Back to Journals » Nature and Science of Sleep » Volume 16
Association Between Preoperative Sleep Disturbance and Postoperative Delirium in Elderly: A Retrospective Cohort Study
Authors Guo H , Li LH, Lv XH, Su FZ, Chen J, Xiao F, Shi M, Xie YB
Received 30 November 2023
Accepted for publication 4 April 2024
Published 16 April 2024 Volume 2024:16 Pages 389—400
DOI https://doi.org/10.2147/NSS.S452517
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Valentina Alfonsi
Hao Guo,1,* Li-Heng Li,2,* Xiao-Hong Lv,1,* Feng-Zhi Su,1,* Jie Chen,1,* Fei Xiao,1,3 Min Shi,1 Yu-Bo Xie1,3
1Department of Anesthesiology, The First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China; 2Department of Anesthesiology, The Guilin Municipal Hospital of Traditional Chinese Medicine, Guangxi, People’s Republic of China; 3Guangxi Key Laboratory of Enhanced Recovery after Surgery for Gastrointestinal Cancer, The First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yu-Bo Xie, Department of Anesthesiology, The First Affiliated Hospital of Guangxi Medical University, No. 22 Shuangyong Road, Nanning, 530000, People’s Republic of China, Tel +86 13977121557, Email [email protected]
Purpose: Postoperative sleep disturbance, characterized by diminished postoperative sleep quality, is a risk factor for postoperative delirium (POD); however, the association between pre-existing sleep disturbance and POD remains unclear. This study aimed to evaluate the association between preoperative sleep disturbance and POD in elderly patients after non-cardiac surgery.
Patients and methods: This retrospective cohort study was conducted at a single center and enrolled 489 elderly patients who underwent surgery between May 1, 2020, and March 31, 2021. Patients were divided into the sleep disorder (SD) and non-sleep disorder (NSD) groups according to the occurrence of one or more symptoms of insomnia within one month or sleep- Numerical Rating Scale (NRS)≥ 6 before surgery. The primary outcome was the incidence of POD. Propensity score matching analysis was performed between the two groups. Multiple logistic regression analysis was performed to identify the risk factors for POD.
Results: In both the unmatched cohort (16.0% vs 6.7%, P=0.003) and the matched cohort (17.0% vs 6.2%, P=0.023), the incidence of POD was higher in the SD group than in the NSD group. In addition, the postoperative sleep quality and the VAS score at postoperative 24 h were significantly lower in the SD group than in the NSD group. Multivariate logistic regression analysis indicated that age (Odds Ratio, 1.13 [95% CI: 1.04– 1.23], P=0.003) and preoperative sleep disturbance (Odds Ratio, 3.03 [95% CI: 1.09– 9.52], P=0.034) were independent risk factors for the development of POD.
Conclusion: The incidence of POD was higher in patients with pre-existing sleep disturbance than those without it. Whether improving sleep quality for preoperative sleep disturbance may help prevent POD remains to be determined.
Keywords: sleep disturbances, postoperative delirium, postoperative sleep quality, postoperative pain, anesthesia
Introduction
Postoperative delirium (POD) is an acute brain dysfunction syndrome characterized by fluctuating disturbances of consciousness, concentration, cognitive function, and perception.1,2 Elderly patients are particularly susceptible to developing POD after surgery due to the presence of major chronic comorbid conditions and a decreased physiological reserve to handle the stress of surgery.3,4 The incidence of POD varies considerably depending on the patient population and surgical type, ranging from 15–25% in elective surgeries to as high as 50–60% in high-risk emergency surgeries.5 While a significant proportion of healthcare professionals perceive delirium patients as agitated, it is noteworthy that hyperactive delirium only accounts for a quarter of such cases. The remaining majority present with hypoactive or “quiet” delirium.6–8 Hypoactive delirium, associated with a less favorable prognosis, may be underdiagnosed due to its less conspicuous presentation.9,10 The manifestations of delirium span a spectrum from mild to extremely severe, with an observed correlation between increased severity and poorer outcomes.1,6–8
POD is associated with worse perioperative outcomes including prolonged hospitalization, functional decline in activities of daily living, persistent cognitive impairment, dementia, and increased hospital readmission and shortened overall survival.11,12 POD develops as a result of a complex interaction between the patient’s baseline vulnerability, which refers to their predisposing risk factors before hospitalization, and precipitating factors or insults, which are events that occur during hospitalization, such as anesthesia and surgery. Some identified susceptibility factors include advanced age, symptoms of cognitive impairment or dementia, and preexisting comorbidities. Sleep disorders, in particular, has frequently been cited as an important potential etiological factor associated with the development of POD.13–17
Sleep disturbance, especially sleep fragmentation, and poor sleep quality are commonly observed in older adults. According to recent studies, a prevalence rate ranging from 15% to 72% of patients has been reported to suffer from sleep disturbance after surgery under general anesthesia, which featured by sleep deprivation, circadian rhythm disruption, and abnormal sleep architecture.18,19 Postoperative sleep disturbance was often attributed to multiple factors, including surgery-related stress and inflammation, pain, high-dose opioids, and environmental aspects such as noise, light, and patient care procedures.20,21 Patients who previously experienced sleep disturbance are more likely to experience lower quality of sleep and are more prone to severe sleep disturbances following surgery.22 Presently, investigations concentrate mainly on the effects of postoperative sleep quality in relation to clinical outcomes, whereas studies regarding preoperative sleep disturbance or interruptions primarily center on acute or chronic pain following surgery and postoperative functional rehabilitation.23–26 The link between preoperative sleep disturbance and postoperative delirium is not clearly understood.
Therefore, we designed a retrospective cohort study to investigate the association between preoperative sleep disturbance and POD in elderly patients. We hypothesized that patients with sleep disturbance before surgery would be at increased risk of POD, accompanied by decreased postoperative sleep quality and increased postoperative pain.
Methods
Study Design and Patient Selection
Using electronic medical records from the First Affiliated Hospital of Guangxi Medical University, this retrospective study examined the association between preoperative sleep disturbance and POD in elderly patients who underwent elective surgery from May 1, 2020, to March 31, 2021. The study data included both structured and unstructured patient information from the hospital’s medical system and anesthesia follow-up system. The Ethics Committee of the First Affiliated Hospital of Guangxi Medical University approved the study protocol (ethical number: 2019 KEY-E-115), which followed the STROBE guidelines.27 The study was conducted in accordance with the Helsinki Declaration, patient data confidentiality was ensured, and written informed consent was waived since this was a retrospective review based on the hospital’s electronic medical records. Inclusion criteria included age during 65 to 90 years and ASA (American Society of Anesthesiologists) class I~IV elective general anesthesia surgery patients, and patients were excluded for cardiac and neurosurgery, severe sleep disorders requiring preoperative hypnotic drugs, a history of restless leg syndrome, periodic limb movement disorder, or obstructive sleep apnea, psychiatric or neurology diagnoses of dementia or Alzheimer’s disease, and consciousness disorders. The types of surgery were classified based on the surgical site into the following categories: general, orthopedic, and urological surgeries.
Preoperative and Intraoperative Data Collection
Patients were categorized into two groups based on their preoperative sleep quality: the Sleep Disturbance group (SD) and the Non-Sleep Disturbance group (NSD). The categorization was based on the presence of one or more symptoms of insomnia such as difficulty in falling asleep, shallow sleep, and early awakening within one month before surgery, and a preoperative visit sleep quality Numerical Rating Scale (NRS) score (0 representing the best possible sleep and 10 the worst; NRS≥6 indicate poor sleep quality).28–30 In addition to patient grouping, we collected demographic details such as gender, age, BMI, preoperative comorbidities, smoking and drinking status, educational level, cognitive function status, and emotional and physical status from the electronic medical record and anesthesia visit system. Upon entering the operating room, routine monitoring including blood pressure, heart rate, pulse oximetry was initiated for all patients. Pertinent intraoperative data such as surgery time, blood loss, hypotension episode, administration of opioid drugs (fentanyl equivalent doses for various opioids) were recorded. Additionally, we noted whether patients received nerve blockade or a dexmedetomidine infusion during surgery.
Postoperative Follow-Up Data Collection
POD was identified using postoperative follow-up data because no specific International Statistical Classification of Diseases and Related Health Problems (ICD) code exists for this condition. All patients were assessed for POD for 7 days after surgery using the Confusion Assessment Method (CAM).31 The CAM assessments were performed by a structured interviewing twice daily (morning between 9:00 and 11:00 a.m., and afternoon between 3:00 and 5:00 p.m.) for 7 postoperative days. The CAM assessment was developed as a screening instrument based on operationalization of Diagnostic and Statistical Manual of Mental Disorders (DSM)-III-R criteria for use by non-psychiatric clinicians. The tool’s algorithm consists of four clinical criteria: acute onset with a fluctuating course, inattention, disorganized thinking, and altered level of consciousness. The determination of delirium requires that both the first and second criterion be present, and either the third or fourth criterion must also be evident. We defined the occurrence of delirium as the patient meeting CAM criteria for delirium on any of the seven postoperative day assessments. In addition, assessors also reviewed the unstructured information for POD in medical records, and interviewed their families for evidence of suspicion of delirium including confusion, agitation, hallucinations, delusions and sedation during assessments.
To evaluate the subjective sleep quality of patients on the 1st, 2nd, and 3rd postoperative nights, we utilized the Numeric Rating Scale (NRS), an 11-point rating system (0 representing the best possible sleep and 10 the worst). Furthermore, the Visual Analog Scale (VAS) was used to evaluate the postoperative pain intensity of patients 24 hours after surgery, where “0” means no pain and “10” implies unbearable pain. If the VAS score was over 4 within 24 hours post-surgery, rescue analgesia with flurbiprofen was prescribed, and the need for rescue analgesia was noted.
Statistical Analysis
Descriptive statistics were presented as mean (standard deviation) or median (interquartile range) for continuous variables, and as counts (percentages) for categorical variables. Based on preoperative assessments, patients were divided into the Sleep Disturbance (SD) and Non-Sleep Disturbance (NSD) groups. Missing data were addressed using the multiple imputation method implemented in SPSS statistical software. The normality and skewness of continuous variables were assessed using Kolmogorov–Smirnov tests, and differences between the SD and NSD groups were compared using one-way ANOVA or Mann–Whitney tests as appropriate. Categorical variables were compared between groups using chi-square tests. The relative risks and 95% confidence intervals for postoperative outcomes were calculated. All data were analyzed using SPSS 22.0 and R statistical software. A two-tailed P value less than 0.05 was considered statistically significant.
Considering potential differences in baseline data between the SD and NSD groups, we also performed a propensity score analysis to further explore the association between preoperative sleep disturbance and POD. Propensity scores for all baseline variables were calculated using logistic regression, taking into account the demographic and clinical characteristics mentioned above. We performed a 1:1 nearest neighbor matching with a caliper value of 0.1. After matching, appropriate statistical tests were used to compare clinical outcomes between the two groups. Adjusting for age, gender, education level, preoperative cognitive impairment and frailty, intraoperative use of dexmedetomidine, surgical type, and intraoperative hypotension, multivariate logistic regression was used to evaluate the effect of preoperative sleep disturbance on delirium. Comparisons of baseline data and outcomes between the two groups were also performed as a non-matched cohort.
Results
A total of 14,409 patients who underwent elective surgery under general anesthesia at the First Affiliated Hospital of Guangxi Medical University from May 1, 2020 to March 31, 2021 were reviewed based on the electronic medical record and anesthesia follow-up system. Of these, 489 met the inclusion criteria, and 108 patients had missing preoperative or intraoperative data and could not be included in the study analysis. Ultimately, 381 patients were included in the final analysis.
Baseline Data
Demographic and preoperative data for all included patients are presented in Table 1. In the unmatched cohort, we identified 128 patients in the SD group and compared them with 253 patients in the NSD group. In the matched cohort, the analysis compared 96 patients in each of the SD and NSD groups (Table 1). The sleep NRS score on preoperative visit was significant difference between two groups (4.0±0.9 vs 7.6±0.9, P<0.001), but the result was not presented because it was not a matched variable. As shown in Table 1, the SMD (Standardized Mean Difference) was significantly reduced after matching, indicating that propensity score matching effectively improved the balance of covariates between the two groups. This improvement in balance made the comparison of two groups more reliable, as the potential impact of confounding factors was minimized.
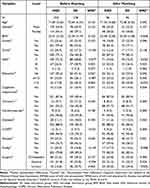 |
Table 1 Baseline Characteristics Before and After Matching |
Intraoperative Data
Intraoperative data are presented in Table 2. In the unmatched cohort, the patients in the SD group presented a significantly shorter surgery time (126.0 vs 145.0, P=0.04), a lower incidence of peripheral nerve block usage (21.1% vs 35.4%, P<0.01), and fewer consumption of fentanyl during surgery compared to patients in the NSD group (0.5 vs 1.2, P=0.01). After matching, no significant differences were found between the NSD and SD groups with respect to intraoperative variables.
 |
Table 2 Intraoperative Clinical Data Before and After Matching |
Comparisons of Outcomes Between the SD and NSD Groups
The outcomes of the SD and NSD groups before and after matching were presented in Table 3. The incidence of POD on the postoperative 7 days was significantly higher in the SD group compared to the NSD group in the unmatched cohort (16.0% vs 6.7%, P=0.003). The odds ratio for the SD group was 2.72 (95% CI: 1.38 to 5.37) compared to the NSD group. Additionally, the postoperative sleep quality score of the SD group was higher on the first and second postoperative days than that of the NSD group (3.8±1.66 vs 4.4±1.87, P<0.001; 3.4±1.68 vs 3.8±1.55, P=0.014, respectively), but was not different on the third day (3.0±1.63 vs 3.1±1.51, P=0.469). Moreover, postoperative pain, as assessed by the VAS score, was found to be significantly higher in the SD group than in the NSD group (3.8±1.56 vs 3.1±1.66, P<0.001), with no significant difference in the percentage of rescue analgesia needed between the two groups after surgery (38% vs 38%, P=0.93).
 |
Table 3 Outcomes Between SD and NSD Groups Before and After Matching |
The compared of the clinical outcomes of two groups after matching were also performed. Compared to the NSD group, the incidence of POD on the 7 days remained significantly higher in the SD group (17.0% vs 6.2%, P=0.023) (Figure 1), the odds ratio of the SD group was 3.0 (95% CI: 1.12 to 8.04). As shown in Figure 2, the postoperative sleep quality score on the first and second postoperative days was significantly higher in the SD group than that in the NSD group (4.4±1.99 vs 3.9±1.56, P=0.03; 3.9±1.61 vs 3.4±1.70, P=0.031, respectively), while there was no difference on the third day (3.3±1.53 vs 2.9±1.63, P=0.093). Likewise, the VAS score on postoperative 24 h among the SD group was significantly higher than the NSD group (3.8±1.62 vs 3.1±1.51, P=0.002) (Figure 3). However, the proportions of patients needing rescue analgesia after surgery were similar between the two groups (41% vs 33%, P=0.30).
 |
Figure 1 The incidence of POD in group SD and group NSD after matching. Abbreviations: SD, sleep disturbance group; NSD, non-sleep disturbance group; POD, postoperative delirium. |
Independent Risk Factors for POD
Multivariate logistic regression analysis in Figure 4 indicated that age (OR 1.13, 95% CI: 1.04 to 1.23) and preoperative sleep disturbance (OR 3.03, 95% CI: 1.09 to 9.25) were significantly associated with the development of POD. Therefore, age and preoperative sleep disturbance were found to be independent risk factors for POD in this particular cohort.
Discussion
This retrospective cohort study demonstrates that patients with preoperative sleep disturbance have a markedly increased likelihood of developing POD subsequent to general anesthesia surgery when compared to patients without sleep disturbance. Multivariate logistic regression analysis revealed that even after adjusting related covariates before and during surgery, sleep disturbance remained an independent risk factor for the occurrence of POD. Furthermore, preoperative sleep disturbance was associated with higher levels of postoperative pain and reduced sleep quality during the first three days following surgery. In summary, our findings underlined the correlation between preoperative sleep disturbance, the presence of POD, diminished postoperative sleep quality, and perioperative pain management.
Accumulating evidence suggests that sleep disturbances are associated with impairments in spatial memory, verbal fluency, attention, and executive function.32–35 Specifically, sleep disturbance was associated with poor cognitive performances and lower volumes of grey matter,36 and the grey matter atrophy in the medial prefrontal cortex (mPFC) is associated with impairment of hippocampal-dependent memory in the elderly.37 In addition, the deposition of Aβ protein in the hippocampus38,39 and increases the ratio of p-tau/total tau,40 which are considered to be related to the occurrence of postoperative cognitive dysfunction,41 have been reported to be related to sleep deprivation. Circadian rhythm disturbances, such as suppression, fragmentation, and delayed acrophase of the 24-hour daily rest-activity rhythms, are features of sleep disturbance that are associated with delirium risk. Delirium cases with suppressed rhythms are more likely to progress to dementia.42,43
Meta-analyses reported sleep disturbance to be a predictor of POD.14,44 However, many of the included studies were conducted on patients with pre-existing obstructive sleep apnea,45–47 it is important to note that obstructive sleep apnea has been demonstrated to increase the risk of POD.48 Consequently, sleep disturbances are anticipated in these patients due to the pathophysiology of obstructive sleep apnea. In contrast to these previous studies, our current findings may differ because we excluded patients with a known history of obstructive sleep apnea. Additionally, it’s worth mentioning that many past studies have primarily focused on evaluating sleep post-surgery.49,50
In our study, we assessed the preoperative sleep quality of patients using the sleep-NRS, which was a subjective measures of sleep quality. In our study, we observed that postoperative sleep quality scores on the first and second postoperative days were significantly higher in the SD group compared to the NSD group. While our findings reveal a statistically significant difference of 0.5 on the NRS, it’s imperative to recognize the potential limitations regarding its clinical significance, particularly within the context of a scale ranging from 1 to 10. Such a modest change may not necessarily equate to meaningful clinical implications. It is paramount to exercise caution when interpreting statistical significance, particularly in the absence of clear clinical relevance, especially when dealing with subjective measures such as the NRS. Clinicians should weigh various factors beyond statistical findings when making informed decisions about patient care in real-world clinical settings.
We found that patients with poor sleep quality (NRS≥6) had a higher incidence of POD than those with good sleep quality. However, we should note that the impact of different sleep characteristics on POD. Our study only focused on the association between insomnia symptoms and POD, and whether the sleep latency, sleep duration, and daytime dysfunction has significantly or not correlated with POD did not been examined. Indeed, the study conduct by Ulsa MC et al has demonstrated that poor sleep burden and worsening trajectory were associated with increased risk for delirium.51 These results suggest that promotion of sleep health may be important for the surgical patients who were susceptibility to delirium.
Although no definitive conclusions can be reached, it is hypothesized that preoperative sleep disturbances may contribute to the development of POD and exacerbate long-term cognitive impairment after discharge.52,53 The present study appears to corroborate this hypothesis since preoperative sleep disturbance significantly increase the incidence of postoperative delirium among elderly patients (17.0% vs 6.2%). Indeed, peri-operative sleep disturbance have been shown to be associated with postoperative cognitive function.54–56 According to a study by Todd et al, patients with preexisting sleep disturbance were significantly more likely to develop POD than those without sleep disturbance (RR 3.90, 95% CI: 2.14 to 7.11).52 Evans et al identified insufficient sleep on the first night after surgery as an early predictor of secondary delirium.57 Concurrently, it has been demonstrated that improving sleep quality in elderly patients can decrease the risk of POD, lending further support to the view that sleep may play a disruptive role in the development of POD.58 Regarding the relationship between perioperative sleep disturbance and POD, we propose a bidirectional effect rather than a definitive causal relationship.
In our study, we discovered that 33.6% of patients had preoperative sleep disturbance, indicating that sleep disturbances are prevalent in elderly patients prior to surgery. This implies that enhancing preoperative sleep quality may be an effective approach for preventing POD. Currently, the prevention and treatment of sleep disturbance mainly consist of various non-pharmacological interventions59–65 and pharmacological treatments, including benzodiazepine hypnotics,66,67 melatonin receptor agonists,68,69 and anesthetics/sedatives.70,71 Prior studies have suggested that dexmedetomidine may reduce the occurrence of POD and improve postoperative sleep quality in elderly patients.72–74 However, our study failed to discover a correlation between the administration of dexmedetomidine and POD, possibly due to the dosage of dexmedetomidine and the vastly distinct surgeries, which could influence the prophylactic effect of dexmedetomidine for POD.
Pain following surgery is an inevitable consequence, leading to diminished sleep quality and disrupted sleep continuity, which are associated with heightened pain sensitivity and decreased cognitive function.75 A multitude of studies suggest a close correlation between sleep disturbances and acute as well as chronic postoperative pain.13,24,26 Bjurstrom et al found that patients with preoperative sleep disturbance who underwent total hip replacement experienced more severe postoperative pain, requiring an increase in opioid use within 24 hours of surgery.24 Our study indicates a close relationship between preoperative sleep disturbance and severe postoperative pain; however, the subjective nature of the VAS score may impact the accurate pain assessment. Despite higher VAS scores, no increase in rescue analgesia was observed in the sleep disturbance group. VAS scores above 4 are typically defined as moderate pain and may necessitate rescue analgesia, yet the average VAS score in the SD group in our study was 3.9. Considering the pain sensitivity of individual, there was no significant change in rescue analgesia requirements among patients. Nonetheless, we hypothesize that preoperative sleep disturbance may be associated with increased postoperative pain intensity and pain interference. Whereas several clinically relevant predictors of poor postoperative pain are interconnected, the actual incidence of clinically meaningful variables such as depression, anxiety, and pain catastrophizing were low, while sleep disturbances were highly prevalent, implicating a strong correlation between sleep quality and pain perception. Pain can be both a cause and a consequence of sleep disturbances This bidirectional relationship between sleep and pain has important implications not only for the clinical management of patients, but also for chronic pain prevention and public health more broadly.76 Given that sleep disturbances can strongly predict pain outcomes, targeting and improving sleep quality may have a beneficial effect on postoperative pain management.
The present study has several limitations. Firstly, being a retrospective cohort study conducted at a single center, and excluding patients with other severe sleep disorders such as restless leg syndrome, periodic limb movement disorder, or obstructive sleep apnea, as well as those with preclinical dementia or Alzheimer’s disease, may limit the generalizability of the results. Therefore, caution should be exercised when interpreting the findings. Future prospective studies with larger cohorts are warranted to provide further insights into the relationship between preoperative sleep disturbances and postoperative delirium. Secondly, the absence of objective sleep quality monitoring, such as polysomnography, is another limitation. While preoperative actigraphy combined with subjective assessment offers a more comprehensive evaluation of sleep quality, utilizing wearable electronic monitoring devices with various sensors could enhance accuracy and identify patients at higher risk of delirium. Given the potential discrepancies between subjective sleep quality ratings and objective sleep assessments, and considering that the complexity of polysomnography monitoring may disrupt patients’ sleep, future studies should consider using wearable electronic monitoring devices equipped with various eye and hand movement sensors.77–79 Thirdly, the potential influence of time as a confounding factor on sleep quality was not explored. Additionally, the absence of involvement of a biostatistician in the analysis process poses another limitation. Despite efforts to adhere to statistical principles, the lack of specialized statistical expertise may have impacted the robustness and interpretation of the findings. Lastly, the study did not investigate the relationship between postoperative sleep quality and POD, as the primary focus was on the association between preoperative sleep disturbances and POD. Given previous research indicating bidirectional influences between postoperative sleep quality, pain, and POD, future randomized controlled trials are warranted to elucidate these relationships.
Conclusion
We conclude that preexisting sleep disturbances are likely associated with postoperative delirium. Whether strategies to improve preoperative sleep quality can benefit patients with preoperative sleep disturbance in postoperative delirium remains to be determined.
Funding
This work was supported by the Special Fund of Neurotoxicity of General Anesthetics and Its Prevention and Treatment Innovation Team of the First Affiliated Hospital of Guangxi Medical University; Guangxi Clinical Research Center for Anesthesiology (No. GK AD22035214); the Key Project of Natural Science Foundation of Guangxi (No. 2020GXNSFDA238025).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Marcantonio ER, Solomon CG. Delirium in hospitalized older adults. New Engl J Med. 2017;377(15):1456–1466. doi:10.1056/NEJMcp1605501
2. Ravi B, Pincus D, Choi S, Jenkinson R, Wasserstein DN, Redelmeier DA. Association of duration of surgery with postoperative delirium among patients receiving hip fracture repair. JAMA Network Open. 2019;2(2):e190111. doi:10.1001/jamanetworkopen.2019.0111
3. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A. 2001;56(3):M146–56. doi:10.1093/gerona/56.3.m146
4. Berenson RA, Horvath J. Confronting the barriers to chronic care management in Medicare. Health Affairs. 2003;2:37–53. doi:10.1377/hlthaff.w3.37
5. Takazawa T, Horiuchi T, Orihara M, et al. Prevention of postoperative cognitive dysfunction by minocycline in elderly patients after total knee arthroplasty: a randomized, double-blind, placebo-controlled clinical trial. Anesthesiology. 2023;138(2):172–183. doi:10.1097/ALN.0000000000004439
6. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi:10.1016/s0140-6736(13)60688-1
7. Marcantonio ER. In the clinic. Delirium. Ann Internal Med. 2011;154(11):. doi:10.7326/0003-4819-154-11-201106070-01006
8. Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA. 2012;308(1):73–81. doi:10.1001/jama.2012.6857
9. Kiely DK, Jones RN, Bergmann MA, Marcantonio ER. Association between psychomotor activity delirium subtypes and mortality among newly admitted post-acute facility patients. J Gerontol Ser A. 2007;62(2):174–179. doi:10.1093/gerona/62.2.174
10. Yang FM, Marcantonio ER, Inouye SK, et al. Phenomenological subtypes of delirium in older persons: patterns, prevalence, and prognosis. Psychosomatics. 2009;50(3):248–254. doi:10.1176/appi.psy.50.3.248
11. POSE Study group. Peri-interventional outcome study in the elderly in Europe: a 30-day prospective cohort study. Euro J Anaesthesiol. 2022;39(3):198–209. doi:10.1097/EJA.0000000000001639
12. Weiss Y, Zac L, Refaeli E, et al. Preoperative cognitive impairment and postoperative delirium in elderly surgical patients - A retrospective large cohort study. Ann Surg. 2022. doi:10.1097/SLA.0000000000005657
13. Luo ZY, Li LL, Wang D, Wang HY, Pei FX, Zhou ZK. Preoperative sleep quality affects postoperative pain and function after total joint arthroplasty: a prospective cohort study. J Orthopaedic Surg Res. 2019;14(1):378. doi:10.1186/s13018-019-1446-9
14. Fadayomi AB, Ibala R, Bilotta F, Westover MB, Akeju O. A systematic review and meta-analysis examining the impact of sleep disturbance on postoperative delirium. Crit Care Med. 2018;46(12):e1204–e1212. doi:10.1097/CCM.0000000000003400
15. Wang X, Hua D, Tang X, et al. The role of perioperative sleep disturbance in postoperative neurocognitive disorders. Nat Sci Sleep. 2021;13:1395–1410. doi:10.2147/NSS.S320745
16. O’Gara BP, Gao L, Marcantonio ER, Subramaniam B. Sleep, pain, and cognition: modifiable targets for optimal perioperative brain health. Anesthesiology. 2021;135(6):1132–1152. doi:10.1097/ALN.0000000000004046
17. Ibala R, Mekonnen J, Gitlin J, et al. A polysomnography study examining the association between sleep and postoperative delirium in older hospitalized cardiac surgical patients. J Sleep Res. 2021;30(5):e13322. doi:10.1111/jsr.13322
18. Gögenur I, Wildschiøtz G, Rosenberg J. Circadian distribution of sleep phases after major abdominal surgery. Br J Anaesth. 2008;100(1):45–49. doi:10.1093/bja/aem340
19. Su X, Wang DX. Improve postoperative sleep: what can we do? Curr Opin Anaesthesiol. 2018;31(1):83–88. doi:10.1097/ACO.0000000000000538
20. Lane T, East LA. Sleep disruption experienced by surgical patients in an acute hospital. Br J Nurs. 2008;17(12):766–771. doi:10.12968/bjon.2008.17.12.30306
21. Pisani MA, Friese RS, Gehlbach BK, Schwab RJ, Weinhouse GL, Jones SF. Sleep in the intensive care unit. Am J Respir Crit Care Med. 2015;191(7):731–738. doi:10.1164/rccm.201411-2099CI
22. Wang J-P, Lu S-F, Guo L-N, Ren C-G, Zhang Z-W. Poor preoperative sleep quality is a risk factor for severe postoperative pain after breast cancer surgery. Medicine. 2019;98(44):e17708. doi:10.1097/md.0000000000017708
23. Boye Larsen D, Laursen M, Simonsen O, Arendt-Nielsen L, Petersen KK. The association between sleep quality, preoperative risk factors for chronic postoperative pain and postoperative pain intensity 12 months after knee and Hip arthroplasty. British J Pain. 2021;15(4):486–496. doi:10.1177/20494637211005803
24. Bjurstrom MF, Irwin MR, Bodelsson M, Smith MT, Mattsson-Carlgren N. Preoperative sleep quality and adverse pain outcomes after total Hip arthroplasty. Eur J Pain. 2021;25(7):1482–1492. doi:10.1002/ejp.1761
25. Ida M, Onodera H, Yamauchi M, Kawaguchi M. Preoperative sleep disruption and postoperative functional disability in lung surgery patients: a prospective observational study. J Anesthe. 2019;33(4):501–508. doi:10.1007/s00540-019-02656-y
26. Varallo G, Giusti EM, Manna C, et al. Sleep disturbances and sleep disorders as risk factors for chronic postsurgical pain: a systematic review and meta-analysis. Sleep Med Rev. 2022;63:101630. doi:10.1016/j.smrv.2022.101630
27. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi:10.1016/s0140-6736(07)61602-x
28. Qiu D, Wang XM, Yang JJ, et al. Effect of intraoperative esketamine infusion on postoperative sleep disturbance after gynecological laparoscopy: a randomized clinical trial. JAMA Network Open. 2022;5(12):e2244514. doi:10.1001/jamanetworkopen.2022.44514
29. Cai J, Chen Y, Hao X, et al. Effect of intraoperative dexmedetomidine dose on postoperative first night sleep quality in elderly surgery patients: a retrospective study with propensity score-matched analysis. Front Med. 2020;7:528. doi:10.3389/fmed.2020.00528
30. Duan G, Wang K, Peng T, Wu Z, Li H. The effects of intraoperative dexmedetomidine use and its different dose on postoperative sleep disturbance in patients who have undergone non-cardiac major surgery: a real-world cohort study. Nat Sci Sleep. 2020;12:209–219. doi:10.2147/NSS.S239706
31. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Int Med. 1990;113(12):941–948. doi:10.7326/0003-4819-113-12-941
32. Zhu B, Dong Y, Xu Z, et al. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis. 2012;48(3):348–355. doi:10.1016/j.nbd.2012.06.022
33. Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36(7):1027–1032. doi:10.5665/sleep.2802
34. Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–1028. doi:10.1016/s1474-4422(14)70172-3
35. Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. doi:10.1001/jama.2011.1115
36. Grau-Rivera O, Operto G, Falcón C, et al. Association between insomnia and cognitive performance, gray matter volume, and white matter microstructure in cognitively unimpaired adults. Alzheimer’s Res Ther. 2020;12(1):4. doi:10.1186/s13195-019-0547-3
37. Mander BA, Rao V, Lu B, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16(3):357–364. doi:10.1038/nn.3324
38. Ju YS, Ooms SJ, Sutphen C, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain. 2017;140(8):2104–2111. doi:10.1093/brain/awx148
39. Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71(8):971–977. doi:10.1001/jamaneurol.2014.1173
40. Guisle I, Gratuze M, Petry S, et al. Circadian and sleep/wake-dependent variations in tau phosphorylation are driven by temperature. Sleep. 2020;43(4). doi:10.1093/sleep/zsz266
41. Liang F, Baldyga K, Quan Q, et al. Preoperative Plasma Tau-PT217 and Tau-PT181 are associated with postoperative delirium. Ann Surg. 2022. doi:10.1097/SLA.0000000000005487
42. Gao L, Li P, Gaykova N, et al. Circadian rest-activity rhythms, delirium risk, and progression to dementia. Ann Neurol. 2023;93(6):1145–1157. doi:10.1002/ana.26617
43. Hu K, Li P, Gao L. Sleep, rest-activity rhythms and aging: a complex web in Alzheimer’s disease? Neurobiol Aging. 2021;104:102–103. doi:10.1016/j.neurobiolaging.2021.02.017
44. Wang H, Zhang L, Zhang Z, et al. Perioperative sleep disturbances and postoperative delirium in adult patients: a systematic review and meta-analysis of clinical trials. Frontiers in Psychiatry. 2020;11:570362. doi:10.3389/fpsyt.2020.570362
45. Flink BJ, Rivelli SK, Cox EA, et al. Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology. 2012;116(4):788–796. doi:10.1097/ALN.0b013e31824b94fc
46. Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76(9):897–905. doi:10.4065/76.9.897
47. Roggenbach J, Klamann M, von Haken R, Bruckner T, Karck M, Hofer S. Sleep-disordered breathing is a risk factor for delirium after cardiac surgery: a prospective cohort study. Critical Care. 2014;18(5):477. doi:10.1186/s13054-014-0477-1
48. Mirrakhimov AE, Brewbaker CL, Krystal AD, Kwatra MM. Obstructive sleep apnea and delirium: exploring possible mechanisms. Sleep Breath. 2014;18(1):19–29. doi:10.1007/s11325-013-0846-z
49. Zhang W-Y, W-l W, J-j G, et al. Risk factors for postoperative delirium in patients after coronary artery bypass grafting: a prospective cohort study. J Crit Care. 2015;30(3):606–612. doi:10.1016/j.jcrc.2015.02.003
50. Maybrier HR, King CR, Crawford AE, et al. Early postoperative actigraphy poorly predicts hypoactive delirium. J Clin Sleep Med. 2019;15(1):79–87. doi:10.5664/jcsm.7576
51. Ulsa MC, Xi Z, Li P, et al. Association of poor sleep burden in middle age and older adults with risk for delirium during hospitalization. J Gerontol Ser A. 2022;77(3):507–516. doi:10.1093/gerona/glab272
52. Todd OM, Gelrich L, MacLullich AM, Driessen M, Thomas C, Kreisel SH. Sleep disruption at home as an independent risk factor for postoperative delirium. J Am Geriatr Soc. 2017;65(5):949–957. doi:10.1111/jgs.14685
53. Lingehall HC, Smulter NS, Lindahl E, et al. Preoperative cognitive performance and postoperative delirium are independently associated with future dementia in older people who have undergone cardiac surgery: a longitudinal cohort study. Crit Care Med. 2017;45(8):1295–1303. doi:10.1097/ccm.0000000000002483
54. Song J, Chu S, Cui Y, et al. Circadian rhythm resynchronization improved isoflurane-induced cognitive dysfunction in aged mice. Exp Neurol. 2018;306:45–54. doi:10.1016/j.expneurol.2018.04.009
55. Ni P, Dong H, Zhou Q, et al. Preoperative sleep disturbance exaggerates surgery-induced neuroinflammation and neuronal damage in aged mice. Mediators Inflammation. 2019;2019:8301725. doi:10.1155/2019/8301725
56. Lu B, Liu RJ, Meng B, et al. Effect of fragmented sleep on postoperative cognitive function and central neuroinflammation. Zhonghua Yi Xue Za Zhi. 2020;100(17):1341–1344. doi:10.3760/cma.j.cn112137-20191215-02734
57. Evans JL, Nadler JW, Preud’homme XA, et al. Pilot prospective study of post-surgery sleep and EEG predictors of post-operative delirium. Clin Neurophysiol. 2017;128(8):1421–1425. doi:10.1016/j.clinph.2017.05.004
58. Kratz T, Heinrich M, Schlauß E, Diefenbacher A. Preventing postoperative delirium. Deutsches Arzteblatt International. 2015;112(17):289–296. doi:10.3238/arztebl.2015.0289
59. Wu XH, Cui F, Zhang C, et al. Low-dose dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit: a pilot randomized controlled trial. Anesthesiology. 2016;125(5):979–991. doi:10.1097/ALN.0000000000001325
60. Le Guen M, Nicolas-Robin A, Lebard C, Arnulf I, Langeron O. Earplugs and eye masks vs routine care prevent sleep impairment in post-anaesthesia care unit: a randomized study. Br J Anaesth. 2014;112(1):89–95. doi:10.1093/bja/aet304
61. Xie H, Kang J, Mills GH. Clinical review: the impact of noise on patients’ sleep and the effectiveness of noise reduction strategies in intensive care units. Critical Care. 2009;13(2):208. doi:10.1186/cc7154
62. Li SY, Wang TJ, Vivienne Wu SF, Liang SY, Tung HH. Efficacy of controlling night-time noise and activities to improve patients’ sleep quality in a surgical intensive care unit. J Clin Nurs. 2011;20(3–4):396–407. doi:10.1111/j.1365-2702.2010.03507.x
63. Obanor OO, McBroom MM, Elia JM, et al. The impact of earplugs and eye masks on sleep quality in surgical ICU patients at risk for frequent awakenings. Crit Care Med. 2021;49(9):e822–e832. doi:10.1097/CCM.0000000000005031
64. Leong RW, Davies LJ, Fook-Chong S, Ng SY, Lee YL. Effect of the use of earplugs and eye masks on the quality of sleep after major abdominal surgery: a randomised controlled trial. Anaesthesia. 2021;76(11):1482–1491. doi:10.1111/anae.15468
65. Scarpa M, Pinto E, Saraceni E, et al. Randomized clinical trial of psychological support and sleep adjuvant measures for postoperative sleep disturbance in patients undergoing oesophagectomy. Br J Surg. 2017;104(10):1307–1314. doi:10.1002/bjs.10609
66. Gong L, Wang Z, Fan D. Sleep quality effects recovery after Total Knee Arthroplasty (TKA)--A randomized, double-blind, controlled study. J Arthropl. 2015;30(11):1897–1901. doi:10.1016/j.arth.2015.02.020
67. Shakya H, Wang D, Zhou K, Luo ZY, Dahal S, Zhou ZK. Prospective randomized controlled study on improving sleep quality and impact of zolpidem after total Hip arthroplasty. J Orthopaedic Surg Res. 2019;14(1):289. doi:10.1186/s13018-019-1327-2
68. Clarkson SJ, Yayac MF, Rondon AJ, Smith BM, Purtill JJ. Melatonin does not improve sleep quality in a randomized placebo-controlled trial after primary total joint arthroplasty. J Am Acad Orthop Surg. 2022;30(2):e287–e294. doi:10.5435/JAAOS-D-21-00243
69. Tanner N, Schultz B, Calderon C, et al. Effectiveness of melatonin treatment for sleep disturbance in orthopaedic trauma patients: a prospective, randomized control trial. Injury. 2022;53(12):3945–3949. doi:10.1016/j.injury.2022.10.011
70. Akeju O, Kim SE, Vazquez R, et al. Spatiotemporal dynamics of dexmedetomidine-induced electroencephalogram oscillations. PLoS One. 2016;11(10):e0163431. doi:10.1371/journal.pone.0163431
71. Akeju O, Hobbs LE, Gao L, et al. Dexmedetomidine promotes biomimetic non-rapid eye movement stage 3 sleep in humans: a pilot study. Clin Neurophysiol. 2018;129(1):69–78. doi:10.1016/j.clinph.2017.10.005
72. Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10054):1893–1902. doi:10.1016/S0140-6736(16)30580-3
73. Skrobik Y, Duprey MS, Hill NS, Devlin JW. Low-Dose nocturnal dexmedetomidine prevents ICU Delirium. A randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2018;197(9):1147–1156. doi:10.1164/rccm.201710-1995OC
74. Qu JZ, Mueller A, McKay TB, et al. Nighttime dexmedetomidine for delirium prevention in non-mechanically ventilated patients after cardiac surgery (MINDDS): a single-centre, parallel-arm, randomised, placebo-controlled superiority trial. EClinicalMedicine. 2023;56:101796. doi:10.1016/j.eclinm.2022.101796
75. Strutz PK, Kronzer V, Tzeng W, et al. The relationship between obstructive sleep apnoea and postoperative delirium and pain: an observational study of a surgical cohort. Anaesthesia. 2019;74(12):1542–1550. doi:10.1111/anae.14855
76. Haack M, Simpson N, Sethna N, Kaur S, Mullington J. Sleep deficiency and chronic pain: potential underlying mechanisms and clinical implications. Neuropsychopharmacology. 2020;45(1):205–216. doi:10.1038/s41386-019-0439-z
77. Leung JM, Tang C, Do Q, Sands LP, Tran D, Lee KA. Sleep Loss the night before surgery and incidence of postoperative delirium in adults 65–95 years of age. Sleep Med. 2023;105:61–67. doi:10.1016/j.sleep.2023.03.015
78. Sugg E, Gleeson E, Baker SN, et al. Sleep and circadian biomarkers of postoperative delirium: protocol for a prospective, observational cohort study. medRxiv. 2023;2023:23295920. doi:10.1101/2023.09.21.23295920
79. Gao L, Li P, Gaba A, Musiek E, Ju YS, Hu K. Fractal motor activity regulation and sex differences in preclinical Alzheimer’s disease pathology. Alzheimer’s Dementia. 2021;13(1):e12211. doi:10.1002/dad2.12211
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



