Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 15
Allopurinol-Induced Stevens–Johnson Syndrome (SJS)
Received 14 August 2023
Accepted for publication 19 September 2023
Published 2 October 2023 Volume 2023:15 Pages 99—105
DOI https://doi.org/10.2147/CPAA.S427714
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Takla R Anis,1 John Meher2
1Pharmacy Department, Henry Mayo Newhall Hospital, Valencia, CA, USA; 2Emergency Department, Henry Mayo Newhall Hospital, Valencia, CA, USA
Correspondence: Takla R Anis, Email [email protected]
Abstract: Allopurinol is a commonly used medication that lowers uric acid production which is essential for gout treatment and prevention. Although many patients tolerate allopurinol therapy without severe complications; Stevens–Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) are life-threatening delayed hypersensitivity reactions that have been reported especially among Asian and African American patients. We describe a case of allopurinol-induced SJS in a 95-year-old Asian female. The patient started allopurinol 13 days prior to presenting to the emergency room (ER). On day 10 of therapy, the patient developed a diffuse erythematous desquamating rash which prompted her to visit the ER after 3 days from the rash onset. This case report describes a rare fatal hypersensitivity reaction that requires rapid identification and treatment in a multi-disciplinary setting.
Keywords: Stevens–Johnson syndrome, allopurinol, genetic testing, hypersensitivity
Introduction
Allopurinol is a common pharmacological agent indicated for treating and preventing gout, which happens due to accumulation of high uric acid levels that crystallize in the joints. Allopurinol primarily works by decreasing the production of uric acid through inhibiting xanthine oxidase, which is the enzyme that converts hypoxanthine to uric acid.1
As gout cases increase, allopurinol is becoming more commonly prescribed.2 This can lead to increased incidences of rare cutaneous adverse reactions like SJS or TEN. Although, the exact mechanism remains unknown, allopurinol-induced cutaneous adverse reactions tend to be caused by a delayed type IV hypersensitivity reaction in a T-cell-mediated fashion.3,4
Allopurinol-induced SJS is more common among patients of Asian descent due to the presence of HLA-B*58:01 allele.5 In their case-control association study, which was performed among multiethnic Malaysian patients, Dyoi et al found that 93.8% of the patients who experienced severe cutaneous adverse reactions tested positive for HLA-B*58:01.6 The purpose of this report is to highlight a rare adverse reaction that could become more prominent with the increased use of allopurinol, which warrants increased awareness by healthcare providers.
Case Description
A 95-year-old female presented to the ER with a desquamating rash with oral, optic, and genital involvement concerning for SJS, Figures 1 and 2. The patient’s past medical history was significant for chronic kidney disease (CKD) stage IV, diastolic congestive heart failure, gout, hyperlipidemia, and hypertension. Upon reviewing the patient’s home medication list by the ER clinical pharmacist and the ER physician, oral allopurinol 100 mg daily was identified as the newest drug which was started 10 days prior to developing this rash. While performing a medication history review, the patient reported having a similar reaction 5 years ago secondary to allopurinol which was discontinued and planned to be stopped indefinitely. Upon admission to the hospital, the patient was started on high-dose steroids with intravenous (IV) methylprednisolone for 3 days, followed by a prolonged steroid taper. Additionally, the patient had ocular complications and decreased vision secondary to sloughed mucosa on the lashes and around the eyelids, Figure 3. Ophthalmology consultation was obtained, and the patient was started on erythromycin 0.5% ointment, ciprofloxacin 0.3% eye drops, prednisolone acetate 1% eye drops, and cyclosporine 0.05% eye drops. On day 3, the patient’s rash started improving and on day 4, the patient was able to open her eyes. During her hospital stay, the patient contracted superimposed cellulitis with Methicillin-resistant staphylococcus aureus bacteremia (MRSA) and was started on IV antibiotics. After 1 month of hospital stay, the patient’s skin lesions were completely resolved. During her hospitalization, our patient also developed acute kidney injury (AKI) requiring hemodialysis.
 |
Figure 1 Detached skin around the neck and chest area. |
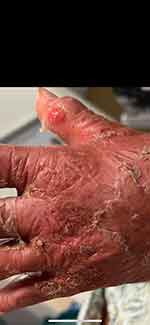 |
Figure 2 Detached skin around the hands area. |
 |
Figure 3 Sloughed mucosa on the lashes and around the eyelids with drainage. |
Discussion
Drug-induced cutaneous reactions like SJS/TEN tend to appear as delayed hypersensitivity reactions. In their review, Su and Chung describe that this happens when the culprit drug acts as an antigen that activates a cascade of immune reactions. As a result, the cytotoxic T lymphocytes and natural killer cells get activated and mount an immune response, which targets the keratinocytes leading to their death.7
We describe a case of a 95-year-old Asian female who developed a delayed hypersensitivity reaction to allopurinol after 10 days from initiating therapy. This is similar to the case presented by Wang et al as their patient developed cutaneous symptoms after 10 days from starting allopurinol.8
While evaluating the cause for this adverse reaction, allopurinol was identified as the culprit drug and a Naranjo score of 8 was calculated as shown in Table 1, which indicates a probable causality.9 Although this diagnosis was mainly a clinical diagnosis; there are diagnostic tools for confirming delayed drug-induced hypersensitivity reactions like the lymphocyte transformation test (LTT). This test detects the memory T-cell response as it quantifies the T-cell proliferation count when the patient’s peripheral mononuclear cells (PMBC) are exposed to the offending agent in vitro.10 This type of testing is not commonly performed in many institutions including our hospital.
 |
Table 1 Naranjo Adverse Drug Reaction Probability Scale for Allopurinol-Induced SJS |
Our patient had several factors that led to poor prognosis like advanced age, detached body surface area >10%, and serum BUN 58 mg/dL. Furthermore, our patient’s Severity-of-Illness Score for Toxic Epidermal Necrolysis (SCORTEN) calculated score was 3, which predicted a 35.3% mortality risk.11
In their study, Minh Duc Do et al identified 7 risk factors to developing cutaneous reactions secondary to allopurinol. Those factors included: female sex, age above 65 years, previous known history of reactions caused by allopurinol, and eGFR <60 mL/min/1.73 m2.12 Our patient had similar risk factors as she was a 95-year-old female with a previous known hypersensitivity reaction to allopurinol and an eGFR of 15 mL/min/1.73 m2.
A recommendation was made by the Burn Center to start our patient on high-dose glucocorticosteroids, starting with IV methylprednisolone 500 mg for 3 days. On day 3 the patient’s rash started improving which indicates potential benefit to using steroids when treating this reaction. In their meta-analysis, Zimmermann et al reported that glucocorticosteroids could possibly show greatest efficacy when administered early or as pulse therapy.13 Future studies on larger patient populations are still needed to determine the ideal treatment for allopurinol-induced SJS and TEN.
Bacteremia has been commonly identified among patients with cutaneous reactions like SJS and TEN. During her hospital stay our patient contracted MRSA bacteremia, for which she was treated with IV vancomycin. Previous studies identified Staphylococcus aureus, Enterococcus faecalis, and Pseudomonas aeruginosa as the most common organisms found in blood cultures among SJS and TEN patients.14 This could guide healthcare providers in selecting appropriate empiric antibiotics.
On her 7th day of hospitalization, our patient developed AKI requiring hemodialysis. Although, our patient had risk factors for developing AKI like receiving IV vancomycin; there has been an association between drug-induced SJS/TEN and developing acute renal failure. In their retrospective study, Hung et al reported that patients with SJS/TEN had a higher incidence of acute renal failure. Additionally, among the group of patients who developed acute renal failure, there was a higher incidence of CKD, cardiac disease, and sepsis.15
Our patient was started on allopurinol 100 mg daily at a Skilled Nursing Facility prior to developing this reaction, which is higher than the recommended dose for her renal function. The American College of Rheumatology recommends starting low-dose allopurinol in patients with CKD and titrating slowly to avoid the risk of developing allopurinol hypersensitivity syndrome.16
Finally, despite the known association between HLA-B*58:01 allele and developing SJS/TEN in Asian patient populations, genetic testing was not performed in our patient prior to starting her on allopurinol. Genetic testing for the HLA-B*58:01 allele is recommended in African American patients and patients of Southeast Asian descent before initiating allopurinol.16 Unfortunately, genetic testing was not performed in our patient while being hospitalized as she was eventually transitioned to comfort care with hospice.
Conclusion
Allopurinol-induced SJS is a fatal hypersensitivity reaction that needs to be identified and treated promptly. Performing a detailed medication history review is warranted to prevent this reaction and to be able to recognize it when it occurs. Many pharmacological agents carry the risk of causing severe hypersensitivity reactions. Therefore, pharmacists and other clinicians play a vital role in practicing pharmacovigilance to identify these reactions. Future large multi-center studies are needed to validate the risk factors and the ideal treatment for drug-induced SJS/TEN.
Ethical Approval
Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent
Written informed consent was obtained from the patient’s next of kin for publication of the case details.
Acknowledgments
We would like to acknowledge the Pharmacy Department and the Emergency Department at Henry Mayo Newhall Hospital for their support in completing this report.
Funding
This article received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stamp LK, Day RO, Yun J. Allopurinol hypersensitivity: investigating the cause and minimizing the risk. Nat Rev Rheumatol. 2016. 12(4):235–242. Erratum in: Nat Rev Rheumatol. 2016 Apr;12(4):i. PMID: 26416594. doi:10.1038/nrrheum.2015.132
2. Russell MD, Yates M, Bechman K, et al. Rising incidence of acute hospital admissions due to gout. J Rheumatol. 2020;47(4):619–623. PMID: 31523046. doi:10.3899/jrheum.190257
3. Bellón T. Mechanisms of severe cutaneous adverse reactions: recent advances. Drug Saf. 2019;42(8):973–992. PMID: 31020549. doi:10.1007/s40264-019-00825-2
4. Yun J, Mattsson J, Schnyder K, et al. Allopurinol hypersensitivity is primarily mediated by dose-dependent oxypurinol-specific T cell response. Clin Exper Allergy. 2013;43(43):1246–1255. doi:10.1111/cea.12184
5. Ferdiana A, Fachiroh J, Oktarina DAM, et al. Allopurinol-induced Stevens-Johnson Syndrome in Javanese Men With Positive HLA-B*58:01. Front Genet. 2022;13:839154. PMID: 35769987; PMCID: PMC9234807. doi:10.3389/fgene.2022.839154
6. Low DE, Nurul-Aain AF, Tan WC, et al. HLA-B*58: 01 association in allopurinol-induced severe cutaneous adverse reactions: the implication of ethnicity and clinical phenotypes in multiethnic Malaysia. Pharmacogenet Genomics. 2020;30(7):153–160. PMID: 32433341. doi:10.1097/FPC.0000000000000408
7. Su SC, Chung WH. Cytotoxic proteins and therapeutic targets in severe cutaneous adverse reactions. Toxins. 2014;6(1):194–210. doi:10.3390/toxins6010194
8. Wang F, Ma Z, Wu X, Liu L. Allopurinol-induced toxic epidermal necrolysis featuring almost 60% skin detachment. Medicine. 2019;98(25):e16078. doi:10.1097/MD.0000000000016078
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi:10.1038/clpt.1981.154
10. Sachs B, Fatangare A, Sickmann A, Glässner A. Lymphocyte transformation test: history and current approaches. J Immunol Methods. 2021;493:113036. doi:10.1016/j.jim.2021.113036
11. Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115(2):149–153. doi:10.1046/j.1523
12. Do MD, Mai TP, Do AD, et al. Risk factors for cutaneous reactions to allopurinol in Kinh Vietnamese: results from a case-control study. Arthritis Res Ther. 2020;22(182). doi:10.1186/s13075-020-02273-1
13. Zimmermann S, Sekula P, Venhoff M, et al. Systemic immunomodulating therapies for Stevens-Johnson Syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2017;153(6):514–522. PMID: 28329382; PMCID: PMC5817620. doi:10.1001/jamadermatol.2016.5668
14. Guerrero-Putz MD, Gomez-Flores M, Ocampo-Candiani J, Alba-Rojas E. Bacteremia in Stevens Johnson Syndrome and toxic epidermal necrolysis: main pathogens and risk factors: a mini review. Austin J Dermatolog. 2021;8(2):1099.
15. Hung CC, Liu WC, Kuo MC, Lee CH, Hwang SJ, Chen HC. Acute renal failure and its risk factors in Stevens-Johnson Syndrome and toxic epidermal necrolysis. Am J Nephrol. 2009;29(6):633–638. doi:10.1159/000195632
16. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res. 2020;72(6):744–760. PubMed 32391934. doi:10.1002/acr.24180
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
