Back to Journals » Drug Design, Development and Therapy » Volume 16
A Review of Herbal Medicine-Based Phytochemical of Garcinia as Molecular Therapy for Breast Cancer
Authors Triyasa KS, Diantini A , Barliana MI
Received 31 January 2022
Accepted for publication 7 September 2022
Published 12 October 2022 Volume 2022:16 Pages 3573—3588
DOI https://doi.org/10.2147/DDDT.S358229
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Anastasios Lymperopoulos
Komang Suma Triyasa,1 Ajeng Diantini,1,2 Melisa Intan Barliana2,3
1Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, Indonesia; 2Center of Excellence in Higher Education for Pharmaceutical Care Innovation, Universitas Padjadjaran, Bandung, Indonesia; 3Department of Biological Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, Indonesia
Correspondence: Melisa Intan Barliana, Department of Biological Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Jl. Ir. Soekarno KM. 21, Jatinangor, Bandung, 45363, Indonesia, Email [email protected]
Abstract: Data from globocan statistic in 2020 indicate that breast cancer has become highest incidence rate of cancer. Estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) are known immunohistochemistry (IHC) markers that mediate cell growth and survival signaling. Furthermore, regulator proteins, receptors, and their downstream signaling pathways have emerged as critical components in breast cancer formation and proliferation, and have become well-established therapeutic targets and the core focus of breast cancer therapy research. Garcinia is a big genus in the Clusiaceae family that contains a wide spectrum of biologically active metabolites for the chemical composition of their isolated fruits, stem barks, seeds, leaves, and roots, have resulted including polyisoprenylated benzophenones, polyphenols, bioflavonoids, xanthones, lactones, and triterpenes. This review article aimed to analyze the potential of Garcinia phytochemicals as a molecular therapy of breast cancer. The results showed that phytochemicals of Garcinia (i.e., α-mangostin, Cambogin, Gambogic Acid [GA], Garcinol, Griffipavixanthone, Friedolanostane triterpenoid, Hexane, Neobractatin, 7-Epiclusianone, xanthochymol - guttiferone E, and isoxanthochymol - cycloxanthochymol) have anticancer properties, including apoptosis, inhibition of proliferation, and metastasis. This review is important to provide information regarding phytochemicals of Garcinia as an alternative treatment for breast cancer patients. This article selected 28 article researches based on inclusion criteria with the keyword “Garcinia” and “Breast cancer”, in English, and available in full text and abstract searching on PubMed.
Keywords: breast cancer, Garcinia spp, molecular therapy, Indonesia
Introduction
Data from Globocan showed that the new cases of breast cancer in 2020 were 11.7% and it became the highest incidence rate of cancer for both sexes in all ages.1 In addition, the International Agency for Research on Cancer (IARC) reported 2.1 million new cases of breast cancer in 2018. Breast cancer is also the top cause of cancer death in women worldwide, with 627,000 fatalities reported in 2018.2
Breast tumor subtyping is traditionally done using immunohistochemistry (IHC) markers such as “estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2).”3 These hormone and growth receptors, which are known to stimulate cell growth and survival signaling, are well-established therapeutic targets for breast cancer treatment and have been the focus of pharmacological research.4
Weinberg et al described six cancer hallmarks in 2000:
maintaining proliferative signals, avoiding growth suppressors, resisting cell death, enabling replicative immortality, initiating angiogenesis, and activating invasion and metastasis.5
A wide range of intracellular chemicals have been discovered as causing cancer cells to proliferate uncontrollably. In malignant cells, for example, “cyclin-dependent kinase (CDK)” overexpression and tumor suppressor protein “(p53), BRCA1 and BRCA2, CDK inhibitors, p21, p27, and p57” downregulation have been discovered.6,7 Protein control of pro-apoptotic “Bcl-2 family members, initiator caspase (e.g., caspase 8/9), effector caspase (e.g., caspase 3), and apoptosis” as a barrier to cancer formation.8 Several important receptors and signaling pathways have emerged as key players in the development and advancement of breast cancer.
“The epidermal growth factor receptor (EGFR), HER2, and Vascular Endothelial Growth factor (VEGF)” are the most prevalent growth factor receptors that are overexpressed in breast cancer cells.9 These receptors may be activated by the Janus kinases, signal transducer and activator of transcription proteins “(JAK/STAT), phosphoinositide 3-kinase (PI3K), protein kinase B (Akt), mammalian target of rapamycin (mTOR), and mitogen-activated protein kinases (MAPK)” pathways. Furthermore tumor cells have been found to exhibit altered expression of many pro-inflammatory transcription factors, including “nuclear factor kpB (NFkpB), activating protein-1 (AP-1), and hypoxia-inducible factor 1 (HIF-1)”.10–14 Chronic inflammation is thought to play a role in both the start and development of cancer.15 As a result, addressing the major aberrant proteins and pathways is a promising strategy to breast cancer treatment.16
Garcinia is a Clusiaceae family genus with approximately 450 species found in tropical Asia, South Africa, and America, as well as Madagascar, New Guinea, and Polynesia.17 For example the fruits of Garcinia have been used in traditional medicine for a variety of purposes, including antifever infusions in Thai folk medicine,18 wound healing and treatment of peptic ulcers in Brazilian folk medicine,17 earache in Thai medicine,19 and ailments such as heat strokes, infections, and edema in the Ayurvedic system of medicine.20
“Polyisoprenylated benzophenones, polyphenols, bioflavonoids, xanthones, lactones, and triterpenes” are among the physiologically active metabolites found in the fruits, stem barks, seeds, leaves, and roots of numerous Garcinia species.21–25 As a result, Garcinia species have been shown to be abundant in compounds that have medicinal properties.26–29 Free-radical scavenging, antiulcer effects,30 cytotoxicity, nitric oxide synthase inhibition,31 cancer chemoprevention,32 induction of apoptosis,33 anti-HIV,34 and trypanocidal properties have been associated to these substances.35 This review aimed to analyze the potential of Garcinia phytochemicals as molecular therapy of breast cancer. This research is important to provide information concerning phytochemicals of Garcinia as an alternative treatment for patients with breast cancer.
Material and Methods
The articles were selected on the basis of inclusion studies published in the PubMed database; articles in English, available in full text and abstract form, consist of the keywords “Garcinia” and “breast cancer.” The sorting processes can be seen in Figure 1.
 |
Figure 1 Prisma chart. Notes: Adapted from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71.66 Creative Commons Attribution (CC BY 4.0) license (https://creativecommons.org/licenses/by/4.0/legalcode). |
Results and Discussion
Globocan showed that new cases of breast cancer in 2020 were 2.261.419 (11.7%) and mortality was 684.996 deaths (6.9%), thus, breast cancer has become the highest incidence rate of cancer.1 Breast tumor subtypes are traditionally classified based on hormonal and growth factor response. In this context, the most clinically important receptors are “ER, PR, and HER2.”4 Cancer cells deregulate these hormonal and growth signals, allowing them to continue proliferative signaling in a variety of ways. They enhance cell surface receptor expression and accumulate activating mutations, resulting in cell surface receptor or downstream signaling pathway activation that is constant.36 Some Garcinia metabolites, such as “Garcinol, α-mangostin, Cambogin, and Gambogic acid” (GA) have exhibited anticancer action in vitro and in vivo, causing apoptosis and cellular cycle arrest, suppression of angiogenesis, and gene expression regulation in carcinogenic cells.37–41 The general pathways of phytochemicals of Garcinia mechanism in cancer targeted therapy can be seen in Figures 2–4.
 |
Figure 4 Cell growth and survival. Mechanism phytochemical of Garcinia affected in ras/raf/MEK, akt/mTOR, and JAK/STAT3 pathway. |
α-Mangostin
Garcinia mangostana extracts were found to contain the anticancer phytochemical α-Mangostin. Kurose et al discovered that α-mangostin induced mitochondrial apoptosis. Increased caspase-3, caspase-8, and caspase-9 activity, as well as increased cytochrome c protein release concentration, support this. The expression of CDK - interacting protein 1 (p21cip1) was upregulated, and Checkpoint Kinase 2 (CHEK2) was tended to increase, resulting in a decrease in CDKs and cyclins, as well as G1-phase arrest and inhibition of cell proliferation, followed by decreases in proliferating cell nuclear antigen (PCNA).42
When compared to proform-HER2 expression, Kritsanawong et al discovered that -Mangostin can reduce Phospho-HER2 (p-HER2) at Tyr1221/1222. This results in a decrease in Nuclear Factor NF-Bp 65, c-Rel, and c-Myc expression while increasing IB Kinase Complex Alpha (IKK) expression. However, activation of p38 and c-Jun N-Terminal Kinase 1/2 (JNK1/2) resulted in the expression of C/EBP Homologous Protein and c-Jun.43
Shibata et al revealed that α-mangostatin promotes mitochondrial apoptosis, G1-phase arrest, and S-phase suppression during the cell cycle. Akt phosphorylation was induced by α-mangostin treatment both in vitro and in vivo, demonstrating that α-mangostin significantly reduces the levels of phospho-Akt-threonine 308 (Thr308); α-Mangostin significantly increased caspase-3, caspase-8, and caspase-9 activity; cytochrome c protein levels in cytosolic fractions were significantly higher in cells treated with α-angostin; and caspase-8-Bid cleavage triggered the mitochondrial pathway.44
α-mangostin also activated caspases-8, −9, and −7, elevated Bax, p53, and cytosolic cytochrome c protein levels, and stimulated Poly (ADP-Ribose) Polymerase (PARP) cleavage while lowering Bid and Bcl-2 protein expression, according to Won et al. Furthermore, apoptosis-inducing factor (AIF) was transferred from the mitochondria to the cytosol and promoted apoptosis in E2-stimulated cells in parallel with non-stimulated cells, lowering the expression of ERa and pS2, an estrogen-responsive gene.45 According to Doi et al, isolated panaxanthone from G. mangostana dramatically boosted caspase-3, caspase-9, and caspase-8 activities, triggered the G1-phase, and lowered the number of cells in both the S- and G2/M-phases.46 α-mangostin reduced 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced MMP-2 and MMP-9 production, as well as cell invasion and migration (Figure 5), according to Lee et al,47
 |
Figure 5 α-Mangostin chemical structure. Notes: Reproduced from:: National Center for Biotechnology Information. PubChem Compound Summary for CID 5281650, alpha-Mangostin. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-Mangostin. Accessed September 15, 2022.66 |
Cambogin
Cambogin compounds (Figure 6) can be found in the branches of Garcinia esculenta. Shen et al reported that cambogin treatment via the NOX enzyme is activated by enhancing p22phox and NADPH Oxidase 1 (NOX1) interaction. The dissociation of Thioredoxin 1 (Trx1) from the activation of the Apoptosis Signal-Regulating Kinase 1 (ASK1) pathway and the induction of mitochondrial network abnormalities resulted from an increase in intracellular and mitochondrial levels of Oxide (O2-) and Hydrogen Peroxide (H2O2), resulting in the dissociation of Thioredoxin 1 (Trx1) from the activation of the Apoptosis.38 According to Shen et al, activation of ASK-1, SAPK/Erk Kinase (SEK1/MKK4), MKK7, and Jun Amino-Terminal Kinases/Stress-Activated Protein Kinase (JNK/SAPK) is necessary for cambogin-induced Reactive Oxygen Species (ROS). Cambogin stimulated the caspase-independent mitochondrial apoptotic pathway, as evidenced by an increase in the ratio of B-Cell Lymphoma Protein 2, Associated X (Bax/Bcl-2), and nuclear translocation of AIF. JNK/SAPK or p38 MPAK activation phosphorylated Activating Transcription Factor 2 (ATF-2) and increased histone H3K9 trimethylation in the Bcl-2 gene promoter’s activator protein 1 (AP-1) binding region.48
 |
Figure 6 Cambogin chemical structure. Notes: Reproduced from: National Center for Biotechnology Information. PubChem Substance Record for SID 426321898, SID 426321898, Source: Google Patents. Available from: https://pubchem.ncbi.nlm.nih.gov/substance/426321898. Accessed September 15, 2022.67 |
Gambogic Acid
The compound GA is abundant in Garcinia hanburyi (Figure 7). According to Wang et al, TNF-Related Apoptosis-Inducing Ligand (TRAIL) and GA increased apoptosis in TRAIL-resistant cells and play a critical role in inducing apoptosis and reducing levels of anti-apoptotic Bcl-2 protein, boosting the interplay of extrinsic and intrinsic apoptosis signaling.40 GA induces apoptosis, according to Zhou et al, who looked at changes in the expression levels of apoptosis-regulating proteins such as cleaved caspase-3, caspase −8, and caspase −9, as well as Bax, while Bcl-2 was decreased and Fas and Fas ligand (FasL) were increased.49 GA depolymerized microtubules and increased JNK1 and p38 phosphorylation, causing G2/M cell-cycle arrest and apoptosis, according to Chen et al,50
 |
Figure 7 GA chemical structure. Notes: Reproduced from: National Center for Biotechnology Information. PubChem Compound Summary for CID 9852185, GA. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/9852185. Accessed September 15, 2022.68 |
According to Wang et al, GA also increase the expression of apoptosis-related proteins FasL, caspase-3, caspase-8, caspase-9, and Bax while suppressing the anti-apoptotic protein Bcl-2.51 GA also caused PARP cleavage, caspase-3, caspase-8, and caspase-9 activation, and an increase in the Bax/Bcl-2 ratio, according to Li et al. Furthemore, GA caused apoptosis via the buildup of ROS and mitochondrial apoptotic pathway, as shown by AIF translocation and cytochrome c (Cyt c) release from mitochondria. By decreasing Akt/mTOR signaling, GA also reduced cell survival.52 GA also prevented tumor invasion and metastasis by reducing MMP-2 and MMP-9 activity, according to Qi et al.53,54
Garcinol
Garcinol (Figure 8) compound can be found in Garcinia morella and Garcinia indica. Choudhury et al reported that Garcinol inhibited the complex polysaccharides like Lipopolysaccharide (LPS) induced increase in cytokine secretion such as Tumor Necrosis Factor Alpha (TNF-α), Interleukin 1 Beta (IL- 1β) by macrophages as inflammatory agent.41 Ahmad et al showed that garcinol causes Mesenchymal-Epithelial Transition (MET) in aggressive breast cancer cells through apoptosis mediated by downregulation of the NF-kB signaling pathway. This is in line with the mesenchymal markers vimentin, ZEB1, and ZEB being downregulated and the epithelial marker E-cadherin being increased, as well as the miRNAs, miR-200, and let-7 families being implicated in the maintenance and control of EMT to MET. The results also show that garcinol has an effect on the Wnt signaling pathway, causing β-catenin to translocate to the nucleus. There is crosstalk between the NF-kB and Wnt signaling pathways when the phosphorylated form of β-catenin increases. GSK-3, the phosphorylation factor for β-catenin, was discovered to be increased, causing β-catenin nuclear translocation to be inhibited and, as a result, Wnt signaling pathways to be inhibited.55
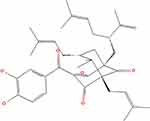 |
Figure 8 Garcinol chemical structure. Notes: Reproduced from: National Center for Biotechnology Information. PubChem Substance Record for SID 444134253, garcinol, Source: A2B Chem. Available from: https://pubchem.ncbi.nlm.nih.gov/substance/444134253. Accessed September 15, 2022.69 |
Garcinol increased Taxol-induced antimitotic activity, reduced caspase-3/iPLA2-stimulated cell repopulation and prevented NF-kB/Twist1-derived pro-inflammatory signaling and pro-metastatic properties, according to Tu et al.56 Chen et al discovered that Garcinol-induced 9-nAChR downregulation may have a direct impact on cyclin D3 gene expression transcriptional regulation. Garcinol inhibits the progression of the cell cycle in human breast cancer cells through regulating the cyclin D3 gene. According to Ahmad et al, garcinol suppressed IL-6-induced STAT-3 phosphorylation as well as the synthesis of urokinase-type plasminogen activator (uPA), VEGF, and matrix metalloproteinase-9 (MMP-9) activator, reducing cell invasion and aggressiveness.57 Garcinol inhibited IL-6-induced STAT-3 phosphorylation and production of urokinase-type plasminogen activator (uPA), VEGF, and matrix metalloproteinase-9 (MMP-9) activator, which reduced cell invasion and aggressiveness, according to Ahmad et al.58 Induction of caspase-mediated apoptosis (Caspase 3, Caspase 9) was also added by Ahmad et al, as evidenced by PARP cleavage. Apoptosis is induced by inactivation of NF-kB signaling and downregulation of its target genes.59
According to Ye et al, garcinol suppressed 17-Estradiol (E2), which elevated ac-H3, ac-H4, and NF-κB/ac-p65 levels. Nuclear translocation of NF-κB/p65, as well as cyclin D1, Bcl-2, and Bcl-xl mRNA and protein expression levels, were decreased in E2-treated cells. In the NF-κB pathway, reduced ac-p65 protein expression is hypothesized to be connected to downregulation of cyclin D1, Bcl-2, and Bcl-xl expression.60
Griffipavixanthone (GPX)
Griffipavixanthone (GPX) is found in Garcinia oblongifolia (Figure 9). According to Ma et al, GPX cleaves caspase-8/9, and PARP GPX increased the mRNA level of the p53 gene and its target genes, and changed Bax expression while Bcl-2 decreased in mitochondria by releasing cytochrome c.40
 |
Figure 9 Griffipavixanthone chemical structure. Notes: Reproduced from: National Center for Biotechnology Information. PubChem Substance Record for SID 382158510, 219649–95-3, Source: BioCrick. Available from: https://pubchem.ncbi.nlm.nih.gov/substance/382158510. Accessed September 15, 2022.70 |
Friedolanostane Triterpenoid
Garcinia celebica fruits contain the triterpenoid compound friedolanostane (Figure 10). Subarnas et al discovered that a compound inhibited the oncogenic protein Akt, resulting in an increase in PARP.61
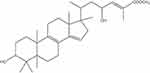 |
Figure 10 Friedolanostane triterpenoid chemical structure. Notes: Reproduced from: Subarnas A, Diantini A, Abdulah R et al. Apoptosis-mediated antiproliferative activity of friedolanostane triterpenoid isolated from the leaves of Garcinia celebica against MCF-7 human breast cancer cell lines. Biomed Rep. 2016;4(1):79–82. doi:10.3892/br.2015.532.61 Copyright © 2015, Spandidos Publications. |
Hexane
The fruits of Garcinia quaesita consist of Hexane (Figure 11). Pathiranage et al reported that the compound increased the activity of caspase 3/7, increased Bax, and decreased Baculoviral Inhibitor of Apoptosis Repeat Containing 5 (BIRC-5).62
 |
Figure 11 Hexane crystal structure. Notes: Reproduced from: National Center for Biotechnology Information. PubChem Compound Summary for CID 8058, Hexane. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Hexane. Accessed September 15, 2022.71 |
Neobractatin (NBT)
Neobractatin (NBT) was found in Garcinia bracteata extract (Figure 12), which inhibited metastasis by decreasing the expressions of pAKT, the EMT markers vimentin and cofilin, and Matrix Metalloproteinase 2 (MMP2).63
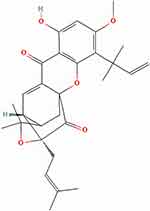 |
Figure 12 Neobractatin chemical structure. Notes: Reproduced from: National Center for Biotechnology Information. PubChem Compound Summary for CID 101508194, Neobractatin. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Neobractatin. Accessed September 15, 2022.72 |
7-Epiclusianone (7-Epi)
The extract of Garcinia gardneriana fruits contains 7-Epiclusianone (7-Epi), which enhances the BAX/BCL-2 ratio when cells accumulate in the G0/G1 phase (Figure 13). 7-Epi reduced the expression of CDK Inhibitor 1A (CDKN1A (p21)) and cyclin E in both cell lines, while decreasing the expression of cyclin D1 and p-ERK in the MCF-7 cell line.64
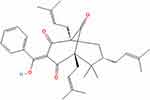 |
Figure 13 7-Epiclusianone chemical structure. Notes: Reproduced from: National Center for Biotechnology Information. PubChem Compound Summary for CID 5471610, 7-Epiclusianone. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/7-Epiclusianone. Accessed September 15, 2022.73 |
S1 (the Regioisomeric Mixture of Xanthochymol and Guttiferone E) and S2 (the Regioisomeric Mixture of Isoxanthochymol and Cycloxanthochymol
These compounds are found in Garcinia xanthochymus (Figure 14). According to Xu et al, S1 and S2 reduced the phosphorylation of STAT3’s upstream kinases, Janus Kinase 2 (JAK2) and Src, as well as the expression of various STAT3-regulated genes, including anti-apoptotic (Bcl-XL, Mcl-1, and survivin), proliferative (cyclin D1), and angiogenic (VEGF) genes.65
 |
Figure 14 (A) S1 (the regioisomeric mixture of xanthochymol and guttiferone E) and (B) S2 (the regioisomeric mixture of isoxanthochymol and cycloxanthochymol chemical structure. Notes: Reproduced from: Xu J, Jin S, Gan F et al. Polycyclic polyprenylated acylphloroglucinols from: Garcinia xanthochymus fruits exhibit antitumor effects through inhibition of the STAT3 signaling pathway. Food Funct. 2020;11(12):10568–10579. doi:10.1039/d0fo02535f.65 © Royal Society of Chemistry 2022. |
The result of this review showed several pieces of research that reported the use of Garcinia phytochemicals as molecular therapy for breast cancer, as seen in Table 1.
 |
Table 1 Review of Molecular Mechanism of Garcinia Phytochemical on Breast Cancer Cells |
Conclusion
On the basis of this review, it can be concluded that Garcinia phytochemical compounds are potential as molecular therapy for breast cancer and have low toxicity to normal cells. This result can be used as an alternative for minimally invasive therapy for patients with breast cancer, since chemotherapy agents have many adverse side effects on healthy cells. The result confirms α-mangostin, Cambogin, GA, Garcinol, Griffipavixanthone, Friedolanostane triterpenoid, Hexane, Neobractatin, 7-Epiclusianone, xanthochymol - guttiferone E, and isoxanthochymol - cycloxanthochymol have anticancer properties including apoptosis, inhibition of proliferation and, metastasis. These phytochemicals can be used as candidates for molecular therapy to improve the health and life expectancy of patients with breast cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Fulford LG, Easton DF, Reis-Filho JS, et al. Specific morphological features predictive for the basal phenotype in grade 3 invasive ductal carcinoma of breast. Histopathology. 2006;49(1):22–34. doi:10.1111/j.1365-2559.2006.02453.x
4. Dai X, Xiang L, Li T, Bai Z. Cancer hallmarks, biomarkers and breast cancer molecular subtypes. J Cancer. 2016;7(10):1281–1294. doi:10.7150/jca.13141
5. Hanahan D, Weinberg RA. The Hallmarks of Cancer Review Evolve Progressively from Normalcy via a Series of Pre. Cell Press; Vol. 100, 2000.
6. Williams GH, Stoeber K. The cell cycle and cancer. J Pathol. 2012;226(2):352–364. doi:10.1002/path.3022
7. Peng L, Xu T, Long T, Zuo H. Association between BRCA status and P53 status in breast cancer: a meta-analysis. Med Sci Monitor. 2016;22:1939–1945. doi:10.12659/MSM.896260
8. Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26(9):1324–1337. doi:10.1038/sj.onc.1210220
9. Masoud V, Pagès G. Targeted therapies in breast cancer: new challenges to fight against resistance. World J Clin Oncol. 2017;8(2):120–134. doi:10.5306/wjco.v8.i2.120
10. Atsaves V, Leventaki V, Rassidakis GZ, Claret FX. AP-1Transcription factors as regulators of immune responses in cancer. Cancers. 2019;11(7):1037. doi:10.3390/cancers11071037
11. Park M, Hong J. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells. 2016;5(2):15. doi:10.3390/cells5020015
12. Peluso I, Yarla NS, Ambra R, Pastore G, Perry G. MAPK signalling pathway in cancers: olive products as cancer preventive and therapeutic agents. Semin Cancer Biol. 2019;56:185–195. doi:10.1016/j.semcancer.2017.09.002
13. Amani H, Ajami M, Nasseri Maleki S, et al. Targeting signal transducers and activators of transcription (STAT) in human cancer by dietary polyphenolic antioxidants. Biochimie. 2017;142:63–79. doi:10.1016/j.biochi.2017.08.007
14. Huang J, Gao L, Li B, et al. Knockdown of Hypoxia-Inducible Factor 1α (HIF-1α) promotes autophagy and inhibits Phosphatidylinositol 3-Kinase (PI3K)/AKT/Mammalian Target of Rapamycin (mTOR) signaling pathway in ovarian cancer cells. Med Sci Monitor. 2019;25:4250–4263. doi:10.12659/MSM.915730
15. Marelli G, Sica A, Vannucci L, Allavena P. Inflammation as target in cancer therapy. Curr Opin Pharmacol. 2017;35:57–65. doi:10.1016/j.coph.2017.05.007
16. Nouri Z, Fakhri S, Nouri K, Wallace CE, Farzaei MH, Bishayee A. Targeting multiple signaling pathways in cancer: the rutin therapeutic approach. Cancers. 2020;12(8):1–34. doi:10.3390/cancers12082276
17. Kumar S, Sharma S, Chattopadhyay SK. The potential health benefit of polyisoprenylated benzophenones from Garcinia and related genera: ethnobotanical and therapeutic importance. Fitoterapia. 2013;89(1):86–125. doi:10.1016/j.fitote.2013.05.010
18. Pornpipat N, Pattalung P, Thongtheeraparp W, Wiriyachitra P, Taylor WC. Xanthones of Garcinia Cowa. Planta Med. 1994;60(04):365–368. doi:10.1055/s-2006-959502
19. Tisdale EJ, Kochman DA, Theodorakis EA. Total synthesis of atroviridin. Tetrahedron Lett. 2003;44(16):3281–3284. doi:10.1016/S0040-4039(03)00629-4
20. Padhye S, Ahmad A, Oswal N, Sarkar FH. Emerging role of Garcinol, the antioxidant chalcone from Garcinia indica Choisy and its synthetic analogs. J Hematol Oncol. 2009;2(1). doi:10.1186/1756-8722-2-38
21. Bennett GJ, Lee HH. Review article number 43 xanthones from guttiferae; Vol 28, 1989.
22. Parveen M, Ud-Din Khan N, Achari~ B, Du-Ita~ PK. A Triterpene from Garcinia Mangostana. Pergamon Press plc; Vol. 30, 1991.
23. Rama Rao AV, Sakma MR, Venkatakaman K, Yemul SS. A Benzophenone and Xanthone with Unusual Hydroxylation Patterns from the Heartwood of Garcinia Pedunculata. Pergamon Press; Vol. 13, 1974.
24. Acuña UM, Dastmalchi K, Basile MJ, Kennelly EJ. Quantitative high-performance liquid chromatography photo-diode array (HPLC-PDA) analysis of benzophenones and biflavonoids in eight Garcinia species. J Food Compos Anal. 2012;25(2):215–220. doi:10.1016/j.jfca.2011.10.006
25. Antony JIX, Josa P, Shankaranarayana ML. Quantitative analysis of (-)hydroxy citric acid and (-)hydroxy citric acid lactone in Garcinia fruits and Garcinia products. J Food Sci Technol. 1998;35:399–402.
26. Gustafson KR, Blunt JW, Munro’ MHG, et al. The Guttiferones, HIY-Inhibitory Benzophenones from Svmuhonia Alobulifera, w Livinastonei, Garcinia Ovalifolia and Clusia Rosea; Vol 48, 1992.
27. Cui J, Hu W, Cai Z, et al. New medicinal properties of mangostins: analgesic activity and pharmacological characterization of active ingredients from the fruit hull of Garcinia mangostana L. Pharmacol Biochem Behav. 2010;95(2):166–172. doi:10.1016/j.pbb.2009.12.021
28. Williams RB, Hoch J, Glass TE, et al. A novel cytotoxic guttiferone analogue from garcinia macrophylla from the Suriname rainforest. Planta Med. 2003;69(09):864–866.
29. Nguyen DC, Timmer TK, Davison BC, McGrane IR. Possible Garcinia cambogia-Induced mania with psychosis: a case report. J Pharm Pract. 2019;32(1):99–102. doi:10.1177/0897190017734728
30. Yamaguchi F, Saito M, Ariga T, Yoshimura Y, Nakazawa H. Free radical scavenging activity and antiulcer activity of garcinol from Garcinia indica fruit rind. J Agric Food Chem. 2000;48(6):2320–2325. doi:10.1021/jf990908c
31. Cruz AJ, Lemos VS, Dos Santos MH, Nagem TJ, Cortes SF. Vascular effects of 7-epiclusianone, a prenylated benzophenone from Rheedia gardneriana, on the rat aorta. Phytomedicine. 2006;13(6):442–445. doi:10.1016/j.phymed.2005.01.014
32. Ito C, Itoigawa M, Miyamoto Y, et al. Polyprenylated benzophenones from Garcinia assigu and their potential cancer chemopreventive activities. J Nat Prod. 2003;66(2):206–209. doi:10.1021/np020372g
33. Pan MH, Chang WL, Lin-Shiau SY, Ho CT, Lin JK. Induction of apoptosis by garcinol and curcumin through cytochrome c release and activation of caspases in human leukemia HL-60 cells. J Agric Food Chem. 2001;49(3):1464–1474. doi:10.1021/jf001129v
34. Piccinelli AL, Cuesta-Rubio O, Chica MB, et al. Structural revision of clusianone and 7-epi-clusianone and anti-HIV activity of polyisoprenylated benzophenones. Tetrahedron. 2005;61(34):8206–8211. doi:10.1016/j.tet.2005.06.030
35. Abe F, Nagafuji S, Okabe H, et al. Trypanocidal constituents in plants 3. Leaves of garcinia intermedia and heartwood of Calophyllum brasiliense. Biol Pharm Bull. 2004;27(1):141–143. doi:10.1248/bpb.27.141
36. Saha T, Solomon J, Samson AO, Gil-Henn H. Invasion and metastasis as a central hallmark of breast cancer. J Clin Med. 2021;10(16):3498. doi:10.3390/jcm10163498
37. Shan T, Ma Q, Guo K, et al. Xanthones from mangosteen extracts as natural chemopreventive agents: potential anticancer drugs. Curr Mol Med. 2011;11(8):666–677. doi:10.2174/156652411797536679
38. Shen K, Lu F, Xie J, et al. Cambogin exerts anti-proliferative and pro-apoptotic effects on breast adenocarcinoma through the induction of NADPH oxidase 1 and the alteration of mitochondrial morphology and dynamics. Oncotarget. 2016;7(31):50596–50611. doi:10.18632/oncotarget.10585
39. Ma Y, Wang Y, Song B. Griffipavixanthone induces apoptosis of human breast cancer MCF-7 cells in vitro. Breast Cancer. 2019;26(2):190–197. doi:10.1007/s12282-018-0912-2
40. Wang S, Xu Y, Li C, et al. Gambogic acid sensitizes breast cancer cells to TRAIL-induced apoptosis by promoting the crosstalk of extrinsic and intrinsic apoptotic signalings. Food Chem Toxicol. 2018;119:334–341. doi:10.1016/j.fct.2018.02.037
41. Choudhury B, Kandimalla R, Elancheran R, Bharali R, Kotoky J. Garcinia morella fruit, a promising source of antioxidant and anti-inflammatory agents induces breast cancer cell death via triggering apoptotic pathway. Biomed Pharmacother. 2018;103:562–573. doi:10.1016/j.biopha.2018.04.068
42. Kurose H, Shibata MA, Iinuma M, Otsuki Y. Alterations in cell cycle and induction of apoptotic cell death in breast cancer cells treated with -mangostin extracted from mangosteen pericarp. J Biomed Biotechnol. 2012;2012. doi:10.1155/2012/672428.
43. Kritsanawong S, Innajak S, Imoto M, Watanapokasin R. Antiproliferative and apoptosis induction of α-mangostin in T47D breast cancer cells. Int J Oncol. 2016;48(5):2155–2165. doi:10.3892/ijo.2016.3399
44. Shibata MA, Iinuma M, Morimoto J, et al. α-Mangostin extracted from the pericarp of the mangosteen (Garcinia mangostana Linn) reduces tumor growth and lymph node metastasis in an immunocompetent xenograft model of metastatic mammary cancer carrying a p53 mutation. BMC Med. 2011;9:1–18. doi:10.1186/1741-7015-9-69
45. Won YS, Lee JH, Kwon SJ, et al. α-Mangostin-induced apoptosis is mediated by estrogen receptor α in human breast cancer cells. Food Chem Toxicol. 2014;66:158–165. doi:10.1016/j.fct.2014.01.040
46. Doi H, Shibata MA, Shibata E, et al. Panaxanthone isolated from pericarp of Garcinia mangostana L. suppresses tumor growth and metastasis of a mouse model of mammary cancer. Anticancer Res. 2009;29(7):2485–2495.
47. Lee YB, Ko KC, Shi M, et al. α-Mangostin, a novel dietary xanthone, suppresses TPA-mediated MMP-2 and MMP-9 expressions through the erk signaling pathway in mcf-7 human breast adenocarcinoma cells. J Food Sci. 2010;75(1):H13–H23. doi:10.1111/j.1750-3841.2009.01407.x
48. Shen K, Xie J, Wang H, et al. Cambogin induces caspase-independent apoptosis through the ROS/JNK pathway and epigenetic regulation in breast cancer cells. Mol Cancer Ther. 2015;14(7):1738–1749. doi:10.1158/1535-7163.MCT-14-1048
49. Zhou J, Luo YH, Wang JR, Lu Bin B, Wang KM, Tian Y. Gambogenic acid induction of apoptosis in a breast cancer cell line. Asian Pac J Cancer Prev. 2013;14(12):7601–7605. doi:10.7314/APJCP.2013.14.12.7601
50. Chen J, Gu HY, Lu N, et al. Microtubule depolymerization and phosphorylation of c-Jun N-terminal kinase-1 and p38 were involved in gambogic acid induced cell cycle arrest and apoptosis in human breast carcinoma MCF-7 cells. Life Sci. 2008;83(3–4):103–109. doi:10.1016/j.lfs.2008.05.003
51. Wang K, Tang Y, Sun M, et al. The mechanism of neogambogic acid-induced apoptosis in human MCF-7 cells. Acta Biochim Biophys Sin (Shanghai). 2011;43(9):698–702. doi:10.1093/abbs/gmr063
52. Li C, Qi Q, Lu N, et al. Gambogic acid promotes apoptosis and resistance to metastatic potential in MDA-MB-231 human breast carcinoma cells. Biochem Cell Biol. 2012;90(6):718–730. doi:10.1139/o2012-030
53. Qi Q, Gu H, Yang Y, et al. Involvement of matrix metalloproteinase 2 and 9 in gambogic acid induced suppression of MDA-MB-435 human breast carcinoma cell lung metastasis. J Mol Med. 2008;86(12):1367–1377. doi:10.1007/s00109-008-0398-z
54. Qi Q, Lu N, Wang XT, et al. Anti-invasive effect of gambogic acid in MDA-MB-231 human breast carcinoma cells. Biochem Cell Biol. 2008;86(5):386–395. doi:10.1139/O08-104
55. Ahmad A, Sarkar SH, Bitar B, et al. Garcinol regulates EMT and Wnt signaling pathways in vitro and in vivo, leading to anticancer activity against breast cancer cells. Mol Cancer Ther. 2012;11(10):2193–2201. doi:10.1158/1535-7163.MCT-12-0232-T
56. Tu SH, Chiou YS, Kalyanam N, Ho CT, Chen LC, Pan MH. Garcinol sensitizes breast cancer cells to Taxol through the suppression of caspase-3/iPLA2 and NF-κB/Twist1 signaling pathways in a mouse 4T1 breast tumor model. Food Funct. 2017;8(3):1067–1079. doi:10.1039/c6fo01588c
57. Chen C-S, Lee C-H, Hsieh C, et al. Nicotine-induced human breast cancer cell proliferation attenuated by garcinol through down-regulation of the nicotinic receptor and cyclin D3 proteins. Breast Cancer Res Treat. 2011;125(1):73–87. doi:10.1007/s10549-010-0821-3
58. Ahmad A, Sarkar SH, Aboukameel A, et al. Anticancer action of garcinol in vitro and in vivo is in part mediated through inhibition of STAT-3 signaling. Carcinogenesis. 2012;33(12):2450–2456. doi:10.1093/carcin/bgs290
59. Ahmad A, Wang Z, Ali R, et al. Apoptosis-inducing effect of garcinol is mediated by NF-κB signaling in breast cancer cells. J Cell Biochem. 2010;109(6):1134–1141. doi:10.1002/jcb.22492
60. Ye X, Yuan L, Zhang L, Zhao J, Zhang CM, Deng HY. Garcinol, an acetyltransferase inhibitor, suppresses proliferation of breast cancer cell line MCF-7 promoted by 17β-estradiol. Asian Pac J Cancer Prev. 2014;15(12):5001–5007. doi:10.7314/APJCP.2014.15.12.5001
61. Subarnas A, Diantini A, Abdulah R, et al. Apoptosis-mediated antiproliferative activity of friedolanostane triterpenoid isolated from the leaves of Garcinia celebica against MCF-7 human breast cancer cell lines. Biomed Rep. 2016;4(1):79–82. doi:10.3892/br.2015.532
62. Colamba Pathiranage V, Lowe JN, Rajagopalan U, et al. Hexane extract of garcinia quaesita fruits induces apoptosis in breast cancer stem cells isolated from triple negative breast cancer cell line MDA-MB-231. Nutr Cancer. 2021;73(5):845–855. doi:10.1080/01635581.2020.1773511
63. Zhang J, Zheng Z, Wu M, et al. The natural compound neobractatin inhibits tumor metastasis by upregulating the RNA-binding-protein MBNL2. Cell Death Dis. 2019;10(8). doi:10.1038/s41419-019-1789-5
64. Lamartine-Hanemann S, Ferreira-Silva GÁ, Horvath R, et al. A tetraprenylated benzophenone 7-epiclusianone induces cell cycle arrest at G1/S transition by modulating critical regulators of cell cycle in breast cancer cell lines. Toxicol Vitro. 2020;68:104927. doi:10.1016/j.tiv.2020.104927
65. Xu J, Jin S, Gan F, et al. Polycyclic polyprenylated acylphloroglucinols from: garcinia xanthochymus fruits exhibit antitumor effects through inhibition of the STAT3 signaling pathway. Food Funct. 2020;11(12):10568–10579. doi:10.1039/d0fo02535f
66. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
67. National Center for Biotechnology Information. PubChem substance record for SID 426321898, SID 426321898, Source: Google Patents. Available from: https://pubchem.ncbi.nlm.nih.gov/substance/426321898.
68. National Center for Biotechnology Information. PubChem compound summary for CID 9852185, Gambogic acid. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/9852185.
69. National Center for Biotechnology Information. PubChem substance record for SID 444134253, garcinol, Source: A2B Chem. Available from: https://pubchem.ncbi.nlm.nih.gov/substance/444134253.
70. National Center for Biotechnology Information. PubChem substance record for SID 382158510, 219649-95-3, Source: bioCrick. Available from: https://pubchem.ncbi.nlm.nih.gov/substance/382158510.
71. National Center for Biotechnology Information. PubChem compound summary for CID 8058, Hexane. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Hexane.
72. National Center for Biotechnology Information. PubChem compound summary for CID 101508194, Neobractatin. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Neobractatin.
73. National Center for Biotechnology Information. PubChem compound summary for CID 5471610, 7-Epiclusianone. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/7-Epiclusianone.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


