Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Analysis of Treatment Efficacy of Intense Pulsed Light (M22) for Meibomian Gland Dysfunction with Demodex Mites
Authors Zhang W, Cao X, Yang L, Duan Y, Zhang W
Received 16 August 2023
Accepted for publication 13 December 2023
Published 27 December 2023 Volume 2023:16 Pages 3743—3751
DOI https://doi.org/10.2147/CCID.S435723
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Weiliang Zhang,1,* Xiaoqin Cao,2,* Lu Yang,3 Yajian Duan,1 Wei Zhang1
1Department of Ophthalmology, Shanxi Bethune Hospital, Taiyuan, People’s Republic of China; 2Department of General Surgery, First Hospital of Shanxi Medical University, Taiyuan, People’s Republic of China; 3Department of Ophthalmology, Changzhi Aier Eye Hospital, Changzhi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lu Yang, Changzhi Aier Eye Hospital, Luyangmen Middle Road, Luzhou District, Changzhi, 046000, People’s Republic of China, Tel +86-3558128120, Email [email protected]
Objective: To investigate the effectiveness of intense pulsed light (M22) in treating patients with meibomian gland dysfunction (MGD) caused by demodex mites.
Methods: A total of 100 patients (100 eyes) diagnosed with demodex mites through microscopic examination at Shanxi Bethune Eye Clinic between June 2021 and May 2023 were selected using convenience sampling. The patients were randomly divided into two groups: an experimental group (n=50) and a control group (n=50). The control group received comprehensive treatment consisting of artificial tears, warm compress, anti-inflammatory eye ointment, hypochlorous acid cleansing, okra cotton pad, and meibomian gland massage. In addition to the comprehensive treatment, the experimental group received intense pulsed light (M22) therapy. After 8 weeks of treatment, the mite clearance rate and cure rate of dry eye were measured for both groups. The recurrence rate of dry eye was also observed 4 weeks after discontinuing M22 treatment.
Results: The experimental group achieved a mite clearance rate of 88.0%, while the control group had a rate of 58.0%, with a statistically significant difference (χ2 = 5.43, P = 0.017). Regarding the cure rate of dry eye, the experimental group showed a rate of 92.0%, while the control group had a rate of 82.0%, with a statistically significant difference (χ2 = 3.61, P = 0.021). In terms of the recurrence rate of dry eye, the experimental group exhibited a rate of 13.04%, while the control group had a rate of 26.83%, with a statistically significant difference (χ2 = 4.18, P = 0.016).
Conclusion: Intense pulsed light (M22) demonstrated superior efficacy in eradicating demodex mites, treating dry eye, and maintaining the treatment’s effectiveness compared to comprehensive treatment with medication in patients suffering from meibomian gland dysfunction with demodex mites.
Keywords: intense pulsed light, meibomian gland dysfunction, dry eye
Introduction
Meibomian gland dysfunction (MGD) is a chronic and diffuse eyelid gland disorder characterized by blockage of the meibomian gland terminal ducts and abnormal quality of meibum secretion. It is recognized as one of the main causes of evaporative dry eye. In addition to dry eye symptoms, MGD can also lead to serious corneal damage, foreign body sensation, burning sensation, pain, blurred vision, photophobia, tearing, and chronic inflammation of the eyelid margins. Prolonged inflammation can further damage the meibomian glands, exacerbating dry eye conditions and creating a vicious cycle.
The etiology and risk factors of MGD include blepharitis, mite infestation, wearing of contact lenses, prolonged uncorrected refractive errors, extensive use of electronic devices, dry environment, air pollution, endocrine abnormalities, rheumatological conditions such as Sjögren’s syndrome, and certain systemic diseases. Among these factors, blepharitis is the most common cause of MGD, followed by mite infestation. However, it is worth noting that mites are detected in as high as 73% of MGD cases associated with blepharitis,1 indicating the significant role of mite infestation in the development of MGD.2
In the treatment of MGD, the effectiveness of single-drug therapy is generally limited. A comprehensive treatment approach, which includes the use of artificial tears, antibiotics, corticosteroids, interferons, local massage, fumigation therapy, nutritional supplementation, and lifestyle or occupational adjustments, has been recognized as the consensus for MGD treatment.3 In recent years, intense pulsed light (IPL) has emerged as an alternative treatment for MGD and has gained increasing attention from clinicians. In our department, the application of IPL (M22) for the treatment of MGD has demonstrated significant benefits in terms of mite elimination, improvement of dry eye symptoms, and long-term treatment outcomes. The research findings are summarized as follows:
Data and Methods
Study Participants
A total of 100 patients (100 eyes) with Demodex confirmed by microscopic examination in the Bethune Ophthalmology Clinic of Shanxi Province between June 2021 and May 2023 were selected by convenience sampling method, 50 patients in the experimental group and 50 patients in the control group. Inclusion criteria: meet the diagnostic criteria for severe MGD-related dry eye as specified in the Chinese Consensus on Dry Eye: Examination and Diagnosis (2020). Exclusion criteria: ① age over 60 years; ② presence of lacrimal gland disorders, active inflammation, eyelid entropion or ectropion, uveitis, and other ocular conditions; ③ history of ocular trauma, chemical burns, ocular surgeries, use of corneal contact lenses, systemic rheumatic or immune diseases, Sjögren’s syndrome, pregnancy, or lactation; ④ history of ocular medication within the week preceding the study. Informed consent was obtained from all participants and their families, and the study was approved by the ethics committee of the hospital.
Research Methods
Severity of Dry Eye
We assessed tear meniscus height, non-invasive tear film break-up time, and meibomian gland examination using an analyzer, and classified meibomian gland function into four levels (Figure 1). The grading criteria were as follows: normal - no missing meibomian glands; mild - less than one-third of meibomian glands missing; moderate - one-third to two-thirds of meibomian glands missing; severe - more than two-thirds of meibomian glands missing. Based on corneal staining with fluorescein sodium (Flu), tear break-up time (BUT), and Schirmer I test (SIT) for basal tear secretion, we divided the severity of dry eye into three levels. Specific SIT measurement method: One end of the standard tear test strip (about 5 mm) was folded into a right angle, one end of the test strip (folded end) was placed in the conjunctival sac in the medial third of the eyelid, and the other end was placed outside the eyelid. At the same time, the patient was told to gently close both eyes, look straight binocularly, and blink; after 5 minutes, the test strip was removed from the lower eyelid, and the wet length of the filter paper was observed and recorded. < 60 years old, normal: infiltration length 10 ~ 25 mm; ≥ 60 years old, normal: wet length < 10 mm, no obvious clinical symptoms. Dry eye < 5 mm. BUT measurement method: BUT measurement method: The patients were measured with fluorescein at a dose and concentration of 1 drop, 20 g/L, and the patients were blinked for 3 times to keep both eyes straight in front of them, and the cornea of the eyes was saccaded with cobalt blue light to observe and record the time from the last blinting to tear film break-up. FL determination method: The lower eyelid conjunctival sac of the patient was touched by fluorescein test strip, and the staining of the patient ‘s cornea under cobalt blue light was also observed, 0 indicating no staining of the corneal epithelium, 1 indicating staining area < 1/3 of the total area, 2 indicating staining area < 1/2 of the total area, and 3 indicating staining area ≥ 1/2 of the total area. The average scores of these three assessments were calculated, and if the score was between 0 and 1 (including 1), it was classified as mild dry eye; between 1 and 2 (excluding 1 but including 2), it was classified as moderate dry eye; between 2 and 3 (excluding 2 but including 3), it was classified as severe dry eye. See Table 1 for details.
 |
Table 1 Score and Grading of Three Tests for Dry Eye |
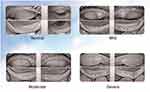 |
Figure 1 Grading of Meibomian Gland Loss. |
Methods of Ocular Mite Examination
Lid margin photography revealed evident blockage of meibomian gland orifices, as shown in Figure 2A, with a significant accumulation of lipid droplets obstructing the orifices of the lower eyelid. The eyelid margin appeared irregular and not smooth. After flushing the conjunctival sac with physiological saline, meibomian gland secretions were collected by massaging the eyelids, and two eyelashes each from the nasal side, central, and temporal side of both upper and lower eyelids, totaling 12 lashes per eye, were plucked and placed on glass slides. After dropwise addition of cedar oil, the samples were examined under a 4x optical microscope for the presence of demodex mites. The mites were further observed under a 10x microscope to confirm their viability (Figure 2B), and it was observed that three live mites were present around the eyelashes, with one of them actively feeding on the hair follicle root tissue. Judge whether there is Demodex infection, if the number of Demodex is ≥ 1, record as infected; if the number of Demodex is < 1, record as no infection.
 |
Figure 2 Ocular Mite Examination Results. Notes: (A) Lid Margin Diffuse Light Photography; (B) Live Demodex Observation under 10x Optical Microscope. |
Treatment Methods
The control group received comprehensive treatment consisting of artificial tears, warm compress, anti-inflammatory eye ointment, hypochlorous acid cleansing, okra cotton pad, and meibomian gland massage. The experimental group received comprehensive treatment along with IPL (M22) therapy, once every two weeks for a total of four sessions. After 8 weeks of treatment, the demodex clearance rate, dry eye cure rate, and dry eye recurrence rate after discontinuation of M22 treatment were measured in both groups.
Cure rate of dry eye:4 cured: eye dryness, burning, foreign body sensation and other discomfort symptoms improved, SIT > 10 mm, FLu = 0; significantly effective: eye discomfort symptoms significantly improved, 10 mm > SIT ≥ 5 mm, FLu = 1; effective: eye discomfort symptoms improved, SIT < 5 mm, FLu = 2; ineffective: a series of eye discomfort symptoms did not improve signs, SIT < 5 mm, FLu = 3. Cure rate% = number cured/total number × 100%.
Demodex clearance rate:5 After 8 weeks, if the number of Demodex mites is < 1, it indicates no infection and Demodex mites are eliminated.
Comprehensive Treatment in the Control Group
Treatment Methods in the Experimental Group
Statistical Analysis
Statistical analysis was conducted using SPSS 26.0 software. Measurement data were expressed as mean ± standard deviation (Means±SD), while count data were expressed as the number of cases and percentages (%). Chi-squared (χ2) test was used for comparison between the two groups, with P<0.05 indicating a statistically significant difference.
Results
Baseline Characteristics of the Study Participants
A total of 100 study participants were included. The experimental group consisted of 29 males and 21 females, aged 18 to 60 years (mean age 45.10±7.42 years). The control group consisted of 30 males and 20 females, aged 17 to 59 years (mean age 43.10±4.42 years). There were 7 and 8 patients with severe dry eye in the control and experimental groups, respectively, 32 and 33 patients with moderate dry eye, and 11 and 9 patients with mild dry eye. There were no statistically significant differences in age, gender, and severity of dry eye between the two groups (P>0.05), as shown in Table 2.
 |
Table 2 Baseline Characteristics of Study Participants |
Comparison of Demodex Clearance Rate Between Two Groups
Comparing the experimental group with the control group, the demodex clearance rate was 88.0% in the experimental group and 58.0% in the control group, with a statistically significant difference (χ2 = 5.43, P = 0.017), as shown in Table 3.
 |
Table 3 Post-Treatment Demodex Examination Results in Experimental and Control Groups (Cases, %) |
Comparison of Dry Eye Treatment Efficacy Between Two Groups
All patients were treated for 8 weeks, and the comprehensive evaluation of tear secretion, tear film break-up time, meibomian gland atrophy, etc. was observed using a dry eye analyzer. The meibomian gland expression ability and meibomian gland lipid quality were also assessed. The experimental group had a higher cure rate for dry eye compared to the control group (92.0% vs 84.0%), with a statistically significant difference (χ2 = 3.61, P = 0.021), as shown in Table 4. Among the patients who were not cured, there were 4 cases in the experimental group, including 1 case of moderate dry eye and 3 cases of mild dry eye, and 8 cases in the control group, including 3 cases of moderate dry eye and 5 cases of mild dry eye.
 |
Table 4 Comparison of Dry Eye Cure Rate Between Two Groups (Cases, %) |
Comparison of Dry Eye Recurrence Rate Between Two Groups
For all cured dry eye patients, the recurrence rate after discontinuation of treatment at 4 weeks was compared. The recurrence rate was 13.04% in the experimental group and was 26.19% in the control group, with a statistically significant difference (χ2 = 4.18, P = 0.016), as shown in Table 5.
 |
Table 5 Comparison of Dry Eye Recurrence Rate Between Two Groups (Cases, %) |
Discussion
This study compared the mite clearance rate and dry eye cure rate in the control group and the experimental group after 8 weeks of treatment, as well as the recurrence rate of dry eye 4 weeks after discontinuation of M22. It was found that the experimental group had higher mite clearance and dry eye cure rates, and a lower recurrence rate compared to the control group.
MGD is the main cause of dry eye, accounting for 53–71% of all dry eye patients, and the incidence rate of MGD increases with age. However, research has shown that7 there are differences in the infection rate of demodex mites among MGD patients of different ages. The proportion of MGD caused by demodex mites is highest in people under 40 years old, while MGD in people over 60 years old is caused by multiple factors. Therefore, the cases in this study were all selected from individuals under 60 years old, and we believe that demodex mite examination is important for middle-aged and young MGD patients. Some studies suggest that males are more susceptible to demodex mite infection, possibly due to male sebum secretion induced by androgens,8,9 while other studies suggest that females have a higher infection rate, possibly due to the effects of cosmetics on demodex mite proliferation.10 However, in this study, we believe that demodex mite infection is related to personal hygiene and local meibomian gland function, without performing statistical analysis of demodex mite infection rates by gender.
In this study, we found that IPL M22 treatment had a good therapeutic effect, which was consistent with the results of other studies.11 The results showed that the clearance rate of Demodex in MDG was very high after IPL M22 intervention, and the possible mechanism was related to the heat produced by IPL M22. Many studies have found12,13 that IPL M22 produces heat much higher than the suitable temperature for mite survival, interferes with the living environment of mites with the mite, coagulates and necrotic proteins inside the mite, and blocks the cascade of inflammation, so as to achieve the purpose of removing mites. After the intervention, the cure rate of dry eye in the experimental group was significantly higher than that in the control group, and the possible mechanisms were the following aspects. M22-optimized IPL treatment showed selective photothermal effects, promoting coagulation and closure of abnormal eyelid capillaries and reducing the release of inflammatory factors, thereby removing a large number of inflammatory sources from the eyelids and meibomian glands.14 In addition, this treatment has thermal radiation effects that soften and increase flow to the meibomian gland,15 resolve meibomian gland obstruction, and reduce microbiological burden, such as Demodex and bacteria. IPL M22 is able to improve the microstructure of the meibomian gland in patients with MGD, and can also change the activity of meibomian acinar cells through light regulation and alleviate the inflammatory response of the gland and surrounding tissues.16–18 This treatment induces an increase in adenosine triphosphate (ATP) production in mitochondria of meibomian gland cells and promotes fibroblast proliferation, thereby improving the microstructure of the meibomian gland.19,20 The M22 optimized IPL therapy improves the meibomian glands and ocular surface microenvironment from multiple aspects, reducing the levels of inflammatory factors in the ocular tissues, thereby improving tear film stability and alleviating dry eye symptoms in patients. Although the symptoms of dry eye were improved after combined therapy with IPL M22, it is unable to cure the condition, and recurrence was observed in both the experimental and control groups. Similar findings were reported in the study conducted by Chen et al.21 Some studies suggest that increasing the number of IPL treatment sessions and extending the comprehensive treatment period to 3–6 months are required for patients with these conditions. However, it is important to note that treatment effectiveness does not accumulate over time. Huang et al22 found that meibomian gland probing (MGP), in combination with IPL therapy, yielded satisfactory results in the treatment of refractory MGD.
Moreover, during the treatment process with IPL M22, close attention needs to be paid to whether patients experience pain or discomfort and skin reddening. If post-treatment symptoms include scab formation, edema, or blisters on the skin, it indicates excessive irradiation with M22 and the treatment parameters should be adjusted accordingly. Additionally, strong light exposure should be avoided after treatment to prevent skin pigmentation. Care should be taken to avoid the eyelashes and eyebrows, and for patients with severe upper eyelid lesions, a protective eye shield should be placed before treatment. Research has found that IPL irradiation can damage hair follicles and cause hair loss. Most importantly, during the treatment, protective measures for the eyes should be taken. During treatment of the lower eyelid, patients should naturally close their eyes or look towards the top of their head, while for treatment of the upper eyelid, patients should keep their eyes closed and gaze towards the tip of their toes. An eye shield should be placed over the cornea to prevent strong IPL light from irradiating the eyeball and causing serious complications such as vitreous opacities, Posner-Schlossman’s syndrome, and uveitis.23
Nonetheless, this study has certain limitations. Firstly, the sample size was small, which may limit the extrapolation of the results. Therefore, in future research, large-sample and multi-center observational cohort studies should be conducted to further validate the findings. Secondly, this study only observed the therapeutic effect of IPL therapy on MGD dry eye. However, there are currently no guidelines or consensus on the interval between each treatment session and the interval between each treatment course. Further research is needed on the treatment period and frequency.
Conclusion
In this study, we found that IPL M22 treatment of patients with meibomian gland dysfunction (MGD) caused by Demodex mites could significantly increase Demodex clearance and dry eye cure rate and reduce dry eye recurrence rate after stopping M22 treatment. Therefore, IPL M22 is safe and effective for Demodex in the treatment of MGD and is recommended to be used in such patients to improve the quality of treatment.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Shanxi Bethune Hospital.
Written informed consent was obtained from all participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
All of the authors had no any personal, financial, commercial, or academic conflicts of interest separately.
References
1. Lu LX, Zang YX, Li SY, et al. Clinical study on the effects of Demodex infection on ocular surface damage after Laisk surgery. Ophthalmol China. 2019;28(6):430–433.
2. Zhang Y, Yi GG, Ke XY, et al. Influence of demodex on ocular surface function in patients with meibomian gland dysfunction. Int Eye Sci. 2019;19(7):1228–1231.
3. Huang LJ, Gao YY, Lai QH, et al. Impact of ocular demodex infection on ocular surface function in patients with meibomian gland dysfunction. Chin Foreign Med Res. 2017;15(27):70–72. doi:10.14033/j.cnki.cfmr.2017.27.038
4. Fu Y, Cen Y, Fu M, et al. Efficacy observation of pranoprofen combined with carboxymethylcellulose sodium eye drops in the treatment of moderate to severe dry eye. Hebei Med J. 2019;25(02):276–280.
5. Jiao H, Wu X, Yang Y, et al. Clinical observation of self-made Yinhua Qingdu Decoction in the treatment of meibomian gland dysfunction associated with Demodex infection. Mod Chin Med Clin. 2018;25(01):
6. Arita R, Morishige N, Shirakawa R, et al. Effects of eyelid warming devices on tear film parameters in normal subjects and patients with meibomian gland dysfunction. Ocul Surf. 2015;13(4):321–330. doi:10.1016/j.jtos.2015.04.005
7. Wang YZ, Huang WZ, Wu M, et al. Relationship between eyelash demodex infection and meibomian gland dysfunction. Guangzhou Med J. 2020;51(1):1–5, 18.
8. Blume-Peytavi U, Kottner J, Sterry W, et al. Age-associated skin conditions and diseases: current perspectives and future options. Gerontologist. 2016;56(Suppl 2):S230–S242. doi:10.1093/geront/gnw003
9. Demirdağ HG, Özcan H, Gürsoy Ş, et al. The effects of sebum configuration on Demodex spp. density. Turk J Med Sci. 2016;46(5):1415–1421. doi:10.3906/sag-1504-77
10. Zeytun E, Karakurt Y. Prevalence and load of demodex folliculorum and Demodex brevis (Acari: demodicidae) in patients with chronic blepharitis in the Province of Erzincan, Turkey. J Med Entomol. 2019;56(1):2–9. doi:10.1093/jme/tjy143
11. Yang SH, Yan ZG, Xu XH, et al. Clinical observation of the optimized pulsed light treatment for meibomian gland dysfunction. Gansu Med J. 2022;41(12):1102–1105.
12. Zhang X, Song N, Gong L. Therapeutic effect of intense pulsed light on ocular demodicosis. Curr Eye Res. 2019;44(3):250–256. doi:10.1080/02713683.2018.1536217
13. Fishman HA, Periman LM, Shah AA. Real-time video microscopy of in vitro demodex death by intense pulsed light. Photobiomodul Photomed Laser Surg. 2020;38(8):472–476. doi:10.1089/photob.2019.4737
14. Tashbayev B, Yazdani M, Arita R, et al. Intense pulsed light treatment in meibomian gland dysfunction: a concise review. Ocul Surf. 2020;18(4):583–594. doi:10.1016/j.jtos.2020.06.002
15. Toyos R, McGill W, Briscoe D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction; a 3-year retrospective study. Photomed Laser Surg. 2015;33(1):41–46. doi:10.1089/pho.2014.3819
16. Yin Y, Liu N, Gong L, et al. Changes in the meibomian gland after exposure to intense pulsed light in Meibomian Gland Dysfunction (MGD) patients. Curr Eye Res. 2018;43(3):308–313. doi:10.1080/02713683.2017.1406525
17. Liu R, Rong B, Tu P, et al. Analysis of cytokine levels in tears and clinical correlations after intense pulsed light treating meibomian gland dysfunction. Am J Ophthalmol. 2017;183:81–90. doi:10.1016/j.ajo.2017.08.021
18. Ruan F, Zang Y, Sella R, et al. Intense pulsed light therapy with optimal pulse technology as an adjunct therapy for moderate to severe blepharitis-associated keratoconjunctivitis. J Ophthalmol. 2019;2019:3143469. doi:10.1155/2019/3143469
19. Mejía LF, Gil JC, Jaramillo M. Intense pulsed light therapy: a promising complementary treatment for dry eye disease. Arch Soc Esp Oftalmol. 2019;94(7):331–336. English, Spanish. doi:10.1016/j.oftal.2019.03.009
20. Ge J, Liu N, Wang X, et al. Evaluation of the efficacy of optimal pulsed technology treatment in patients with cataract and Meibomian gland dysfunction in the perioperative period. BMC Ophthalmol. 2020;20(1):111. doi:10.1186/s12886-020-01357-5
21. Qiao C, Li L, Wang H, et al. Adverse events of intense pulsed light combined with meibomian gland expression versus meibomian gland expression in the treatment of meibomian gland dysfunction. Lasers Surg Med. 2021;53(5):664–670. doi:10.1002/lsm.23339
22. Huang X, Qin Q, Wang L, et al. Clinical results of Intraductal Meibomian gland probing combined with intense pulsed light in treating patients with refractory obstructive Meibomian gland dysfunction: a randomized controlled trial. BMC Ophthalmol. 2019;19(1):211. doi:10.1186/s12886-019-1219-6
23. Vigo L, Taroni L, Bernabei F, et al. Ocular surface workup in patients with meibomian gland dysfunction treated with intense regulated pulsed light. Diagnostics. 2019;9(4):147. doi:10.3390/diagnostics9040147
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
