Back to Journals » Nursing: Research and Reviews » Volume 14
An Applicable Framework for Understanding Successful Aging of People Living with HIV and Comorbid Chronic Obstructive Pulmonary Disease
Authors Byun JY , Chapman Lambert C, Fazeli PL, Goodin BR , Iyer AS , Kempf MC, Wise JM, Lee Y, Batey DS, Vance DE
Received 29 July 2023
Accepted for publication 20 December 2023
Published 3 January 2024 Volume 2024:14 Pages 1—14
DOI https://doi.org/10.2147/NRR.S432977
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Jun Y Byun,1 Crystal Chapman Lambert,1 Pariya L Fazeli,1 Burel R Goodin,2 Anand S Iyer,1,3 Mirjam-Colette Kempf,1 Jenni M Wise,1 Yookyong Lee,4 David Scott Batey,5 David E Vance1
1School of Nursing, University of Alabama at Birmingham, Birmingham, AL, USA; 2School of Medicine, Washington University in St. Louis, St. Louis, MO, USA; 3Division of Pulmonary, Allergy, and Critical Care Medicine, University of Alabama at Birmingham, Birmingham, AL, USA; 4Department of Social Work, College of Arts and Sciences, University of Alabama at Birmingham, Birmingham, AL, USA; 5School of Social Work, Tulane University, New Orleans, LA, USA
Correspondence: Jun Y Byun, School of Nursing, University of Alabama at Birmingham, 1701 University Boulevard, Birmingham, AL, 35294-4410, USA, Tel +1 205 427 5878, Email [email protected]
Abstract: Chronic obstructive pulmonary disease (COPD) is a fatal chronic disease experienced by 10% of people living with HIV (PLWH). As the population of older PLWH continues to increase, the prevalence of COPD is anticipated to grow as well. Despite this concerning trend, there is a lack of theoretical models to guide clinical practice and research on aging with HIV and COPD. To address this gap, this article describes the Baltes and Baltes’ SOC (Selective Optimization with Compensation) Model as a guiding framework for understanding successful aging of PLWH with COPD. The article highlights eight areas of aging (eg, length of life, biological health, mental health, cognitive efficiency, social competence, productivity, personal control, and life satisfaction) with HIV and with COPD and the potential synergetic effects of having two critical chronic diseases. Drawing from a synthesis of literature, we adapted the SOC model to include dedicated areas specifically tailored for HIV, COPD, and the intersectional areas impacted by both conditions. Furthermore, we integrated bidirectional relationships within the eight areas of aging into the model, aiming to enhance the practical application of the modified model. Based on the identified gaps in the literature, implications for clinical practice, research, and future theoretical developments are provided.
Keywords: COPD, HIV, cognitive efficiency, length of life, successful aging
Introduction
As life expectancy of people living with HIV (PLWH) increases, many of them are facing lung function decline because of systemic and pulmonary inflammation, persistence of HIV in the lung, smoking, oxidative stress, lung microbiome, and lung metabolome.1,2 Among respiratory diseases in PLWH, chronic obstructive pulmonary disease (COPD) is the most common non-communicable disease with a prevalence of 10–23%, which is higher than that of the general population (7–10%).2,3 As one of the third leading causes of death worldwide, COPD is an irreversible chronic respiratory disease characterized by dyspnea, cough, and sputum and more likely to occur for individuals over 40 years of age due to prolonged exposure to risk factors (eg, exposure to particles such as smoking, workplace dust, and fumes) and respiratory diseases (eg, asthma, chronic bronchitis).4 For PLWH, onset of COPD is earlier than the general population, indicating that COPD will be a part of their aging experience even earlier than the general population.5 In addition to early onset, COPD in PLWH showed more frequent exacerbation,6,7 which is significantly related to poor prognosis and high mortality rate in COPD.4
Studies regarding COPD in HIV are prevalent in pathology and epidemiology. A high smoking rate in PLWH contributes to a high prevalence of COPD in HIV.1 Even after adjusting for smoking, HIV infection is significantly related to the development of COPD, as it causes chronic systematic inflammation, chronic innate immune activation, and abnormalities in immune function.1 Aligned with concerns around the high prevalence of COPD, integrating COPD screening into HIV care is suggested.8 Although many studies have discovered the causes of COPD in HIV and ways to diagnose COPD early for a better prognosis, there is a paucity of studies regarding what it is like to age with HIV and COPD. Drummond et al9 studied the quality of life (QoL) of individuals with and at risk for HIV, which showed worse QoL for individuals with COPD than those without COPD. Akgun et al10 discovered physical limitation is worse in PLWH with COPD compared to people living with either HIV or COPD. Considering the number of PLWH over 50 years of age is expected to increase to 73% in 2030,11 which may increase the number of older PLWH with COPD, studies for a comprehensive understanding of aging with HIV and COPD are significantly needed.
Aging is a complex concept that requires the consideration of diverse areas. Research into aging began with seeking to understand what it means to age well, in other words, how we can define “successful aging”.12 According to the model of successful aging developed by Rowe and Kahn13 to promote positive aging against disease, disability, and chronological age, successful aging consists of a low probability of disease and disability, a high physical and cognitive function level, and active engagement in life. In this model, individuals are either on usual or successful status,13 rather than on continuous process. Critiques of the model of successful aging point out that having a disease or disability is not always considered a failure of aging for older adults, indicating aging experience needs to be considered individually and continuously within their own context.12 Building on this, in considering the vast models found in the literature, we chose Baltes and Baltes Selective Optimization with Compensation (SOC) Model as a framework because it is very dynamic (ie, considers several types of adaptation), widely accepted, parsimonious, and could be applied to different areas of aging (eg, cognitive efficiency, biological health, etc.).
The purpose of this article was to identify a conceptual framework for understanding the aging of PLWH with COPD, explore what is known about aging in PLWH and in COPD, and extrapolate that to those aging with both diseases. Baltes’ and Baltes’ SOC Model guided this examination,14 providing a framework to evaluate what we do and do not know about aging in this population.
Baltes and Baltes’ SOC Model
Baltes and Baltes developed a psychological model called the SOC Model as a conceptualization of successful aging.14 In the SOC Model, aging is an ongoing process with three main constructs: 1) selection of individualized goal, 2) optimization of mean, and 3) compensation for loss due to aging. This approach allows individuals to improve their aging experience according to their own context, aging with HIV and COPD.
In this article, aging of PLWH with COPD was discussed in eight different areas suggested by Baltes and Baltes: (a) length of life, (b) biological health, (c) mental health, (d) cognitive efficiency, (e) social competence, (f) productivity, (g) personal control, and (h) life satisfaction.14 Using the SOC Model, these eight areas compensate for each other forming an individual’s aging-experience. If someone retains ability or function in each of these conceptual areas and is able to adapt to losses, then ideally, one is considered to be aging successfully; however, if someone is deficient in an area that cannot be compensated from other areas, then successful aging overall may be diminished, as individual areas can have downstream effects.
To reiterate, we chose this SOC Model as a guide for several reasons. First, it is parsimonious with distinct concepts and is a well-renowned theory in gerontology research validated in HIV by Vance et al.15 Second, the eight areas interact synergistically to affect successful aging. This structure allows different areas to have an adaptive and compensatory effect. For example, if one’s biological health is compromised due to having poor vision and heart disease, but one experiences a great deal of social competence, productivity, personal control, and life satisfaction, then that might be enough to compensate for such poor biological health and still rate oneself as aging successfully. The SOC model and eight areas can serve as the basis for further research and intervention development.
Methods
In this article, the SOC Model served as a guiding framework for the literature search and analysis. The selection of keywords within areas of aging was informed by the work of Vance et al;15,16 life expectancy, comorbidities, depressive symptoms, cognitive impairment, stigma, employment, self-management, and QoL. Vance et al15,16 adapted the SOC Model for older PLWH identifying topics that warrant further exploration and intervention. To address the limited literature available on aging with HIV and comorbid COPD, a synthesis of literature was conducted for HIV and COPD, respectively; Medical Subject Heading (MeSH) terms for HIV and COPD were also combined to locate the few studies on HIV with COPD. Moreover, we conducted case studies17 and analyzed HIV/COPD clinic data (690 PLWH, including 102 PLWH with COPD)18 that have further informed our discussion of this model. Integrating these findings, extrapolation and potential research questions were suggested. Applications of the SOC Model were illustrated in hypothetical case examples, demonstrating how the eight areas can compensate for others and describing potential interventions.
Eight Areas of Aging
The conceptual framework for understanding aging of PLWH with COPD comprises eight areas: (a) length of life, (b) biological health, (c) mental health, (d) cognitive efficiency, (e) social competence, (f) productivity, (g) personal control, and (h) life satisfaction (see Figure 1).
Length of Life
Length of life refers to the duration of living that an individual expects or experiences. In a meta-analysis of eight cohort studies estimating the life expectancy of PLWH treated with combination antiretroviral therapy (cART),19 the life expectancy in high-income countries ranged from 63 to 67 years depending on their age of starting cART. Yet, it is difficult to generalize the results because it included PLWH with different cART initiation times or treatment effects. In a collaborative analysis of 18 European and North American HIV cohorts,20 researchers found that PLWH diagnosed with HIV at 20 years of age who started cART and maintained a CD4 count above 300 cells/μL a year later had a life expectancy of 78 years, which is similar to the life expectancy of the total US population (78 years).21 Yet, in a sample of 17,995 PLWH, for well-treated PLWH, Helleberg et al22 found that smoking reduced more life years than HIV, indicating that smoking cessation is critical to longevity among PLWH.
For people with COPD, COPD severity and smoking status significantly impact the length of life. In a sample of 532 people with COPD, Chen et al23 found that people with severe COPD lost 9 years of life compared to 6 years in those with moderate COPD. In a sample of 204 people with COPD, Bai et al24 found that the mortality rate was significantly higher in smokers (65%) compared to non-smokers (43%) over five years.
Even though the gap in life expectancy between PLWH and the general population is gradually narrowing, it might change when PLWH develop COPD. COPD and related characteristics (eg, poor pulmonary function, abnormalities in computed tomography) are independent predictors of all-cause and lung disease-related mortality for PLWH.25,26 This aligns with the fact that smoking significantly impacts life expectancy in both diseases.22,24 It is evident that we need to find ways to reduce the cumulative effect of cigarette smoking on the length of life in older PLWH with COPD.
Biological Health
Although advances in cART have significantly reduced the percentage of deaths of PLWH from AIDS-associated diseases, the incidence of chronic conditions, such as COPD, cardiovascular disease, cancer, chronic kidney disease, and diabetes, has increased as the life expectancy of PLWH has increased.27 A likely reason for these chronic conditions is the low-grade inflammation caused by HIV infection that slowly damages tissues and DNA.28 Maciel et al29 conducted a cross-sectional study of 208 PLWH 50 years of age or older who were matched with 208 uninfected older adults. These researchers found that PLWH experienced a higher rate of comorbidity than uninfected persons. In addition, the duration of HIV infection and treatment significantly influenced multimorbidity. In a study investigating PLWH over 75 years of age, 82% of them had at least one comorbidity, and 58% had two or more comorbidities.30
COPD is a disease caused by irreversible airway changes due to unique organ structures or chronic inflammatory responses and has the characteristics of geriatric syndrome.4 When assessing the severity of COPD, the level of airflow limitation is measured using spirometry (forced expiratory volume graded 1–4).4 The severity of symptoms plus history of acute exacerbations are then used to grade burden using GOLD groups A (less symptomatic/few exacerbations) through D (very symptomatic/frequent exacerbations).4 People with COPD continue to experience chronic respiratory distress in their daily lives, and when symptoms worsen, oxygen saturation decreases, and carbon dioxide accumulates, leading to an exacerbation that directly threatens their lives.4 The high frequency of acute exacerbations significantly affects mortality in people with COPD. People with COPD have comorbidities such as cardiovascular disease, lung cancer, diabetes, and osteoarthritis.4
Lung abnormalities observed in PLWH include emphysema, bronchiectasis, diffusion impairment, and air obstruction, and these are directly or indirectly related to the development of COPD in PLWH.2 HIV infection exerts a significant effect on the frequency of acute exacerbations of COPD.6 Also, since both HIV and COPD diagnoses are irreversible and have a lasting effect on the individual’s biological health, the degree of physical limitation and frailty are exacerbated when older adults experience HIV and COPD together.2,10 Akgun et al10 compared physical limitation and frailty levels between 3538 PLWH and 3606 individuals without HIV, and worse physical limitation and frailty were shown in those with both HIV and COPD. COPD strongly impacted physical limitation and frailty more than HIV, indicating focusing on treating COPD symptoms can effectively improve the physical functions of PLWH. As such, having HIV and COPD have a more negative impact on biological health than having either, and other components of biological health need to be explored to fully understand this population.
Mental Health
Mental health refers to psychological, emotional, and social well-being,31 and it can affect all aspects of one’s life. A common mental health issue observed in both HIV and COPD is depression. In a cross-sectional comparative study of 122 PLWH and 94 adults without HIV, Rooney et al32 examined the relationship among depressive symptoms, QoL, and positive psychological factors such as successful aging, grit, and resilience. These researchers observed that 58% of PLWH experienced elevated depressive symptoms, which was significantly higher than adults without HIV (33%). Specifically, PLWH with elevated depressive symptoms experienced lower levels of QoL, successful aging, grit, and resilience, compared to PLWH with lower depressive symptoms. Similarly, Olson et al33 identified that more severe depressive symptoms are related to worse QoL among 296 PLWH who were 50 years of age or older. Interestingly, QoL was not related to indicators of HIV status (ie, diagnosed duration, viral suppression). These studies demonstrated that the mental health of PLWH exerts a significant impact on their life satisfaction and successful aging, and that poor mental health may be a function of other processes aside from HIV severity (eg, stigma).
A mental health challenge for people with COPD is depression. In a multicenter prospective cross-sectional study of 80 individuals with COPD (age 66 ± 8.3), Jang et al34 found that depressive symptoms were significantly associated with worse QoL along with dyspnea, a high number of exacerbations (eg, emergency department visits, hospitalizations), and poor exercise capacity. Kayhan et al35 investigated the prevalence of major depression in people with long-term oxygen therapy-dependent COPD and the effect that depression had on them regarding adherence to treatment. Sixty-three percent of the participants had major depression, and 90.6% of participants, who were noncompliant with treatment, had major depression. These results indicate that depression in people with COPD can significantly affect adherence to treatment.
Both COPD and HIV correlate to depressive symptoms or depression at a high rate, negatively affecting factors beneficial to people with chronic disease, such as QoL, treatment compliance, resilience, grit, and successful aging. Thus, we hypothesize that one’s mental health would worsen when diagnosed with both HIV and COPD.
Cognitive Efficiency
Cognitive efficiency refers to an individual’s ability to learn, problem solve, or achieve goals using mental resources.36 Older PLWH experience cognitive decline due to neuronal injury from aging, neuroinflammation, polypharmacy, substance abuse, comorbidities, or current antiretroviral toxicities.37 Sheppard et al38 observed that both accentuated (more severe) and accelerated (at an earlier onset) neurocognitive aging may occur in PLWH. Accentuated aging was observed in PLWH 50–65 years of age who performed worse cognitively compared to adults without HIV of the same age. Accelerated aging was observed in PLWH 50–65 years who performed at the same level compared to adults without HIV 65 and older. Reflecting these points, in the Multicenter AIDS Cohort Study that followed men who had sex with men with (n=2278) and without HIV (n=2808), Goodkin et al39 found that age and HIV severity were factors significantly affecting cognition in all cognition domains (ie, information processing speed, executive function, episodic memory, working memory, and motor function).
People with COPD also may experience cognitive impairment due to hypoxemia, hypercapnia, and systematic inflammation.40 COPD impairs gas exchange in the lungs, leading to hypoxemia and hypercapnia.4,40,41 Hypoxemia disrupts brain metabolism, which can lead to neuronal injury.40,41 Lung impairments in COPD can negatively affect various domains of cognition, such as memory and learning, attention, psychomotor speed, visuospatial and constructional abilities, executive skills, and language skills.41 In a cross-sectional study of 90 people with and 90 people without COPD, Cleutjens et al42 found that 56.7% of those with COPD experienced general cognitive impairment, compared to 13.3% of those without COPD. Specifically, people with COPD showed more impairments in cognitive flexibility (43.3% vs 12.2%), psychomotor speed (17.8% vs 3.3%), and planning (17.8% vs 1.1%) compared to those without COPD.
PLWH and people with COPD experience cognitive impairment at a high rate due to aging and the disease itself, with some overlap in process (eg, inflammation). Therefore, we infer with a high probability that the onset of cognitive impairment may be earlier and/or more severe in people who experience both diseases. Hence, it is essential to screen cognitive functions for PLWH with COPD and provide appropriate interventions to them so that cognitive functions can be maintained or improved.
Social Competence
Social competence refers to an individual’s ability in building interpersonal relationships.43 Stigma or discrimination is a factor that hinders an individual’s social competence; this threatens successful aging of PLWH. Older PLWH may experience HIV-related stigma as well as stigma related to being older, LGBTQ+, and history of substance use.44 In a study of 914 older PLWH, Brennan et al45 found 31% felt isolated; furthermore, greater isolation was related to increased stigma, depression, and loneliness and decreased well-being. Such stigma can impact other areas of successful aging. For example, in a sample of 512 older PLWH, Lam et al46 found those with higher HIV-related stigma experience less cognitive efficiency and poorer mental health.
Similarly, people with COPD experience stigma due to excessive symptoms such as coughing and expelling sputum as well as visible treatment equipment such as carrying oxygen tanks. In this clinical population, stigma is often related to their history of smoking, which is the main cause of the disease. In a qualitative study of 12 older adults with late-stage COPD,47 participants expressed that their healthcare provider or family members blamed them for having the disease because of their smoking history, and they described their self-image as “the body as a mirror of shame”. These participants with COPD reported feeling as if they were being stared at when coughing and shortness of breath were severe. In fact, some people are reluctant to use inhalers or oxygen therapy tools in public places, either because of their own embarrassment or the presence of their family members. Such COPD-related stigma lowers individuals’ self-care behavior (eg, avoiding using the inhaler in public) and their use of medical resources (eg, avoiding doctor’s judgement), in turn lowering their QoL.48
Although the stigmas experienced by people with HIV or COPD are derived from different etiologies, both stigmas negatively affect physical and mental health. This prompts the question, “Do PLWH with COPD experience more elevated stigma compared to those with HIV or COPD alone?” We hypothesize that the combined stigma may create a more intense stigma that can compromise social competence, productivity, personal control, and life satisfaction. Such stigma may be exacerbated further by stigmatizing factors (ie, older age, low socioeconomic status, LGBTQ+) which can intensify stigma, depression, and loneliness. Clearly, studies on disease-related intersectional stigma in people experiencing both HIV and COPD are needed.
Productivity
Productivity refers to one’s participation in social and meaningful activities (eg, employment, community membership) that benefits self and others.49 For our purposes, employment is a major focus of productivity as it exerts unique benefits for successful aging. Vance et al50 examined the personal benefits that employment provides for PLWH: 1) increasing social competence, 2) increasing cognitive efficiency, 3) preventing depression, 4) establishing routines that can prevent lethargy, 5) engaging with people and society, and 6) providing meaning to one’s life. Despite these advantages, in a conceptual framework on employment among women living with HIV, Wise et al51 found that reduced social power and culturally entrenched discrimination and stigma hinder adequate social support for them to find employment, leading to 60% of unemployment. Likewise, other studies show that only around 30% of PLWH are employed full or part-time.50 This means that the majority of PLWH do not have equal access to benefits of employment, impacting their ability to age successfully over time.
For older adults with COPD, the change in productivity is significant. In a sample of 56,052 adults with and without COPD, Fitzsimmons et al52 found that only 39.4% of people with COPD were employed, while 72.1% of those without COPD were employed. COPD often results in breathlessness, coughing, and sputum, requiring rest or inhaler use after physical exertion,4 thereby diminishing job performance in physically demanding occupations. In a multicenter study of over 2100 people with COPD, higher COPD Assessment Test scores, a standard measurement of symptomatic burden in COPD, were significantly related to lower work productivity.53 In a sample of 314 people with COPD, Solem et al54 found that people with COPD experiencing a severe exacerbation had more work-related activity impairment than those with a moderate exacerbation (72.0% ± 22.4%, 51.3% ± 28.1%, respectively).
Based on the literature, the following question is posited – Is productivity compromised more for PLWH with COPD? As mentioned in the biological health section, PLWH with COPD frequently experience acute exacerbations of COPD, possibly reducing the quantity and quality of their productivity. Additional factors such as depression, anxiety, and stigma can also reduce or impede the amount of work that can be achieved. However, older PLWH with COPD can compensate for reduced productivity in areas other than employment. The compensation might be achieved in activities such as attending church or providing childcare with lower physical demands.
Personal Control
Personal control refers to the ability to promote positive outcomes or prevent negative effects on safety, care, and well-being for one’s self.55 For those living with chronic diseases such as HIV and COPD, self-management in daily life, in addition to intervention and treatment by healthcare providers, has a great influence on their health outcomes. In an observational cross-sectional study of 467 PLWH, Fuster-RuizdeApodaca et al56 found that a higher level of self-management was related to a higher level of QoL. In an interventional study of 359 HIV-positive men who have sex with men (186 in an intervention group and 173 in a control group), Zhang et al57 applied a self-management program consisting of informative sessions (eg, symptom management, HIV-related emotion management, use of medications, communication skills) and individualized action plans with feedback. For the intervention group, physical function, cognitive function, vitality, and QoL significantly improved compared to the control group; likewise, the level of anxiety was significantly reduced.
Various interventional studies to promote self-management in people with COPD demonstrated a similar outcome as PLWH. In a review article of 12 randomized controlled trials related to nurse-driven self-management programs for people with COPD, Helvaci and Gok Metin58 found that physical health outcomes (eg, symptoms, 6-minute walking distance test) and psychosocial health outcomes (eg, QoL, anxiety, depression) showed greater improvement in the intervention group. In addition, in an interventional study of 154 people with COPD, Wang et al40 found that improvement in self-management led to less hospital readmissions and emergency department visits as well as higher exercise capacity and QoL.
Based on the literature, we hypothesize that it is important for PLWH with COPD to engage in self-care strategies to avoid the negative synergistic effects of having HIV and COPD. Boucher et al59 discussed that applying the self-management program to PLWH will play out the following roles: 1) effective management of comorbid diseases, 2) improving the support from peers or society, and 3) reducing perceived stigma. Despite these advantages, studies and interventions related to self-management that reflect the characteristics of HIV with COPD are lacking. Therefore, it is crucial to develop tailored interventions that consider the unique aspects of both HIV and COPD to enhance personal control among older PLWH with COPD.
Life Satisfaction
Life satisfaction refers to how an individual feels or thinks about his or her life.60 This concept is important enough to be a target indicator for treatment or intervention for PLWH or people with COPD who keep living with a disease that has no cure. In previous studies, life satisfaction has been measured with QoL as the main indicator, and it includes both the physical and mental aspects. In a sample of 1422 women including older (≥50 years) and younger (<50 years) women living with HIV, Kteily-Hawa et al61 found that older women with HIV had a lower level of physical health-related QoL, which was associated with resilience and mental health. In a cross-sectional study of 331 PLWH, 119 people with type 1 diabetes mellitus, 2114 people with type 2 diabetes mellitus, and 250 people with rheumatoid arthritis, Engelhard et al62 found that PLWH experienced significantly lower mental health-related QoL than people with other chronic conditions. Severe comorbidity, a history of AIDS, and a longer duration of cART were associated with lower level of physical health-related QoL of PLWH.62
Similarly, for people with COPD, age and disease have a significant effect on QoL. In a prospective observational longitudinal study of 543 people with COPD, Esteban et al63 found that age was significantly related to QoL along with inhaler use, smoking, lung function, physical activity, and body mass index. In a sample of 1012 participants, including 328 controls and 684 people with respiratory diseases (eg, COPD, current asthma, past asthma, chronic bronchitis, allergic rhinitis, and non-allergic rhinitis), Cappa et al64 found that people with COPD showed the poorest QoL among those with respiratory diseases. Also, people with COPD experienced a lower level of QoL than the control group.64
Both HIV and COPD are associated with a poorer QoL compared to other similar chronic diseases. Furthermore, age was a significant factor affecting QoL. In a previous study, Drummond et al9 found that individuals with or at risk for HIV experienced a lower level of QoL for those with COPD compared to those without COPD. Since the treatment of HIV and COPD has improved significantly over the past ten years and life expectancy has risen, we suggest that a deeper understanding of the current status on the QoL of PLWH with COPD is needed.
Hypothetical Case Example
To illustrate the applicability of the Baltes and Baltes’ SOC Model for understanding aging with HIV and COPD, hypothetical case examples are provided.
Michael, 66, Male, African American, Gay (Positive Example)
Michael is a 66-year-old African American man and gay. At 35 years old, he was diagnosed with HIV; fortunately, he immediately began cART, successfully suppressing his viral load. He worked as a bank teller in an urban area until he retired at age 60. Afterwards, he traveled extensively with his long-term partner John and was actively engaged in their church. He had diabetes and hypertension for 20 years, but having high health literacy, he exercised regularly and adhered to treatment guidelines from his health provider, which enabled him to manage both conditions well. The only detrimental behavior to his health was daily smoking.
For Michael at 63, COPD “ambushed” him as he said. Michael, who thought that the breathing difficulties he felt while walking resulted from natural aging, he did not see a respiratory physician until a few months after he could not “catch his breath” going up the stairs. Along with the COPD diagnosis, his physician prescribed him an inhaler and warned smoking could be the biggest cause of his condition. Frustrated, he realized he had to live with breathing difficulty for the rest of his life. He felt ashamed that his smoking likely caused his COPD and tried to hide this new diagnosis from family and friends; he only informed his trusted partner of the COPD diagnosis and explained the nature of the disease (eg, inhaler use, long-term prognosis). One year later, Michael stopped smoking with John’s support. Two years after his COPD diagnosis, he still struggled with confusion and despair. However, with John’s support, Michael slowly let go of his grief by reaching deeper into his faith, leaning on John, and disclosing to his family and friends about COPD. Several medications, inhalers, and shortness of breath are always with him, but he has grown into a stronger man who appreciates life.
Ginger, 55, Female, White (Negative Example)
Ginger, a 55-year-old single White woman, lives alone in a rural area. When she was 38, she was diagnosed with HIV and started cART immediately. Sadly, her viral load has been sporadically suppressed over the years due to poor medication adherence. Her lack of medication adherence may be attributed to the lack of knowledge and stigma of HIV diagnosis, to which she has self-medicated with excessive alcohol and smoking. After she turned 50, she was diagnosed with depression and liver fibrosis. Despite these diagnoses, she was apathetic in changing her behaviors and received only intermittent care and no follow-up for her conditions.
Working on a farm, she collapsed from sudden shortness of breath about a year ago and was taken to the emergency room. Diagnosed with COPD, her overall physical condition was poor, making farm work impossible. After losing her job, she became financially burdened and could not afford car insurance, which prompted her to go to the clinic even less often. She felt guilty and ashamed because of what her doctors told her about the consequences of her smoking and drinking. She stopped using the inhaler in public places to hide her COPD diagnosis. Ginger—who lost her job after being diagnosed with COPD, goes to the clinic less frequently, avoids meeting people, and still cannot stop drinking and smoking—is approaching a health crisis for HIV, COPD, depression, and liver cirrhosis while also managing multiple intersectional stigmas related to HIV, COPD, liver disease, and poverty.
Tiara, 55, Female, African American (Intervention Example)
Tiara is a 60-year-old African American woman diagnosed with HIV at 33 and COPD at 50. She moved to a new city and met a nurse practitioner, Jasmine, at an HIV clinic. During her first visit, using the SOC Model, Jasmine assessed Tiara’s biological health, mental health, cognitive efficiency, social competence, productivity, personal control, and life satisfaction. Tiara experienced severe shortness of breath, depressive symptoms, elevated stigma, poor self-care behavior (eg, spotty medication adherence), and reduced QoL.
Jasmine intervened in specific areas of aging that can also impact other areas; based on the SOC Model’s concept of compensating, intervening in one area could impact another area needed for successful aging. She focused mainly on intervening in shortness of breath, stigma, and self-care behavior, expecting that addressing these areas could positively affect depressive symptoms and QoL. First, Jasmine provided education sessions on how HIV and COPD can affect eight areas of aging in the SOC Model, along with self-care strategies. Second, Jasmine referred Tiara to a pulmonologist and social worker for appropriate treatment and needed support. Third, Jasmine connected her to a peer support group, a regular gathering of PLWH with respiratory diseases. On the next clinic visit, Tiara showed significant improvements. With educational sessions, Tiara learned the importance of self-care behavior and medication adherence. The pulmonologist and social worker helped her to find the best ways to manage her symptoms with inhalers and oxygen therapy, along with financial support. Moreover, joining the peer support group allowed her to share concerns and gain needed insights from peers, reducing stigma. Improvements in shortness of breath, self-care behavior, and stigma led to improvement in depressive symptoms and QoL.
Discussion
Utilizing the SOC model and integrating the eight areas proposed by Baltes and Baltes,14 we conducted a comprehensive synthesis of literature on aging with HIV and COPD. In the literature, a correlation among eight distinct areas emerged, suggesting that interventions in one domain could positively impact others, ultimately benefiting the aging experience of PLWH with COPD. Moreover, the literature highlighted distinct causes of diminished health outcomes from HIV and COPD, respectively, underscoring the necessity for a tailored approach when caring for older individuals with both conditions. Although the original SOC model offered a foundational understanding of goal selection, mean optimization, and loss compensation in the context of aging, it did not specifically include areas of aging in the model. To address this gap, Vance et al15,16 adapted the SOC model for older PLWH, incorporating the eight areas of aging and introducing practical variables for examination. However, this model faced challenges in illustrating the combined influence of both HIV and COPD.
In response, we refined the SOC model to address these challenges. Our modified model depicts bidirectional relationships among the eight areas of aging and incorporates dedicated sections for HIV, COPD, and overlapping areas influenced by both conditions (see Figure 2). This modification ensures a more comprehensive representation, making it practical and straightforward to target specific areas of aging when providing care. Our modifications and the synthesis of the literature guided us in formulating suggestions for future practices, research, and theoretical development, with the aim of addressing gaps in the literature on aging with HIV and COPD.
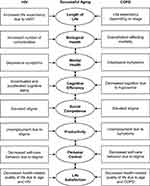 |
Figure 2 Modified Selective Optimization with Compensation Model in the Context of Aging with HIV and COPD. Abbreviations: COPD, chronic obstructive pulmonary disease; cART, combination antiretroviral therapy. Notes: This figure elucidates a modified Selective Optimization with Compensation (SOC) model, illustrating the respective impact of eight areas of aging in HIV and COPD on an individual’s successful aging. It depicts bi-directional relationships among the different areas, providing a foundation for individuals to select goals from these areas, optimize the means to achieve those goals, and compensate for losses in areas affected by aging with HIV and COPD. Data from Baltes and Baltes.14 |
Nursing Implications for Practice
Older PLWH with COPD require a holistic approach related to their clinical care, and nurses are uniquely positioned to monitor their aging experience. The nurse’s role in healthcare may volley from advocate to interventionist. As advocates for older PLWH with COPD, nurses must support the patient-provider relationship to assure optimal prognosis of HIV and COPD. This support is important for patients who may feel shame or stigma about their COPD symptoms, as well as for providers who may become frustrated with patients’ resistance to behavior change. Further, nurses can provide information to PLWH regarding potential lung function decline in aging with HIV for those who may need COPD treatment but are unaware of their lung status.
In the role of interventionists, nurses are directly involved in routine assessment of the aging with HIV and COPD. Using the SOC Model as a guide, nurses can work with older PLWH with COPD to evaluate each area of aging and intervene appropriately as needed. Assessments can be done during medical appointments or check-in calls. For example, Rubtsova et al65 used a single-item 10-point scale to assess self-rated successful aging with HIV (1=least successful to 10=most successful). This measure was sensitive to predicting protective attributes (eg, resilience, personal mastery, optimism, spirituality) and psychological distress (eg, anxiety, depression, loneliness, stigma); in other words, simple assessments can reveal larger insights about patients’ well-being across multiple areas of aging. Notably, standardized instruments are easily added to patient-reported outcomes (PROs) batteries and can be self-administered via electronic devices or adapted to nurse-patient semi-structured interviews.
Nurses may also facilitate evidence-based or evidence-informed strategies to improve aging with HIV and COPD. Once a patient’s assessment of aging is established, the nurse can determine which of the eight areas need intervention and which can be called upon to support the others. For example, if one is experiencing poor biological health (ie, poor breathing), one can quit smoking by rallying personal control (ie, resilience) and social competence (ie, gathering support from family and friends) to change this area of aging for the better. Building and maintaining resilience is a strategy that can exert a significant impact on mental health, cognitive efficiency, social competence, productivity, and life satisfaction simultaneously.66
Implications for Research
Nurse researchers are well-positioned to research the aging of PLWH with COPD through multidimensional approaches, exploring the dynamic in areas of aging. Nurse researchers can advance the fields of HIV and COPD through their participation in the following specific actions. First, more information about PLWH with COPD in areas of aging is needed, and varied research methods can be utilized to gather this information. For example, qualitative research allows PLWH with COPD to share their lived experiences; large-scale, quantitative research provides inferential statistical analyses to answer questions on this population; and longitudinal studies are encouraged to track changes in PLWH with COPD as they age through various stages of life. Second, nurse researchers, using a theoretical perspective such as the risk and resilience perspective, can help examine what protective factors are associated with aging among PLWH with COPD. Moreover, risk factors that hinder this population from aging successfully should be explored further to inform appropriate interventions.
Third, there are areas that require further attention from nurse researchers on HIV and COPD. One of the neglected areas that can have a unique influence across multiple areas of aging is spirituality, including religion (ie, biopsychosocial spirituality). Including spirituality in treatment plans can strengthen a holistic approach to care for older PLWH with COPD. Although spirituality affects cognitive focus, stress, resilience, biological health, and length of life in serious illnesses,67 its impact on the health of PLWH with COPD is equivocal. More research is needed to better understand how spirituality integrates with various areas of aging. Another area that will help shed light on the aging of older PLWH with COPD is intersectional stigmas associated with age, race, ethnicity, gender identity, sexual orientation, substance use, socioeconomic status, and comorbidities. Studying the intersectionality of stigmas from the different characteristics of older PLWH with COPD will be the basis for comprehensive understanding and developing interventions that may support their successful aging.
Implications for Future Theoretical Development
Efforts are needed to enhance future theoretical development. First, various conceptual frameworks can be explored to find which approach(es) can help better understand older PLWH with COPD. By examining and merging different frameworks (eg, risk and resilience frameworks), researchers can identify the most suitable theoretical perspectives that provide valuable insights into this population’s unique challenges and needs. Second, while conducting research and practice utilizing the SOC Model, areas or relationships outside the conceptual framework may arise, leading to the necessity of creating a more dynamic and interactive framework. In this case, theory development may be needed through qualitative study (eg, grounded theory), which includes nuanced experiences and perspectives of older PLWH with COPD. Third, collaborations among interdisciplinary researchers (eg, social worker, psychologist, nurse, physician) are critical in advancing the knowledge on the aging of PLWH with COPD. Fourth, while the SOC Model may serve as a foundation for understanding the aging of PLWH with COPD, researchers can refine and apply this model to understand aging of individuals with different comorbidities. When practicing the model in research and theoretical development for vulnerable populations—older PLWH with COPD—it is imperative for nurse researchers to prioritize ethical considerations. Obtaining informed consent from participants is essential to ensure their autonomy and rights are respected throughout the process.
Conclusion
In this article, the aging of PLWH with COPD was explored through eight areas based on the Baltes and Baltes’ SOC Model with implications for practice and research. In each of the eight areas, the unique characteristics or common characteristics of individuals with each of the two diseases were described; length of life (eg, life expectancy), biological health (eg, comorbidities), mental health (eg, depressive symptoms), cognitive efficiency (eg, cognitive impairment), social competence (eg, stigma), productivity (eg, low employment), personal control (eg, self-care behavior), and life satisfaction (eg, QoL). Nurses and researchers can be better informed about COPD as a serious HIV comorbidity and ready to advocate and develop essential strategies to achieve optimal health outcomes among older PLWH. Therefore, the need remains for continuous research and interest in how aging is perceived by individuals and what factors can positively change it.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This article was supported by: an NIH/NINR R21-award (1R21NR016632-01; ClinicalTrials.gov [NCT03122288]; PI: Vance) titled “Individualized-Targeted Cognitive Training in Older Adults with HAND“; an NIH/NIMH R01-award (1R01MH106366-01A1; ClinicalTrials.gov [NCT02758093]; PI: Vance) titled “An RCT of Speed of Processing Training in Middle-aged and Older Adults with HIV“; an NIH/NIA R00-award (AG048762; PI: Fazeli) titled “A Novel Neurorehabilitation Approach for Cognitive Aging with HIV”; and an NIA/NIH K76 (AG064327; PI: Iyer) titled “Aging Well with COPD through Geriatrics-Palliative Care”.
Disclosure
Dr Anand Iyer reports grants from NIH, during the conduct of the study; grants from NIH, personal fees from AstraZeneca, outside the submitted work. Dr David Batey reports grants from the National Institute on Allergy and Infectious Diseases, grants from the National Institute on Mental Health, grants from the National Institute on Nursing Research, grants from the National Institute on Alcohol Abuse and Alcoholism, independent contractor for Birmingham AIDS Outreach, independent contractor for Association of Nurses in AIDS Care, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Cribbs SK, Crothers K, Morris A. Pathogenesis of HIV-related lung disease: immunity, infection, and inflammation. Physiol Rev. 2020;100(2):603–632. doi:10.1152/physrev.00039.2018
2. Byanova K, Kunisaki KM, Vasquez J, Huang L. Chronic obstructive pulmonary disease in HIV. Expert Rev Respir Med. 2021;15(1):71–87. doi:10.1080/17476348.2021.1848556
3. Bigna JJ, Kenne AM, Asangbeh SL, Sibetcheu AT. Prevalence of chronic obstructive pulmonary disease in the global population with HIV: a systematic review and meta-analysis. Lancet Glob Health. 2018;6(2):e193–e202. doi:10.1016/s2214-109x(17)30451-5
4. Global Initiative for Chronic Obstructive Pulmonary Disease. Global strategy for prevention, diagnosis and management of COPD: 2023 report; 2023. Available from: https://goldcopd.org/2023-gold-report-2/.
5. Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130(5):1326–1333. doi:10.1378/chest.130.5.1326
6. Lambert AA, Kirk GD, Astemborski J, Mehta SH, Wise RA, Drummond MB. HIV infection is associated with increased risk for acute exacerbation of COPD. J Acquir Immune Defic Syndr. 2015;69(1):68–74. doi:10.1097/qai.0000000000000552
7. Depp TB, McGinnis KA, Kraemer K, et al. Risk factors associated with acute exacerbation of chronic obstructive pulmonary disease in HIV-infected and uninfected patients. AIDS. 2016;30(3):455–463. doi:10.1097/qad.0000000000000940
8. Shirley DK, Kaner RJ, Glesby MJ. Screening for chronic obstructive pulmonary disease (COPD) in an urban HIV clinic: a pilot study. AIDS Patient Care STDS. 2015;29(5):232–239. doi:10.1089/apc.2014.0265
9. Drummond MB, Kirk GD, McCormack MC, et al. HIV and COPD: impact of risk behaviors and diseases on quality of life. Qual Life Res. 2010;19(9):1295–1302. doi:10.1007/s11136-010-9701-x
10. Akgün KM, Tate JP, Oursler KK, et al. Association of chronic obstructive pulmonary disease with frailty measurements in HIV-infected and uninfected Veterans. AIDS. 2016;30(14):2185–2193. doi:10.1097/qad.0000000000001162
11. Smit M, Brinkman K, Geerlings S, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15(7):810–818. doi:10.1016/s1473-3099(15)00056-0
12. Martin P, Kelly N, Kahana B, et al. Defining successful aging: a tangible or elusive concept? Gerontologist. 2015;55(1):14–25. doi:10.1093/geront/gnu044
13. Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37(4):433–440. doi:10.1093/geront/37.4.433
14. Baltes PB, Baltes MM. Psychological perspectives on successful aging: the model of selective optimization with compensation. In: Successful Aging: Perspectives from the Behavioral Sciences. Cambridge University Press; 1990:1–34.
15. Vance DE, Blake BJ, Brennan-Ing M, DeMarco RF, Fazeli PL, Relf MV. Revisiting successful aging with HIV through a revised biopsychosocial model: an update of the literature. J Assoc Nurses AIDS Care. 2019;30(1):5–14. doi:10.1097/jnc.0000000000000029
16. Vance DE, McGuinness T, Musgrove K, Orel NA, Fazeli PL. Successful aging and the epidemiology of HIV. Clin Interv Aging. 2011;6:181–192. doi:10.2147/cia.S14726
17. Byun JY, Chapman Lambert C, Fazeli PL, Iyer AS, Batey DS, Vance DE. An exploration of intersectional stigma of older people living with HIV and COPD in Alabama: a qualitative study of three cases.
18. Byun JY. Aging with HIV and Comorbid Chronic Obstructive Pulmonary Disease. University of Alabama at Birmingham; 2023.
19. Teeraananchai S, Kerr SJ, Amin J, Ruxrungtham K, Law MG. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med. 2017;18(4):256–266. doi:10.1111/hiv.12421
20. Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4(8):e349–e356. doi:10.1016/s2352-3018(17)30066-8
21. Arias E, Tejada-Vera B, Ahmad F. Provisional life expectancy estimates for January through June, 2020; 2021. Available from: https://www.cdc.gov/nchs/data/vsrr/vsrr015-508.pdf.
22. Helleberg M, May MT, Ingle SM, et al. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS. 2015;29(2):221–229. doi:10.1097/qad.0000000000000540
23. Chen CZ, Shih CY, Hsiue TR, et al. Life expectancy (LE) and loss-of-LE for patients with chronic obstructive pulmonary disease. Respir Med. 2020;172:106132. doi:10.1016/j.rmed.2020.106132
24. Bai JW, Chen XX, Liu S, Yu L, Xu JF. Smoking cessation affects the natural history of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:3323–3328. doi:10.2147/copd.S150243
25. Gingo MR, Nouraie M, Kessinger CJ, et al. Decreased lung function and all-cause mortality in HIV-infected individuals. Ann Am Thorac Soc. 2018;15(2):192–199. doi:10.1513/AnnalsATS.201606-492OC
26. Triplette M, Justice A, Attia EF, et al. Markers of chronic obstructive pulmonary disease are associated with mortality in people living with HIV. AIDS. 2018;32(4):487–493. doi:10.1097/qad.0000000000001701
27. Webel AR, Schexnayder J, Cioe PA, Zuñiga JA. A review of chronic comorbidities in adults living with HIV: state of the science. J Assoc Nurses AIDS Care. 2021;32(3):322–346. doi:10.1097/jnc.0000000000000240
28. Bloch M, John M, Smith D, Rasmussen TA, Wright E. Managing HIV-associated inflammation and ageing in the era of modern ART. HIV Med. 2020;21(Suppl 3):2–16. doi:10.1111/hiv.12952
29. Maciel RA, Klück HM, Durand M, Sprinz E. Comorbidity is more common and occurs earlier in persons living with HIV than in HIV-uninfected matched controls, aged 50 years and older: a cross-sectional study. Int J Infect Dis. 2018;70:30–35. doi:10.1016/j.ijid.2018.02.009
30. Bernaud C, Sécher S, Michau C, et al. HIV-infected patients aged above 75 years. Med Mal Infect. 2020;50(1):43–48. doi:10.1016/j.medmal.2019.04.001
31. Centers for Disease Control and Prevention. About mental health. Centers for Disease Control and Prevention; 2023. Available from: https://www.cdc.gov/mentalhealth/learn/index.htm#:~:text=What%20is%20mental%20health%3F,others%2C%20and%20make%20healthy%20choices.
32. Rooney AS, Moore RC, Paolillo EW, et al. Depression and aging with HIV: associations with health-related quality of life and positive psychological factors. J Affect Disord. 2019;251:1–7. doi:10.1016/j.jad.2019.03.025
33. Olson B, Vincent W, Meyer JP, et al. Depressive symptoms, physical symptoms, and health-related quality of life among older adults with HIV. Qual Life Res. 2019;28(12):3313–3322. doi:10.1007/s11136-019-02271-0
34. Jang SM, Kim KU, Na HJ, et al. Depression is a major determinant of both disease-specific and generic health-related quality of life in people with severe COPD. Chron Respir Dis. 2019;16:1479972318775422. doi:10.1177/1479972318775422
35. Kayhan F, Ilik F, Karamanli H, Cemal Pazarli A, Kayhan A. Major depression in long-term oxygen therapy dependent chronic obstructive pulmonary disease. Perspect Psychiatr Care. 2018;54(1):6–10. doi:10.1111/ppc.12169
36. Hoffman B, Schraw G, McCrudden MT. Cognitive efficiency. In: Seel NM, editor. Encyclopedia of the Sciences of Learning. Springer US; 2012:590–593.
37. Winston A, Spudich S. Cognitive disorders in people living with HIV. Lancet HIV. 2020;7(7):e504–e513. doi:10.1016/s2352-3018(20)30107-7
38. Sheppard DP, Iudicello JE, Morgan EE, et al. Accelerated and accentuated neurocognitive aging in HIV infection. J Neurovirol. 2017;23(3):492–500. doi:10.1007/s13365-017-0523-2
39. Goodkin K, Miller EN, Cox C, et al. Effect of ageing on neurocognitive function by stage of HIV infection: evidence from the multicenter AIDS cohort study. Lancet HIV. 2017;4(9):e411–e422. doi:10.1016/s2352-3018(17)30098-x
40. Wang T, Mao L, Wang J, Li P, Liu X, Wu W. Influencing factors and exercise intervention of cognitive impairment in elderly patients with chronic obstructive pulmonary disease. Clin Interv Aging. 2020;15:557–566. doi:10.2147/cia.S245147
41. Andreou G, Vlachos F, Makanikas K. Effects of chronic obstructive pulmonary disease and obstructive sleep apnea on cognitive functions: evidence for a common nature. Sleep Disord. 2014;2014:768210. doi:10.1155/2014/768210
42. Cleutjens FA, Franssen FM, Spruit MA, et al. Domain-specific cognitive impairment in patients with COPD and control subjects. Int J Chron Obstruct Pulmon Dis. 2017;12:1–11. doi:10.2147/copd.S119633
43. American Psychological Association. APA dictionary of psychology. American Psychological Association; 2023. Available from: https://dictionary.apa.org/social-competence.
44. Johnson Shen M, Freeman R, Karpiak S, Brennan-Ing M, Seidel L, Siegler EL. The intersectionality of stigmas among key populations of older adults affected by HIV: a thematic analysis. Clin Gerontol. 2019;42(2):137–149. doi:10.1080/07317115.2018.1456500
45. Brennan-Ing M, Seidel L, Karpiak SE. Social support systems and social network characteristics of older adults with HIV. Interdiscip Top Gerontol Geriatr. 2017;42:159–172. doi:10.1159/000448561
46. Lam A, Mayo NE, Scott S, Brouillette MJ, Fellows LK. HIV-related stigma affects cognition in older men living with HIV. J Acquir Immune Defic Syndr. 2019;80(2):198–204. doi:10.1097/qai.0000000000001898
47. Jerpseth H, Knutsen IR, Jensen KT, Halvorsen K. Mirror of shame: patients experiences of late-stage COPD. A qualitative study. J Clin Nurs. 2021;30(19–20):2854–2862. doi:10.1111/jocn.15792
48. Chin ED, Armstrong D. Anticipated stigma and healthcare utilization in COPD and neurological disorders. Appl Nurs Res. 2019;45:63–68. doi:10.1016/j.apnr.2018.12.002
49. McColl M, Law M, Stewart D. The Theoretical Basis of Occupational Therapy.
50. Vance DE, Cody SL, Yoo-Jeong M, Jones GL, Nicholson WC. The role of employment on neurocognitive reserve in adults with HIV: a review of the literature. J Assoc Nurses AIDS Care. 2015;26(4):316–329. doi:10.1016/j.jana.2015.04.003
51. Wise JM, Vance DE, Heaton K, et al. Employment and occupational productivity among women living with HIV: a conceptual framework. J Assoc Nurses AIDS Care. 2021;32(1):37–46. doi:10.1097/jnc.0000000000000202
52. Fitzsimmons K, Pechter E, Sparer-Fine E. Chronic obstructive pulmonary disease and employment among Massachusetts adults. Prev Chronic Dis. 2020;17:E144. doi:10.5888/pcd17.200116
53. Ding B, Small M, Bergström G, Holmgren U. COPD symptom burden: impact on health care resource utilization, and work and activity impairment. Int J Chron Obstruct Pulmon Dis. 2017;12:677–689. doi:10.2147/copd.S123896
54. Solem CT, Sun SX, Sudharshan L, Macahilig C, Katyal M, Gao X. Exacerbation-related impairment of quality of life and work productivity in severe and very severe chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2013;8:641–652. doi:10.2147/copd.S51245
55. Thompson SC. Perceived control. National Institutes of Health. Available from: https://cancercontrol.cancer.gov/sites/default/files/2020-06/perceived_control.pdf.
56. Fuster-RuizdeApodaca MJ, Sánchez-Vega N, Galindo MJ, et al. The influence of patient experience with healthcare on the health-related quality of life of people living with HIV: an observational cross-sectional survey. Infect Dis Ther. 2019;8(3):369–382. doi:10.1007/s40121-019-0252-3
57. Zhang P, Gao J, Wang Y, Sun Q, Sun X. Effect of chronic disease self-management program on the quality of life of HIV-infected men who have sex with men: an empirical study in Shanghai, China. Int J Health Plann Manage. 2019;34(3):1055–1064. doi:10.1002/hpm.2874
58. Helvaci A, Gok Metin Z. The effects of nurse-driven self-management programs on chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Adv Nurs. 2020;76(11):2849–2871. doi:10.1111/jan.14505
59. Boucher LM, O’Brien KK, Baxter LN, Fitzgerald ML, Liddy CE, Kendall CE. Healthy aging with HIV: the role of self-management support. Patient Educ Couns. 2019;102(8):1565–1569. doi:10.1016/j.pec.2019.02.019
60. Diener E, Lucas RE, Oishi S. Subjective well-being: the science of happiness and life satisfaction. Handbook Posit Psychol. 2002;2:63–73.
61. Kteily-Hawa R, Andany N, Wang Y, et al. Quality of life of older women living with HIV: comparative assessment of physical and mental health-related markers using a large Canadian Sexual and Reproductive Health Cohort Study. HIV Res Clin Pract. 2019;20(2):35–47. doi:10.1080/15284336.2018.1554373
62. Engelhard EAN, Smit C, van Dijk PR, et al. Health-related quality of life of people with HIV: an assessment of patient related factors and comparison with other chronic diseases. AIDS. 2018;32(1):103–112. doi:10.1097/qad.0000000000001672
63. Esteban C, Arostegui I, Aramburu A, et al. Predictive factors over time of health-related quality of life in COPD patients. Respir Res. 2020;21(1):138. doi:10.1186/s12931-020-01395-z
64. Cappa V, Marcon A, Di Gennaro G, et al. Health-related quality of life varies in different respiratory disorders: a multi-case control population based study. BMC Pulm Med. 2019;19(1):32. doi:10.1186/s12890-019-0796-8
65. Rubtsova AA, Wingood G, Ofotokun I, et al. Psychosocial mechanisms of self-rated successful aging with HIV: a structural equation model. AIDS Behav. 2021;25:2875–2885. doi:10.1007/s10461-021-03340-7
66. Cannon DL, Sriram KB, Liew AW, Sun J. Resilience factors important in health-related quality of life of subjects with COPD. Respir Care. 2018;63(10):1281–1292. doi:10.4187/respcare.05935
67. Balboni TA, VanderWeele TJ, Doan-Soares SD, et al. Spirituality in serious illness and health. JAMA. 2022;328(2):184–197. doi:10.1001/jama.2022.11086
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

