and was shown to be effective in treating and preventing bleeding episodes in pediatric patients ≤6 years old with hemophilia B. The findings from this study, therefore, provide further support to the continued use of plasma-derived FIX concentrates such as IMMUNINE® in the management of patients with hemophilia B.
Abbreviations
AE, adverse event; BU, Bethesda units; ED, exposure day; FIX, factor IX; ITT, intent to treat; PASS, post-authorization safety surveillance; PP, per protocol; SAE, serious adverse event; SD, standard deviation; WFH, World Federation of Hemophilia.
Data Sharing Statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results reported in this article, will be made available within 3 months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
Ethics Approval and Informed Consent
The study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice E6, Title 21 of the US Code of Federal Regulations, the European Clinical Trial Directive, and applicable national and local regulatory requirements, including European regulations pertaining to PASS studies. This study was also conducted in accordance with the Declaration of Helsinki. Approval from Institutional Review Boards/Independent Ethics Committees, listed in Supporting Information, was obtained for the study protocol and informed consent form. All patients or their legally authorized representative signed an informed consent form prior to entering the study.
Acknowledgments
Under the direction of the authors, medical writing support was provided by Sarah Morgan, PhD, employee of Excel Scientific Solutions, Inc., a member of the Envision Pharma Group (Fairfield, Connecticut, USA), and was funded by Takeda Development Center Americas, Inc., Lexington, Massachusetts, USA.
Material from this manuscript was presented at the International Society on Thrombosis and Haemostasis (ISTH) Congress; July 09–13, 2022. Abstract title: A post-authorization safety surveillance study to report clinical experience with purified factor IX concentrate in pediatric patients with hemophilia B. https://abstracts.isth.org/abstract/a-post-authorization-safety-surveillance-study-to-report-clinical-experience-with-purified-fix-concentrate-in-pediatric-patients-with-hemophilia-b/.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Baxalta Innovations GmbH, Vienna, Austria, a Takeda company.
Disclosure
Zoran Igrutinović reports being an investigator for this post-authorization safety surveillance study, which was funded by Baxalta Innovations GmbH, Vienna, Austria, a Takeda company. Hélène Louise Hooimeijer has no interests that might be perceived as posing a conflict or bias for this study. Karim Kentouche reports being an investigator for this post-authorization safety surveillance study, which was funded by Baxalta Innovations GmbH, Vienna, Austria, a Takeda company. Jaco Botha and Marta Kokot-Kierepa are employees of Takeda Pharmaceuticals International AG, and Takeda stockholders. Peter L. Turecek is an employee of Baxalta Innovations GmbH, a Takeda company, and a Takeda stockholder. Hanna T. Gazda is an employee of Takeda Development Center Americas, Inc., a Takeda company, and a Takeda shareholder. The authors report no other conflicts of interest in this work.
References
1. Berntorp E, Fischer K, Hart DP, et al. Haemophilia. Nat Rev Dis Primers. 2021;7(1):45. doi:10.1038/s41572-021-00278-x
2. Srivastava A, Santagostino E, Dougall A, et al. WFH Guidelines for the Management of Hemophilia panelists and co-authors. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(Suppl 6):1–158. doi:10.1111/hae.14046
3. Castaman G. The benefits of prophylaxis in patients with hemophilia B. Expert Rev Hematol. 2018;11(8):673–683. doi:10.1080/17474086.2018.1489719
4. Turecek PL, Abbühl B, Tangada SD, et al. Nonacog gamma, a novel recombinant factor IX with low factor IXa content for treatment and prophylaxis of bleeding episodes. Expert Rev Clin Pharmacol. 2015;8(2):163–177. doi:10.1586/17512433.2015.1011126
5. Thomas KB, Urbancik W, Turecek PL, et al. Continuous infusion of FVIII and FIX concentrates: in vitro analysis of clinically relevant parameters. Haemophilia. 1999;5(1):17–25. doi:10.1046/j.1365-2516.1999.00210.x
6. Kavakli K, Smith L, Kuliczkowski K, et al. Once-weekly prophylactic treatment vs. on-demand treatment with nonacog alfa in patients with moderately severe to severe haemophilia B. Haemophilia. 2016;22(3):381–388. doi:10.1111/hae.12878
7. Windyga J, Lissitchkov T, Stasyshyn O, et al. Pharmacokinetics, efficacy and safety of BAX326, a novel recombinant factor IX: a prospective, controlled, multicentre Phase I/III study in previously treated patients with severe (FIX level <1%) or moderately severe (FIX level ≤2%) haemophilia B. Haemophilia. 2014;20(1):15–24. doi:10.1111/hae.12228
8. Collins PW, Quon DVK, Makris M, et al. Pharmacokinetics, safety and efficacy of a recombinant factor IX product, trenonacog alfa in previously treated haemophilia B patients. Haemophilia. 2018;24(1):104–112. doi:10.1111/hae.13324
9. Valentino LA, Rusen L, Elezovic I, et al. Multicentre, randomized, open-label study of on-demand treatment with two prophylaxis regimens of recombinant coagulation factor IX in haemophilia B subjects. Haemophilia. 2014;20(3):398–406. doi:10.1111/hae.12344
10. Takeda Canada Inc. IMMUNINE® VH: factor IX concentrate (Human). Product Monograph; 2021. Available from: https://assets-dam.takeda.com/raw/upload/v1662721792/legacy-dotcom/siteassets/en-ca/home/what-we-do/our-medicines/product-monographs/immunine-vh/immunine-vh-pm-en.pdf.
11. Takeda Manufacturing Austria AG. IMMUNINE 1200 IU: powder and solvent for solution for injection or infusion. Human blood coagulation factor IX. Package Leaflet; 2020. Available from: https://file.wuxuwang.com/hma/AT_H_0177_002_FinalPL_1of3.pdf.
12. Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763–1774. doi:10.1007/s12325-018-0805-y
13. Negrier C, Voisin S, Baghaei F, et al. Global post-authorization safety surveillance study: real-world data on prophylaxis and on-demand treatment using FEIBA (an activated prothrombin complex concentrate). Blood Coagul Fibrinolysis. 2016;27(5):551–556. doi:10.1097/mbc.0000000000000525
14. Gascoigne EW, Dash CH, Harman C, Wilmot D. A retrospective survey on the safety of Replenate, a high-purity factor VIII concentrate. Pharmacoepidemiol Drug Saf. 2004;13(4):243–252. doi:10.1002/pds.956
15. Gascoigne EW, Dash CH, Harman C, Wilmot D. A retrospective survey on the safety of replenine, a high-purity factor IX concentrate. Pharmacoepidemiol Drug Saf. 2004;13(3):187–195. doi:10.1002/pds.911
16. Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Treatment Guidelines Working Group on behalf of the World Federation of Hemophilia. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47. doi:10.1111/j.1365-2516.2012.02909.x
17. Mancuso ME, Mahlangu JN, Pipe SW. The changing treatment landscape in haemophilia: from standard half-life clotting factor concentrates to gene editing. Lancet. 2021;397(10274):630–640. doi:10.1016/s0140-6736(20)32722-7
18. Collins PW, Young G, Knobe K, et al; Paradigm 2 Investigators. Recombinant long-acting glycoPEGylated factor IX in hemophilia B: a multinational randomized Phase 3 trial. Blood. 2014;124(26):3880–3886. doi:10.1182/blood-2014-05-573055
19. Powell JS, Pasi KJ, Ragni MV, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313–2323. doi:10.1056/NEJMoa1305074
20. Santagostino E, Martinowitz U, Lissitchkov T, et al. Long-acting recombinant coagulation factor IX albumin fusion protein (rIX-FP) in hemophilia B: results of a phase 3 trial. Blood. 2016;127(14):1761–1769. doi:10.1182/blood-2015-09-669234
 © 2024 Takeda Pharmaceuticals International AG. This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 Takeda Pharmaceuticals International AG. This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
