Back to Journals » Clinical Interventions in Aging » Volume 19
Association Between Intraoperative Noradrenaline Infusion and Outcomes in Older Adult Patients Undergoing Major Non-Cardiac Surgeries: A Retrospective Propensity Score-Matched Cohort Study
Authors Yang YJ, Feng YM, Wang TX, Wang JY, Pang QY , Liu HL
Received 18 September 2023
Accepted for publication 31 January 2024
Published 9 February 2024 Volume 2024:19 Pages 219—227
DOI https://doi.org/10.2147/CIA.S440902
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Ya-Jun Yang,1,* Yu-Mei Feng,1,* Tong-Xuan Wang,1 Jing-Yun Wang,2 Qian-Yun Pang,1 Hong-Liang Liu1
1Department of Anesthesiology, Chongqing University Cancer Hospital, Chongqing, People’s Republic of China; 2School of Medicine, Chongqing University, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hong-Liang Liu; Qian-Yun Pang, Department of Anesthesiology, Chongqing University Cancer Hospital, No. 181, Hanyu Road, Shapingba District, Chongqing, 400030, People’s Republic of China, Tel +86 13883686721, Email [email protected]
Background: Noradrenaline (NA) is commonly used intraoperatively to prevent fluid overload and maintain hemodynamic stability. Clinical studies provided inconsistent results concerning the effect of NA on postoperative outcomes. As aging is accompanied with various diseases and has the high possibility of the risk for postoperative complications, we hypothesized that intraoperative NA infusion in older adult patients undergoing major non-cardiac surgeries might potentially exert adverse outcomes.
Methods: In this retrospective propensity score-matched cohort study, older adult patients undergoing major non-cardiac surgeries were selected, 1837 receiving NA infusion during surgery, and 1072 not receiving NA. The propensity score matching was conducted with a 1:1 ratio and 1072 patients were included in each group. The primary outcomes were postoperative in-hospital mortality and complications.
Results: Intraoperative NA administration reduced postoperative urinary tract infection (OR:0.124, 95% CI:0.016– 0.995), and had no effect on other postoperative complications and mortality, it reduced intraoperative crystalloid infusion (OR:0.999, 95% CI:0.999– 0.999), blood loss (OR: 0.998, 95% CI: 0.998– 0.999), transfusion (OR:0.327, 95% CI: 0.218– 0.490), but increased intraoperative lactate production (OR:1.354, 95% CI:1.051– 1.744), and hospital stay (OR:1.019, 95% CI:1.008– 1.029).
Conclusion: Intraoperative noradrenaline administration reduces postoperative urinary tract infection, and does not increase other postoperative complications and mortality, and can be safely used in older adult patients undergoing major non-cardiac surgeries.
Keywords: noradrenaline, outcome, older adult, non-cardiac surgery
Introduction
Noradrenaline (NA), a strong adrenergic α1 and mild β1 agonist, can counteract the vasodilation induced by general anesthesia and treat intraoperative hypotension,1 and it is commonly used intraoperatively to prevent fluid overload and maintain hemodynamic stability. Clinical studies revealed that NA increased postoperative wound infection and mortality in high-risk surgeries,2–4 but others showed that intraoperative restrictive fluid therapy combined with NA administration decreased postoperative complications and hospital stay in radical cystectomy,5 and NA infusion was not the risk factor for postoperative acute kidney injury.6 Animal studies revealed that NA impaired microcirculation and inhibited tissue oxygen extraction,7,8 These inconsistent results may result from the heterogeneity of NA on microcirculation and organ function,9 but whether NA can be safely used intraoperatively is still an unresolved issue.
Older adult patients are more likely to suffer hypotension during surgery.10 Aging is accompanied with various diseases and has the high possibility of the risk for postoperative complications, and many adverse events are associated with major surgeries, including stroke, myocardial infarction (MI), or even death.11 We hypothesized that intraoperative NA infusion in older adult patients might cause adverse outcomes.
Thus, in this study, we investigated the association between NA and postoperative in-hospital outcomes, tried to figure out the safety of intraoperative NA administration in older adult patients undergoing major non-cardiac surgery.
Materials and Methods
This retrospective cohort study was conducted at Chongqing University Cancer Hospital, and approved by the Ethics Committee of Chongqing University Cancer Hospital (approval number: CZLS2018-024; approval date: 2018-1-30), the informed consent was waived due to the anonymous nature of data.
Participants
The eligible participants included older adult patients who underwent major non-cardiac surgeries. Major non-cardiac surgery referred to the surgical procedure associated with significant fluid shifts that required postoperative hospitalization,12 which included open resection of organs, thoracic surgery, intracranial surgery, spinal surgery, large joint replacement, laparoscopic surgery. The inclusion criteria included: patients age ≥65 years, surgical duration ≥2 hours, and elective surgery under general anesthesia. The exclusion criteria included emergency operation, intraoperative massive bleeding (>2000mL), and patients with preexisting organ failure.
Data Extraction
The data were extracted from the medical database of Chongqing University Cancer Hospital from January 2018 to December 2021, which included sex, age, ASA (American Society of Anesthesiology) physical status classification, surgical types, comorbidities, fluid replacement, transfusion, urinary output, NA dosage, lactate production (the difference of lactate level between the end of surgery and baseline), postoperative in-hospital complications and mortality and hospital stay. In NA group, a bolus of Ringer’s solution was administered at a dose of 4–6 mL/kg during the induction of general anesthesia, and norepinephrine was infused till the end of surgery, the infusion rate was 0.04–0.1μg/kg/min, Ringer’s solution was infused at 5–6mL/kg/h intraoperatively, and the mean artery pressure (MAP) target was set above 60 mmHg, when hypotension (MAP < 60mmHg) occurred at any time during surgery and anesthesia, the primary strategy was to adjust NA infusion rate, a bolus of crystalloid (200–250 mL) was administered if needed, which was decided by the attending anesthesiologist. In non-NA group, a bolus of Ringer’s solution was administered at a dose of 8–10 mL/kg during the induction of general anesthesia, and Ringer’s solution was infused at 8–12mL/kg/h afterwards till the end of surgery, when MAP < 60 mmHg at any time during surgery and anesthesia, a bolus of 200–250 mL of Ringer’s solution was administered and repeatedly when necessary, ephedrine was administered if needed. In all patients, colloid was infused when blood loss exceeding 500mL or managing hypotension if necessary.
A balanced salt crystalloid solution of 1000–1500 mL and 500 mL 5% glucose was routinely infused postoperatively for the surgical patients per day until the normal oral intake recovered.
Outcomes
The primary outcomes were postoperative in-hospital mortality and complications, including stroke, myocardial infarction (MI), arrhythmia, pulmonary edema, pulmonary infection, respiratory failure, acute kidney injury (AKI), urinary tract infection, wound infection, anastomotic leakage, and thrombosis. AKI was defined according to the classification of the Acute Kidney Injury Network (AKIN) based on changes in plasma creatinine levels over 72 postoperative hours.13 The secondary outcomes were intraoperative crystalloid infusion, blood loss, transfusion, lactate production, and hospital stay.
Statistical Analysis
The patients were grouped as receiving NA and not receiving NA, the basic characteristics between groups were balanced using propensity score matching (PSM) with R program language 4.1.2. PSM was performed using a one-to-one nearest neighbor matching algorithm without replacement, the caliper was 0.2. The predictors for PSM were age, sex, BMI, smoking, ASA physical status, comorbidities, and surgical types. Categorized variables were presented as absolute numbers and proportions, and were compared using Fisher exact test. Continuous variables were presented as median (interquartile range), those of normal distribution were compared using t test, and those of non-normal distribution were compared using Mann–Whitney test. Logistic regression was performed for intraoperative fluid replacement, transfusion, blood loss, urinary output, intraoperative lactate increase, postoperative in-hospital complications and mortality. Odds ratio (OR) and 95% confidence interval (CI) were achieved. A P value less than 0.05 was considered statistically significant, and the statistical analysis was conducted using Stata MP 14 (64-bit) software.
Results
Figure 1 showed the procedure of patients selection. A total of 3466 older adult patients undergoing elective major non-cardiac surgery were identified, and 2909 patients were eventually included. Of those, 1837 receiving intraoperative NA infusion, and 1072 not receiving NA infusion. The propensity score matching was conducted with a 1:1 ratio, and 1072 patients were eventually included in either group.
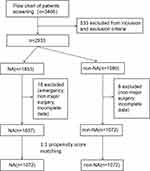 |
Figure 1 Flowchart of patients screening. |
The baseline characteristics before and after propensity score matching were presented in Table 1. The sex composition, age, ASA composition, the proportions of patients with diabetes mellitus (DM), smoking, gynecological surgery, orthopedic surgery, gastrointestinal surgery, thoracic surgery, and neurosurgery were significantly different between groups; after propensity score matching, the patients characteristics were well balanced.
 |
Table 1 The Characteristics in NA and Non-NA Cohort Before and After Matching |
The in-hospital mortality and complications after surgery in the unmatched and matched cohort were presented in Table 2. No difference existed in in-hospital mortality between NA and non-NA group (P=0.506). NA reduced postoperative urinary tract infection (P=0.019), but had no impact on other postoperative in-hospital complications. The OR with 95% CI in NA group with reference to non-NA in the logistic regressions for in-hospital mortality and postoperative complications in the matched cohort were shown in Figure 2 and Supplemental Table 1. Patients receiving NA had a lower likelihood of urinary tract infection (OR: 0.124, 95% CI: 0.016–0.995).
 |
Table 2 The Incidence of Postoperative in-Hospital Complications in Unmatched and Matched Cohorts |
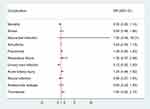 |
Figure 2 The Odds ratios (ORs) in matched cohorts for NA with reference to non-NA in postoperative in-hospital complications. NA reduced the risk of urinary tract infection. |
The ORs in NA with reference to non-NA from logistic regression model for intraoperative infusion, transfusion, bleeding, arterial lactate levels, and hospital stay were presented in Figure 3 and Supplemental Table 2 after propensity score matching. NA infusion reduced intraoperative crystalloid infusion (OR: 0.999,95% CI:0.999–0.999), colloid infusion (OR:1.000,95% CI: 0.999–1.000), blood loss (OR: 0.998, 95% CI: 0.998–0.999), transfusion (OR: 0.327, 95% CI: 0.218–0.490), and urinary output (OR: 0.999, 95% CI: 0.999–0.999), but increased intraoperative lactate production (OR: 1.354, 95% CI: 1.051–1.744) and hospital stay (OR: 1.019, 95% CI: 1.008–1.029).
 |
Figure 3 The Odds ratios (ORs) in matched cohort for NA with reference to non-NA in intra- and post-operative outcomes. Abbreviation: RBC, red blood cell. |
Discussion
In this retrospective propensity score-matched cohort study, we investigated the association between intraoperative NA infusion and postoperative in-hospital outcomes in older adult patients undergoing major non-cardiac surgeries. From the results, intraoperative NA administration could reduce the volume of intraoperative fluid therapy, blood loss and RBC transfusion, and postoperative urinary tract infection, but had no effect on other postoperative complications despite the increased intraoperative lactate production and extended hospital stay.
Clinical studies have confirmed that intraoperative NA administration prevents fluid overload and counteract intraoperative hypotension,1 and promotes platelet aggregation and clot firmness in surgical patients.14 Adrenergic α1 receptor exists on the surface of platelets, and NA can promote platelet aggregation by activating α1 receptor and ADP pathway,13 so as to reduce surgical bleeding. Our previous meta-analysis showed that intraoperative restrictive fluid therapy reduced postoperative infectious complications in abdominal surgery.15 In this study, intraoperative NA administration significantly reduced the volume of fluid therapy, which might contribute to the lower rate of urinary tract infection in NA cohort.
It was reported that intraoperative NA administration at a rate of 0.05 or 0.075 μg/kg/min could maintain blood pressure more stable than 0.025 μg/kg/min after spinal block in cesarean section.16 In this study, the intraoperative NA infusion rate was 0.04–0.1μg/kg/min, which could maintain the stability of blood pressure and reduce fluid replacement, but increased intraoperative lactate production. NA infusion at 0.05 μg/kg/min in cesarean section could slightly increase maternal lactate level after delivery, although there did not exist significance.17 In our study, intraoperative NA infusion at 0.04–0.1μg/kg/min significantly increased lactate production. As the duration of cesarean section was short and NA was infused for a very short period, but the infusion was much longer in our study. NA activates α1 receptor, contracts arterioles, and impairs mesenteric microcirculation,18 in addition, NA inhibited oxygen extraction in tissues in animal studies.8 All these mechanisms might contribute to the increased lactate production during surgery.
One study reported that intraoperative restrictive fluid therapy combined with NA administration decreased postoperative complications and hospital stay in radical cystectomy,5 but our study showed that intraoperative NA infusion extended the hospital stay by 1 day compared with non-NA cohort, and a further study is needed to explore the reason. Another study found that combination of intraoperative restrictive fluid therapy and NA infusion increased the risk of AKI after radical cystectomy, and NA was not a risk factor.6 In our study, intraoperative NA infusion and restrictive fluid therapy did not affect cardiac, pulmonary, and other in-hospital complications. So, NA infusion at a rate of 0.04–0.1μg/kg/min is relatively safe during major non-cardiac surgery in older adult patients despite the increased intraoperative lactate production and hospital stay.
Various definitions of intraoperative hypotension exist in different studies when using the absolute level of MAP, the criteria included MAP <70 mmHg, or <65mmHg, or <60 mmHg, or <55mmHg.19 According to Perioperative Quality Initiative consensus statement, MAP <60–70 mmHg was defined as hypotension, and associated with postoperative adverse outcomes.20 So we used MAP < 60 mmHg as intraoperative hypotension in our study.
The incidence of perioperative stroke is 0.1–1.0% in non-cardiac, nonvascular, nonneurological surgeries.21,22 In our study, the incidence of postoperative in-hospital stroke was 1.6% in NA cohort, and 1.7% in non-NA cohort, which were higher than those in previous studies. Several reasons might be involved in it, the patients undergoing neurosurgery were included in our study, and it is higher in patients undergoing neurosurgery (1.5%),23 the patients underwent major non-cardiac surgeries, and most of them were cancer patients, and the patients were from Southwest of China, who might be at a higher risk of perioperative stroke, but it needs to be testified.
As NA is a strong adrenergic α1 agonist and has a very high property of constricting arteries and veins, the major concern of peripheral administration is extravasation which might cause severe and long-lasting skin damage.24 One recent retrospective cohort study found that peripherally administered NA did not result in more adverse events including skin necrosis.25 In our study, NA was administered mostly via a central venous line, we might be cautious to use peripheral route for NA infusion.
This study has some limitations, the advanced monitoring parameters such as cardiac output and pulse pressure variation are not common in our clinical practice, and the related data cannot be presented; the consistency within each cohort cannot be completely ensured, such as the infusion rate of NA or crystalloid during surgery, the strategy to manage hypotension in each cohort, and this is a single-center retrospective cohort study, the results cannot be extrapolated to other centers; in this study, we only investigated postoperative in-hospital complications, which was a relatively short-term period, and further studies are needed to explore long-term complications associated with intraoperative NA infusion.
Conclusions
Intraoperative noradrenaline administration reduces postoperative urinary tract infection, and does not increase other postoperative complications and mortality, and can be safely used in older adult patients undergoing major non-cardiac surgeries.
Abbreviations
NA, Noradrenaline; MI, myocardial infarction; MAP, mean artery pressure; AKI, acute kidney injury; ASA, American Society of Anesthesiology; DM, diabetes mellitus.
Data Sharing Statement
All the data can be obtained by contacting the corresponding author.
Ethics Approval and Informed Consent
This retrospective cohort study was conducted at Chongqing University Cancer Hospital, and approved by the Ethics Committee of Chongqing University Cancer Hospital (approval number: CZLS2018-024; approval date: 2018-1-30), the informed consent was waived due to the anonymous nature of data. This study complies with the Declaration of Helsinki for medical studies.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Key R & D project of Chongqing Science and Technology Bureau, No: cstc2020jscx-dxwtB0010 and the Key R & D project of the Ministry of Science and Technology of China, No: 2018YFC0116704.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hiltebrand LB, Koepfli E, Kimberger O, Sigurdsson GH, Brandt S. Hypotension during fluid-restricted abdominal surgery: effects of norepinephrine treatment on regional and microcirculatory blood flow in the intestinal tract. Anesthesiology. 2011;114(3):557–564. doi:10.1097/ALN.0b013e31820bfc81
2. Huette P, Moussa MD, Beyls C, et al. Association between acute kidney injury and norepinephrine use following cardiac surgery: a retrospective propensity score-weighted analysis. Ann Intensive Care. 2022;12(1):61. doi:10.1186/s13613-022-01037-1
3. Chen JL, Chen YL, Qi B, et al. Impact of Intraoperative Norepinephrine Support on Living Donor Liver Transplantation Outcomes: a Retrospective Cohort Study of 430 Children. Front Pharmacol. 2020;11:1254. doi:10.3389/fphar.2020.01254
4. Joosten A, Rinehart J, Van der Linden P, et al. Computer-assisted Individualized Hemodynamic Management Reduces Intraoperative Hypotension in Intermediate- and High-risk Surgery: a Randomized Controlled Trial. Anesthesiology. 2021;135(2):258–272. doi:10.1097/ALN.0000000000003807
5. Wuethrich PY, Burkhard FC, Thalmann GN, Stueber F, Studer UE. Restrictive deferred hydration combined with preemptive norepinephrine infusion during radical cystectomy reduces postoperative complications and hospitalization time: a randomized clinical trial. Anesthesiology. 2014;120(2):365–377. doi:10.1097/ALN.0b013e3182a44440
6. Furrer MA, Schneider MP, Löffel LM, Burkhard FC, Wuethrich PY. Impact of intra-operative fluid and noradrenaline administration on early postoperative renal function after cystectomy and urinary diversion: a retrospective observational cohort study. Eur J Anaesthesiol. 2018;35(9):641–649. doi:10.1097/EJA.0000000000000808
7. Hebert MT, Marshall JM. Direct observations of responses of mesenteric microcirculation of the rat to circulating noradrenaline. J Physiol. 1985;368:393–407. doi:10.1113/jphysiol.1985.sp015864
8. Beckh K, Otto R, Ji S, Jungermann K. Control of oxygen uptake, microcirculation and glucose release by circulating noradrenaline in perfused rat liver. Biol Chem Hoppe-Seyler. 1985;366(7):671–678. doi:10.1515/bchm3.1985.366.2.671
9. Schneider AG, Goodwin MD, Schelleman A, Bailey M, Johnson L, Bellomo R. Contrast-enhanced ultrasonography to evaluate changes in renal cortical microcirculation induced by noradrenaline: a pilot study. Crit Care. 2014;18(6):653. doi:10.1186/s13054-014-0653-3
10. Südfeld S, Brechnitz S, Wagner JY, et al. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017;119(1):57–64. doi:10.1093/bja/aex127
11. Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative Major Adverse Cardiovascular and Cerebrovascular Events Associated With Noncardiac Surgery. JAMA Cardiol. 2017;2(2):181–187. doi:10.1001/jamacardio.2016.4792
12. Rossouw E, Chetty S. Acute kidney injury after major non-cardiac surgery: incidence and risk factors. S Afr Med J. 2023;113(3):135–140. doi:10.7196/SAMJ.2023.v113i3.16783
13. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi:10.1186/cc5713
14. Singh S, Damén T, Dellborg M, Jeppsson A, Nygren A. Intraoperative infusion of noradrenaline improves platelet aggregation in patients undergoing coronary artery bypass grafting: a randomized controlled trial. J Thromb Haemost. 2019;17(4):657–665. doi:10.1111/jth.14408
15. Pang Q, Liu H, Chen B, et al. Restrictive and liberal fluid administration in major abdominal surgery. Saudi Med J. 2017;38(2):123–131. doi:10.15537/smj.2017.2.15077
16. Hasanin AM, Amin SM, Agiza NA, et al. Norepinephrine Infusion for Preventing Postspinal Anesthesia Hypotension during Cesarean Delivery: a Randomized Dose-finding Trial. Anesthesiology. 2019;130(1):55–62. doi:10.1097/ALN.0000000000002483
17. Feng K, Wang X, Feng X, et al. Effects of continuous infusion of phenylephrine vs. norepinephrine on parturients and fetuses under LiDCOrapid monitoring: a randomized, double-blind, placebo-controlled study. BMC Anesthesiology. 2020;20:229. doi:10.1186/s12871-020-01145-0
18. Chiarandini P, Pompei L, Costa MG, et al. Effects of catecholamines on microcirculation during general inhalation anesthesia. J CardiothoracVascAnesth. 2013;27(6):1239–1245. doi:10.1053/j.jvca.2013.03.036
19. Weinberg L, Li SY, Louis M, et al. Reported definitions of intraoperative hypotension in adults undergoing non-cardiac surgery under general anaesthesia: a review. BMC Anesthesiology. 2022;22(1):69. doi:10.1186/s12871-022-01605-9
20. Sessler DI, Bloomstone JA, Aronson S, et al. Perioperative quality initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122(5):563–574. doi:10.1016/j.bja.2019.01.013
21. Bateman BT, Schumacher HC, Wang S, et al. Perioperative acute ischemic stroke in noncardiac and nonvascular surgery: incidence, risk factors, and outcomes. Anesthesiology. 2009;110(2):231–238. doi:10.1097/ALN.0b013e318194b5ff
22. Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114(6):1289–1296. doi:10.1097/ALN.0b013e318216e7f4
23. Spence J, LeManach Y, Chan MTV, et al. Association between complications and death within 30 days after noncardiac surgery. CMAJ. 2019;191(30):E830–E837. doi:10.1503/cmaj.190221
24. Alexander CM, Ramseyer M, Beatty JS. Missed Extravasation Injury from Peripheral Infusion of Norepinephrine Resulting in Forearm Compartment Syndrome and Amputation. Am Surg. 2016;82(7):e162–e163. doi:10.1177/000313481608200713
25. Pancaro C, Shah N, Pasma W, et al. Risk of Major Complications After Perioperative Norepinephrine Infusion Through Peripheral Intravenous Lines in a Multicenter Study. Anesth Analg. 2020;131(4):1060–1065. doi:10.1213/ANE.0000000000004445
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
