Back to Journals » Clinical Interventions in Aging » Volume 18
A Predictive Model for Contrast-Induced Acute Kidney Injury After Percutaneous Coronary Intervention in Elderly Patients with ST-Segment Elevation Myocardial Infarction
Authors Qiu H, Zhu Y , Shen G, Wang Z, Li W
Received 9 January 2023
Accepted for publication 16 March 2023
Published 22 March 2023 Volume 2023:18 Pages 453—465
DOI https://doi.org/10.2147/CIA.S402408
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Zhi-Ying Wu
Hang Qiu,1 Yinghua Zhu,1 Guoqi Shen,1 Zhen Wang,1 Wenhua Li1,2
1Institute of Cardiovascular Diseases, Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 2Department of Cardiology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China
Correspondence: Wenhua Li, Department of Cardiology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China, Tel +86 18052268293, Email [email protected]
Purpose: Development and validation of a nomogram model to predict the risk of Contrast-Induced Acute Kidney Injury (CI-AKI) after emergency percutaneous coronary intervention (PCI) in elderly patients with acute ST-segment elevation myocardial infarction (STEMI).
Patients and Methods: Retrospective analysis of 542 elderly (≥ 65 years) STEMI patients undergoing emergency PCI in our hospital from January 2019 to June 2022, with all patients randomized to the training cohort (70%; n=380) and the validation cohort (30%; n=162). Univariate analysis, LASSO regression, and multivariate logistic regression analysis were used to determine independent risk factors for developing CI-AKI in elderly STEMI patients. R software is used to generate a nomogram model. The predictive power of the nomogram model was compared with the Mehran score 2. The area under the ROC curve (AUC), calibration curves, and decision curve analysis (DCA) was used to evaluate the prediction model’s discrimination, calibration, and clinical validity, respectively.
Results: The nomogram model consisted of five variables: diabetes mellitus (DM), left ventricular ejection fraction (LVEF), Systemic immune-inflammatory index (SII), N-terminal pro-brain natriuretic peptide (NT-proBNP), and highly sensitive C-reactive protein(hsCRP). In the training cohort, the AUC is 0.84 (95% CI: 0.790– 0.890), and in the validation cohort, it is 0.844 (95% CI: 0.762– 0.926). The nomogram model has better predictive ability than Mehran score 2. Based on the calibration curves, the predicted and observed values of the nomogram model were in good agreement between the training and validation cohort. Decision curve analysis (DCA) and clinical impact curve showed that the nomogram prediction model has good clinical utility.
Conclusion: The established nomogram model can intuitively and specifically screen high-risk groups with a high degree of discrimination and accuracy and has a specific predictive value for CI-AKI occurrence in elderly STEMI patients after PCI.
Keywords: nomogram, elderly, risk prediction model, ST-segment elevation myocardial infarction, percutaneous coronary intervention, contrast-induced acute kidney injury
Introduction
Cardiovascular disease has been the leading cause of death worldwide. With the acceleration of population aging, the number of elderly patients with acute ST-segment elevation myocardial infarction (STEMI) is also increasing, posing a considerable challenge to social health care.1 Percutaneous coronary intervention (PCI) is currently the mainstay of treatment for patients with acute STEMI, but contrast-induced acute kidney injury (CI-AKI) occurs in 15–35% of patients after PCI.2
CI-AKI, a vital complication after PCI, is the third leading cause of hospital-acquired renal failure after renal artery hypoperfusion and nephrotoxicity of drugs.3 CI-AKI is closely associated with adverse clinical outcomes, more extended hospital stays, and higher medical costs.4,5 The increasing aging process means that more elderly patients are undergoing PCI. The reduction of nephrons, insufficient renal function reserve, and more comorbidities in elderly patients make the elderly more sensitive to CI-AKI, with a higher probability of CI-AKI and a worse prognosis than the general population.6,7 Due to no effective treatment measures available for CI-AKI in clinical work, it is of great value to accurately identify high-risk elderly patients.8
Various risk scores for developing CI-AKI after PCI have been established in recent years.9–11 The Mehran score and the updated Mehran 2 CI-AKI risk score (Mehran Score 2 for short) have been widely validated.12,13 However, many risk scores have limitations, such as the low prediction accuracy of risk score models, the inclusion of variables not known before surgery, and the inappropriate definition of CI-AKI, leading to limited applicability in routine clinical practice.12,14
Nomogram is a simple and personalized visualization tool that combines the weighted scores of each risk factor to predict the occurrence of a specific outcome. Compared with the traditional prediction model, the nomogram model is more intuitive, easier to operate and calculate, and can integrate more prognostic factors to achieve individualized prediction, making it a potentially ideal model for predicting the incidence and prognosis of various diseases.15 Therefore, this study established a prediction model of CI-AKI after emergency PCI in elderly patients with STEMI and verified its clinical effectiveness. Risk stratification was performed in elderly patients to provide more accurate guidance and a scientific basis for the early prevention of CI-AKI in elderly patients with STEMI after PCI.
Materials and Methods
Subjects
Between January 2019 to June 2022, 638, elderly patients (≥ 65 years old) with acute STEMI who underwent emergency PCI at Xuzhou Medical University Hospital were retrospectively analyzed (Figure 1).
 |
Figure 1 The study flow diagram. |
Inclusion criteria: (1) Patients diagnosed with STEMI according to “the fourth universal definition of myocardial infarction”:16 (i): Troponins at least one occasion above the 99th percentile of the upper limit of the reference value; (ii): New ST-segment elevation occurred at J point in two adjacent leads, with ≥2 mm in lead V2-V3 (male, ≥40 years old); ≥1.5 mm (in women, regardless of age); Other leads were ≥1.0 mm. (2) Patients aged ≥65 years, and (3) Patients undergoing emergency PCI.
Exclusion criteria: 1. Chronic renal dialysis patients or those with severe hepatic abnormalities; 2. Inflammation (including active infection, systemic inflammation, and autoimmune diseases); 3. Patients with incomplete clinical data; 4. Active malignant tumor; 5. Hematological disorders; 6. Exposure to radiocontrast or nephrotoxic drugs within 48h or 72h preoperatively. Finally, 542 patients remained in the study sample, which included 361 men. The mean age was 73.53 ± 6.02, and all patients were randomized to the training cohort (70%; n=380) and the validation cohort (30%; n=162).
PCI Method and Medications
PCI was performed by experienced interventional cardiologists based on standard clinical practice. All patients received a loading dose of aspirin 300mg, clopidogrel 300mg or ticagrelor 180 mg, and 100 U/kg of intravenous heparin before PCI. The contrast agent used was a low-osmolar nonionic contrast agent with an osmotic concentration of 600–800 mOsm/Kg. After the procedure, depending on the patient’s underlying physical condition, the patient was given an appropriate amount of fluid hydration by an interventional cardiologist to facilitate the metabolism of the contrast agent in the body.17
Clinical Endpoints
Diagnostic criteria for CI-AKI depend on the Kidney Disease: Improving Global Outcomes (KDIGO): an increase in serum creatinine (SCr) ≥26.5 μmol/L within 48 hours, or ≥1.5 times baseline SCr within seven days, or urine output <0.5 mL·kg−1·h for six consecutive hours after the administration of contrast agents.18,19
Data Collection
The basic clinical characteristics of the patients were collected, including basic information, previous diseases, surgery-related information, and medication. Antecubital venous blood samples were collected before and after PCI, and the blood samples were uniformly tested and reported in the hospital laboratory. The SII was derived by multiplying the neutrophil count by the platelet count divided by the lymphocyte count.20 Mehran score 2: 8 points for STEMI; 1 point for eGFR (30 ~ 59 mL.min−1 per 1.732 m), 4 points for eGFR (<30 mL.min−1 per 1.732 m); 2 points for left ventricular ejection fraction (LVEF) <40%;1 point for diabetes mellitus without insulin therapy; 2 points for diabetes mellitus treated with insulin; 1 point for hemoglobin <110 g/L; 1 point for fasting basal glucose ≥8.33 mmol/L; 1 point for congestive heart failure; 1 point for age ≥75 years. The total score of the above variables was the Mehran score 2.13
Statistical Analysis
SPSS version 26.0 and the R software package (version 4.1.2) were used to analyze the data statistically. The continuous data were tested by normality test, the data by normal distribution were expressed by  , and Student’s t-test analyzed the comparison between groups. Continuous variables that did not follow the normal distribution were represented as M (Q1, Q3), and a comparison between groups was performed using the Mann–Whitney U-test. Categorical data were expressed as n (%), and comparison between groups was analyzed using the χ²-test. Since the original values of NT-proBNP and SII were skewed, natural logarithm-transformed values were used for the statistical analysis. LASSO regression was used to screen non-zero coefficient features, and multivariate logistic regression was used to analyze the independent predictors of CI-AKI in elderly STEMI patients after PCI. The prediction model was constructed and evaluated in terms of discrimination ability, calibration ability, and clinical validity. The DeLong test was also used to compare the area under the ROC curve of the nomogram model with Mehran score 2. The Net Reclassification Improvement (NRI) and Integrated Discrimination Improvement (IDI) were calculated to determine how much the model improves the prediction accuracy and ability compared to Mehran score 2.
, and Student’s t-test analyzed the comparison between groups. Continuous variables that did not follow the normal distribution were represented as M (Q1, Q3), and a comparison between groups was performed using the Mann–Whitney U-test. Categorical data were expressed as n (%), and comparison between groups was analyzed using the χ²-test. Since the original values of NT-proBNP and SII were skewed, natural logarithm-transformed values were used for the statistical analysis. LASSO regression was used to screen non-zero coefficient features, and multivariate logistic regression was used to analyze the independent predictors of CI-AKI in elderly STEMI patients after PCI. The prediction model was constructed and evaluated in terms of discrimination ability, calibration ability, and clinical validity. The DeLong test was also used to compare the area under the ROC curve of the nomogram model with Mehran score 2. The Net Reclassification Improvement (NRI) and Integrated Discrimination Improvement (IDI) were calculated to determine how much the model improves the prediction accuracy and ability compared to Mehran score 2.
Results
Training Cohort Baseline Characteristics
CI-AKI occurred in 13.9% (53/380) of the 380 elderly STEMI patients in the training cohort. The two groups’ clinical features and laboratory indicators are shown in Table 1 and Table 2. Significant differences were found between the CI-AKI and non-CI-AKI groups regarding clinical characteristics and laboratory indicators, including diabetes mellitus, LVEF, diuretics use, lymphocyte, platelet count, hsCRP, SII, CK-MB, cTnT, NT-proBNP, Basal blood glucose and Mehran score 2 (P < 0.05).
 |
Table 1 Comparison of Clinical Features Among the Training Cohort |
 |
Table 2 Comparison of Laboratory Indicators in the Training Cohort |
LASSO Regression and Multiple Logistic Regression Analysis
LASSO regression showed that, When λ takes the minimum value of 0.0208, 14 factors, including DM, LVEF, Ln SII, Ln NT-proBNP, hsCRP, diuretics use, Stent number per patient, monocyte count, platelet count, total cholesterol, HDL-C, sdLDL-C, lipoprotein (a), CK-MB were significant predictors (Figure 2A and B). Multivariate logistic regression analysis showed that DM, LVEF, Ln SII, Ln NT-proBNP, and hsCRP predicted CI-AKI independently (Table 3).
 |
Table 3 Multivariate Logistic Regression Analysis for the CI-AKI After PCI in Elderly Patients with STEMI in Training Cohort |
Establishment of the Nomogram
Five variables, including DM, LVEF, Ln SII, Ln NT-proBNP, and hsCRP, were included in this study to establish a nomogram model for predicting CI-AKI in elderly STEMI patients after PCI (Figure 3). First, the ROC curve was plotted to verify the model’s discriminatory ability. The AUC of the nomogram model in the training cohort was 0.84 (95% CI: 0.790–0.890) (Figure 4A), indicating that the model is differentiated well. Meanwhile, Bootstrap internal validation was adopted, and a 1000 times simple resampling method was used for internal validation of the model. The result of the Hosmer-Lemeshow test was χ2=7.161 (P=0.519, P > 0.05), which proved that the model was well-calibrated. The calibration curve showed good consistency between the observed value (Apparent) and the predicted values of the model (Ideal), indicating that the model has a good calibration ability (Figure 5A).
 |
Figure 3 The nomogram for predicting the occurrence of CI-AKI after PCI in patients elderly with STEMI. |
 |
Figure 4 ROC curve of the nomogram for predicting CI-AKI after PCI in elderly patients with STEMI. (A) ROC curve of the training set; (B) ROC curve of the validation set. |
 |
Figure 5 Calibration curve of the nomogram for the training set (A) and the validation set (B). |
Comparison of Nomogram with Mehran Score 2
According to the DeLong test, the AUC of Mehran score 2 was 0.674 (95CI: 0.625–0.721), and the nomogram model has better predictive ability than Mehran score 2 (Z=4.122, P ≤0.001) (Figure 6). The Net Reclassification Improvement (NRI) was 0.1764 [95% CI: (0.0701,0.2828), p < 0.01] by comparing the nomogram model with the Mehran score 2, and the proportion of correct reclassification in the new model was 17.64% higher than that in the Mehran score 2. The Integrated Discrimination Improvement (IDI) was 0.1577[95% CI: (0.1026, 0.2128), p < 0.001], and the predictive ability of the new model was improved by 15.77% compared with that of the Mehran score 2.
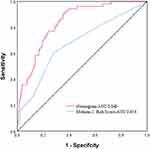 |
Figure 6 The receiver operator characteristic curves of the nomogram and the Mehran Risk Score 2. |
Validation of the Nomogram
In the validation cohort, CI-AKI occurred in 21 patients (12.9%), and the AUC of the nomogram model was 0.844 (95% CI: 0.762–0.926) (Figure 4B). The Hosmer-Lemeshow test result was χ2=6.646 (P=0.575, P > 0.05). The plotted calibration curves showed good consistency and fit of the nomogram prediction model (Figure 5B).
Clinical Utility of the Nomogram Model
Finally, the DCA curves of the nomogram model in the training and the validation cohorts were plotted to verify the clinical validity of the model (Figure 7A and B). Compared to the two extreme cases, the net benefit of the nomogram was significantly higher in the training and validation cohorts. In addition, the clinical impact curve was further drawn, and the red line represents the number of people judged as high risk by the model under different probability thresholds (Figure 8A and B). The blue lines represent the number of people the model judges as high risk and who experienced an outcome event at various probability thresholds. In conclusion, the nomogram has good clinical utility.
 |
Figure 7 Decision curve analysis for the training cohort (A) and the validation cohort (B). |
 |
Figure 8 The clinical impact curve of the training cohort (A) and the validation cohort (B) are drawn based on the nomogram. |
Discussion
With the further acceleration of population aging, an increasing number of elderly patients worldwide are undergoing procedures using contrast agents.21 CI-AKI is a complication of undergoing PCI and is one of the most common causes of hospital-acquired renal insufficiency.3 The poor clinical prognosis due to CI-AKI, as well as more extended hospital stays and increased treatment costs, have been important limitations for using contrast angiography, especially in high-risk patients with STEMI. Although the pathogenesis of CI-AKI has not been fully elucidated, it has been confirmed that it may be related to nephrotoxicity, inflammatory activation, oxidative stress, production of reactive oxygen species and renal medulla ischemia, and other factors.4,22 Therefore, early and accurate risk stratification and individualized preventive measures are of great clinical value.
The probability of CI-AKI in elderly patients after PCI is significantly increased, and the reason may be the substantial loss of nephrons caused by advanced age.6 At the same time, with the increase of age, vascular stiffness increases, and vascular endothelial function decreases in elderly patients, which reduces the possibility of rapid recovery of postoperative creatinine and increases the risk of CI-AKI in elderly patients.23–25 The prognosis of CI-AKI in elderly patients is worse than that in the general population, specifically manifested in the significantly increased incidence of cardiovascular adverse events and 1-year death after CI-AKI. In past studies, a series of risk stratification models combining laboratory indicators and surgical data have been validated. The Mehran score is a classic risk score, but its clinical application is limited due to the timeliness of the model and the exclusion of patients with acute myocardial infarction. Recently, Mehran developed a newer and simpler Mehran risk score 2, which includes eight easily accessible clinical variables (age, coronary artery disease subtype, eGFR, diabetes mellitus, congestive heart failure, hemoglobin, fasting plasma glucose, and LVEF).13 However, most of these models classified the study population into risk categories (low, intermediate, or high risk). They did not explain how assigning risk categories would affect diagnostic or therapeutic decisions. At the same time, the previous CI-AKI risk-scoring models do not include inflammation-related indicators, which ignores the critical role of inflammation in the occurrence and development of CI-AKI.26
In this study, we developed and internally validated an elderly patient-specific risk model for predicting CI-AKI risk in elderly patients with STEMI undergoing PCI. We found that CI-AKI occurred in 74 of 542 elderly patients (13.6%) with STEMI after PCI in this study. Diabetes mellitu, decreased LVEF, increased NT-proBNP, increased SII, and increased hsCRP are independent risk factors for CI-AKI in elderly STEMI patients after emergency PCI. The prediction model of CI-AKI risk based on these five indicators showed good discrimination and calibration.
Previous studies have shown that diabetes is closely related to CI-AKI, and patients with diabetes are significantly more likely to develop CI-AKI than those without diabetes. Diabetes amplifies the susceptibility to underlying chronic kidney disease. The high glucose state of diabetic patients enhances the release of oxidative stress and ROS, which further induces the increased synthesis of vasoactive substances such as endothelin and angiotensin II. These mechanisms further lead to renal vasoconstriction, altered renal microcirculation, and distal ischemia. Ischemia can lead to increased oxygen free radicals and ROS formation, forming a vicious cycle.27,28 Our study results indicated that those with low LVEF were at an increased risk of developing CI-AKI. As a result of long-term low cardiac output, the tissues and organs of the patient may be hypoperfused, leading to impaired renal function.29
SII is a comprehensive inflammatory index introduced in recent years, which can more comprehensively reflect patients’ immune and inflammatory status. The study of AliBag˘cı pointed out that a high preoperative level of SII has predictive value for CI-AKI, which is consistent with the results of our research.30 Previous studies have shown that many neutrophils will mediate renal tubular injury when the body’s inflammatory activity increases. The release of large quantities of anti-inflammatory factors will lead to lymphocyte apoptosis, and the body’s immune and antioxidant defense is impaired, leading to endothelial dysfunction.31–33 Platelet activation can also secrete various pro-inflammatory cytokines to participate in the body’s immune process. The severe inflammatory response can lead to collective microcirculation disorders, increased platelet destruction, and reduced renal blood flow and oxygen supply.34,35 Therefore, increased SII levels may affect the occurrence and development of CI-AKI through the inflammatory pathway and hyperactive coagulation pathway.34
The results of previous studies have shown that NT-proBNP levels increase with age and that the occurrence of CI-AKI after PCI in elderly STEMI patients is associated with high NT-pro BNP levels, similar to the results of this study.36,37 NT-pro BNP is a stable biomarker synthesized and secreted in response to myocardial ischemia, cardiac pressure overload, or ventricular dilatation.38 NT-proBNP has diuretic and natriuretic effects and vasodilatory and adverse inotropic effects. It inhibits myocardial contractility by inhibiting Ca2+ ATPase in the sarcoplasmic reticulum of cardiomyocytes and weakening the impact of catecholamines, which aggravates renal hemodynamic changes and renal medullary ischemia, leading to decreased renal blood flow and perfusion. Consequently, the sympathetic nervous system and renin-angiotensin-aldosterone system are activated, forming a vicious cycle of renal vasoconstriction.37,39 At the same time, CI-AKI may be associated with the decrease in cardiac output caused by the increase of NT-ProBNP, the active tubule-bulb feedback, the growth of intra-abdominal pressure, and the expansion of venous vascular bed volume. Their interaction leads to kidney congestion, which further aggravates kidney damage.40
This study showed that high sensitivity C-reactive protein (hsCRP) was an independent risk factor for CI-AKI, and the hsCRP in patients with CI-AKI is higher than that without CI-AKI. As an inflammatory marker, hsCRP is an essential predictor of the onset and prognosis of cardiovascular and cerebrovascular diseases.41,42 The level of hsCRP is closely related to the body’s inflammatory state. High hsCRP can mediate the expression of adhesion molecules, reduce the production of NO, and damage the antioxidant defense function of endothelial cells, resulting in endothelial dysfunction, which has been considered to be an important factor in the occurrence of CI-AKI.41,43
The nomogram model constructed in this study has good discrimination ability and accuracy in the training and validation cohort. Its effectiveness and applicability have been verified to a certain extent, making our model more clinically appealing. Medical workers can predict the incidence of CI-AKI after PCI according to the sum of the corresponding scores of various risk factors. According to the results, medical workers should strengthen the management of high-risk patients, such as adequate hydration, alkalization treatment, the use of relevant preventive drugs, and other ways to prevent CI-AKI after PCI actively.
Limitations
Our study has some limitations. Firstly, this was a single-center retrospective observational study, and there may be selection bias in including research subjects. Secondly, patients were not stratified by age for further analysis. Finally, the sample size of this study needs to be increased. Although internally validated, multi-centers and large sample sizes are required to evaluate the model’s value.
Conclusion
Our prediction model has good discrimination and accuracy for the elderly population, which allows physicians to assess the risk of CI-AKI through simple calculations before PCI and take relevant preventive measures to reduce the incidence of CI-AKI. It is worthy of further promotion and application.
Ethics Consent
The study was conducted following the principles in the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (ethics number: XYFY2022-KL123-01). The review committee waived the requirement for written informed consent because the study was a retrospective analysis. Before analysis, all confidential patient information was removed.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas. Have drafted or written, or substantially revised or critically reviewed the article. Have agreed on the journal to which the article will be submitted. Reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage, and agree to take responsibility and be accountable for the contents of the article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: a Report From the American Heart Association. Circulation. 2020;141(9):e139–e596. doi:10.1161/cir.0000000000000757
2. Silvain J, Nguyen LS, Spagnoli V, et al. Contrast-induced acute kidney injury and mortality in ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2018;104(9):767–772. doi:10.1136/heartjnl-2017-311975
3. Fähling M, Seeliger E, Patzak A, Persson PB. Understanding and preventing contrast-induced acute kidney injury. Nat Rev Nephrol. 2017;13(3):169–180. doi:10.1038/nrneph.2016.196
4. Mehran R, Dangas GD, Weisbord SD. Contrast-Associated Acute Kidney Injury. N Engl J Med. 2019;380(22):2146–2155. doi:10.1056/NEJMra1805256
5. Pan HC, Wu XH, Wan QL. Analysis of the risk factors for contrast-induced nephropathy in over-aged patients receiving coronary intervention. Exp biol med. 2018;243(12):970–975. doi:10.1177/1535370218799973
6. Denic A, Lieske JC, Chakkera HA, et al. The substantial loss of nephrons in healthy human kidneys with aging. J Am Soc Nephrol. 2017;28(1):313–320. doi:10.1681/asn.2016020154
7. Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80(1):93–104. doi:10.1038/ki.2010.531
8. Chandiramani R, Cao D, Nicolas J, Mehran R. Contrast-induced acute kidney injury. Cardiovasc Interv Ther. 2020;35(3):209–217. doi:10.1007/s12928-020-00660-8
9. Silver SA, Shah PM, Chertow GM, Harel S, Wald R, Harel Z. Risk prediction models for contrast induced nephropathy: systematic review. BMJ. 2015;351:h4395. doi:10.1136/bmj.h4395
10. Allen DW, Ma B, Leung KC, et al. Risk Prediction Models for Contrast-Induced Acute Kidney Injury Accompanying Cardiac Catheterization: systematic Review and Meta-analysis. Can J Cardiol. 2017;33(6):724–736. doi:10.1016/j.cjca.2017.01.018
11. Efe SC, Keskin M, Toprak E, et al. A Novel Risk Assessment Model Using Urinary System Contrast Blush Grading to Predict Contrast-Induced Acute Kidney Injury in Low-Risk Profile Patients. Angiology. 2021;72(6):524–532. doi:10.1177/00033197211005206
12. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393–1399. doi:10.1016/j.jacc.2004.06.068
13. Mehran R, Owen R, Chiarito M, et al. A contemporary simple risk score for prediction of contrast-associated acute kidney injury after percutaneous coronary intervention: derivation and validation from an observational registry. Lancet. 2021;398(10315):1974–1983. doi:10.1016/s0140-6736(21)
14. Tsai TT, Patel UD, Chang TI, et al. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the National Cardiovascular Data Registry Cath-PCI Registry. J Am Heart Assoc. 2014;3(6):e001380. doi:10.1161/jaha.114.001380
15. Park SY. Nomogram: an analogue tool to deliver digital knowledge. J Thorac Cardiovasc Surg. 2018;155(4):1793. doi:10.1016/j.jtcvs.2017.12.107
16. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–2264. doi:10.1016/j.jacc.2018.08.1038
17. Nijssen EC, Rennenberg RJ, Nelemans PJ, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, Phase 3, controlled, open-label, non-inferiority trial. Lancet. 2017;389(10076):1312–1322. doi:10.1016/s0140-6736(17)30057-0
18. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84. doi:10.1159/000339789
19. Kellum JA, Lameire N, Aspelin P. Section 4: contrast-induced AKI. Kidney International Supplements. 2012;2(1):69–88. doi:10.1038/kisup.2011.34
20. Erdoğan M, Erdöl MA, Öztürk S, Durmaz T. Systemic immune-inflammation index is a novel marker to predict functionally significant coronary artery stenosis. Biomark Med. 2020;14(16):1553–1561. doi:10.2217/bmm-2020-0274
21. Mettler FA, Mahesh M, Bhargavan-Chatfield M, et al. Patient Exposure from Radiologic and Nuclear Medicine Procedures in the United States: procedure Volume and Effective Dose for the Period 2006-2016. Radiology. 2020;295(2):418–427. doi:10.1148/radiol.2020192256
22. Hossain MA, Costanzo E, Cosentino J, et al. Contrast-induced nephropathy: pathophysiology, risk factors, and prevention. Saudi j Kidney Dis Transplantation. 2018;29(1):1–9. doi:10.4103/1319-2442.225199
23. Stojanović SD, Fiedler J, Bauersachs J, Thum T, Sedding DG. Senescence-induced inflammation: an important player and key therapeutic target in atherosclerosis. Eur Heart J. 2020;41(31):2983–2996. doi:10.1093/eurheartj/ehz919
24. Jia G, Aroor AR, Jia C, Sowers JR. Endothelial cell senescence in aging-related vascular dysfunction. Biochimica et biophysica acta Mol dis. 2019;1865(7):1802–1809. doi:10.1016/j.bbadis.2018.08.008
25. Çınar T, Karabağ Y, Ozan Tanık V, Çağdaş M, Rencüzoğulları İ, Öz A. The investigation of TIMI risk index for prediction of contrast-induced acute kidney injury in patients with ST elevation myocardial infarction. Acta Cardiol. 2020;75(1):77–84. doi:10.1080/00015385.2018.1551263
26. Yuan Y, Qiu H, Hu X, et al. Predictive value of inflammatory factors on contrast-induced acute kidney injury in patients who underwent an emergency percutaneous coronary intervention. Clin Cardiol. 2017;40(9):719–725. doi:10.1002/clc.22722
27. Li Y, Ren K. The Mechanism of Contrast-Induced Acute Kidney Injury and Its Association with Diabetes Mellitus. Contrast Media Mol Imaging. 2020;2020:3295176. doi:10.1155/2020/3295176
28. Qin YH, Yan GL, Ma CL, Tang CC, Ma GS. Effects of hyperglycaemia and elevated glycosylated haemoglobin on contrast-induced nephropathy after coronary angiography. Exp Ther Med. 2018;16(1):377–383. doi:10.3892/etm.2018.6183
29. Wang K, Li HL, Bei WJ, et al. Association of left ventricular ejection fraction with contrast-induced nephropathy and mortality following coronary angiography or intervention in patients with heart failure. Ther Clin Risk Manag. 2017;13:887–895. doi:10.2147/tcrm.S137654
30. Bağcı A, Aksoy F, Baş HA. Systemic Immune-Inflammation Index May Predict the Development of Contrast-Induced Nephropathy in Patients With ST-Segment Elevation Myocardial Infarction. Angiology. 2022;73(3):218–224. doi:10.1177/00033197211030053
31. Butt K, D’Souza J, Yuan C, et al. Correlation of the Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) with Contrast-Induced Nephropathy in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Interventions. Cureus. 2020;12(12):e11879. doi:10.7759/cureus.11879
32. Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi:10.1155/2009/137072
33. Ösken A, Öz A, Keskin M, et al. The association between neutrophil-to-lymphocyte ratio and contrast-induced acute kidney injury in patients with carotid artery stenting. Vascular. 2021;29(4):550–555. doi:10.1177/17085381211012562
34. Jansen MP, Emal D, Teske GJ, Dessing MC, Florquin S, Roelofs JJ. Release of extracellular DNA influences renal ischemia reperfusion injury by platelet activation and formation of neutrophil extracellular traps. Kidney Int. 2017;91(2):352–364. doi:10.1016/j.kint.2016.08.006
35. Gameiro J, Fonseca JA, Jorge S, Gouveia J, Lopes JA. Neutrophil, lymphocyte and platelet ratio as a predictor of mortality in septic-acute kidney injury patients. Nefrologia. 2020;40(4):461–468. doi:10.1016/j.nefro.2019.11.006
36. Wang K, Li HL, Chen LL, et al. Association of N-terminal pro-brain natriuretic peptide with contrast-induced acute kidney injury and long-term mortality in patients with heart failure and mid-range ejection fraction: an observation study. Medicine. 2017;96(10):e6259. doi:10.1097/md.0000000000006259
37. Lin KY, Wu ZY, You ZB, et al. Pre-Procedural N-Terminal Pro-B Type Natriuretic Peptide Predicts Contrast-Induced Acute Kidney Injury and Long-Term Outcome in Elderly Patients After Elective Percutaneous Coronary Intervention. Int Heart J. 2018;59(5):926–934. doi:10.1536/ihj.17-573
38. Li X, Liu C, Mao Z, Qi S, Song R, Zhou F. Brain Natriuretic Peptide for Predicting Contrast-Induced Acute Kidney Injury in Patients with Acute Coronary Syndrome Undergoing Coronary Angiography: a Systematic Review and Meta-Analysis. J Interv Cardiol. 2020;2020:1035089. doi:10.1155/2020/1035089
39. Okamoto R, Ali Y, Hashizume R, Suzuki N, Ito M. BNP as a Major Player in the Heart-Kidney Connection. Int J Mol Sci. 2019;20(14):48. doi:10.3390/ijms20143581
40. Afsar B, Ortiz A, Covic A, Solak Y, Goldsmith D, Kanbay M. Focus on renal congestion in heart failure. Clin Kidney J. 2016;9(1):39–47. doi:10.1093/ckj/sfv124
41. Guedeney P, Claessen BE, Kalkman DN, et al. Residual Inflammatory Risk in Patients With Low LDL Cholesterol Levels Undergoing Percutaneous Coronary Intervention. J Am Coll Cardiol. 2019;73(19):2401–2409. doi:10.1016/j.jacc.2019.01.077
42. Shacham Y, Leshem-Rubinow E, Steinvil A, Keren G, Roth A, Arbel Y. High sensitive C-reactive protein and the risk of acute kidney injury among ST elevation myocardial infarction patients undergoing primary percutaneous intervention. Clin Exp Nephrol. 2015;19(5):838–843. doi:10.1007/s10157-014-1071-1
43. Fujii H, Li SH, Szmitko PE, Fedak PW, Verma S. C-reactive protein alters antioxidant defenses and promotes apoptosis in endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2006;26(11):2476–2482. doi:10.1161/01.Atv.0000242794.65541.02
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

