Back to Journals » Clinical Interventions in Aging » Volume 19
Association Between Quality of Life Defined by EuroQol Group 5 Dimension and Composite Inferior Outcome Among Inpatients with Cirrhosis
Authors Hui Y, Wang H, Guo G, Yang W, Zhang X, Yang J, Yang F, Wang X, Fan X, Cui B, Chen X, Jiao H, Sun C
Received 21 November 2023
Accepted for publication 13 March 2024
Published 21 March 2024 Volume 2024:19 Pages 551—560
DOI https://doi.org/10.2147/CIA.S444842
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Yangyang Hui,1,* Han Wang,2,* Gaoyue Guo,1,* Wanting Yang,1 Xuqian Zhang,3 Jie Yang,1 Fang Yang,4 Xiaoyu Wang,1 Xiaofei Fan,1 Binxin Cui,5 Xin Chen,1 Huanli Jiao,2 Chao Sun1,5
1Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China; 2Department of Health Management, Tianjin Hospital, Tianjin, People’s Republic of China; 3Department of Gastroenterology and Hepatology, China Aerospace Science & Industry Corporation 731 Hospital, Beijing, People’s Republic of China; 4Department of Digestive System, Baodi Clinical College of Tianjin Medical University, Tianjin, People’s Republic of China; 5Department of Gastroenterology, Tianjin Medical University General Hospital Airport Hospital, Tianjin Airport Economic Area, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huanli Jiao; Chao Sun, Tel +8613642060733 ; +8613820846027, Fax +86-022-27813550, Email [email protected]; [email protected]
Purpose: The utility of the EuroQol Group 5 Dimension (EQ-5D) measuring health-related quality of life (HRQoL) has been verified; however, knowledge gaps remain concerning predictive performance in cirrhosis. We aimed to identify the optimal threshold for risk stratification and the pronounced domain in the EQ-5D linked to inferior outcomes.
Patients and Methods: The X-tile project was used to obtain a threshold, considering the composite outcome of 1-year all-cause mortality or readmission. A restricted cubic spline (RCS) was performed to test the non-linear relationship between the EQ-5D utility value and the primary outcome. Six multivariate Cox regression models incorporating EQ-5D utility value and each of the five domains were constructed.
Setting/Participants: Totally, 420 patients with cirrhosis were recruited.
Results: The median utility value of the study population was 0.77 and 59.8% reported impairment in minimal one EQ-5D domain. RCS indicated a linear relationship between the utility value and composite inferior outcome. X-tile pinpointed a utility value of 0.59 stratifying populations into high- and low-risk groups based on the outcome. Inpatients with cirrhosis and deteriorated HRQoL (utility value ≤ 0.59) were at higher risk of death or readmission (adjusted HR: 2.18, P < 0.001). Furthermore, mobility and usual activities were the most pronounced domains associated with composite inferior outcome.
Conclusion: A utility value ≤ 0.59 can identify cirrhotic inpatients exhibiting compromised HRQoL and mortality/readmission risk. It is tempting to reverse the decreased HRQoL by applying longitudinal measurements and keeping surveillance on utility value, while interventions appear to mainly focus on improving mobility and usual activities.
Keywords: EQ-5D, liver cirrhosis, mortality, readmission, disability-adjusted life year
Introduction
It should be clinically noted that the global burden of liver cirrhosis continues to rise, evident by 1.48 million cases of death in 2019, accounting for an increase of 8.1% relative to those in 2017.1 Furthermore, concerns have also been addressed by steep increase in public health burden attributed to cirrhosis and other chronic liver diseases. In 2019, the percentage of disability-adjusted life year (DALY) for cirrhosis ranked 12th responsible for 2.8% of all diseases and pathological entities among populations aged from 25 to 49 years.2 Although the DALY for HBV/HCV cirrhosis decreased from 2010 to 2019, a rapidly increasing trend of alcohol-associated and metabolic dysfunction-associated steatotic liver disease (MASLD) cirrhosis is anticipated wherein the latter may construct mainstream in the near future due to the epidemic of obesity and diabetes mellitus.3 Moreover, accumulating evidence suggests that there is an increasing trend in the age of patients with cirrhosis around China.4,5 Another report derived from China’s Disease Surveillance Points system clearly reveals a substantial proportion of end-stage liver disease cases with the average age of death at 63.37 years, in particular, cirrhosis at 62.32 years and liver cancer at 63.57 years.6 These findings are in alignment with a recent nationwide survey in Japan, implicating a mean age approximately 2 years older when comparing patients between 2015 and 2017 to patients between 2008 and 2010 (68.2 years vs 66.4 years).7 Notably, all healthcare facilities and practitioners are supposed to face a grave situation since the “real” disease burden of cirrhosis is currently underestimated. From economic and health resource perspectives, hospitalization costs, admission rates, and median length of stay tend to increase, according to the latest report derived from the National Inpatient Sample data.8 Beyond traditional disease complications, it is also crucial to take other psychophysiologic perturbations or multidimensional parameters into account with the purpose of enhancing therapeutic approaches and medical care in the context of cirrhosis.9
Ensuring that patients with serious illnesses receive patient-centered care is a cornerstone of medical care. To specify and streamline this care to be patient-centered, a prerequisite refers to what matters to the patients.10 Accordingly, patients’ health-related quality of life (HRQoL), a key determinant of patient-reported outcomes, is of increasing interest for its clinical importance and prognostic role in a wide range of advanced chronic liver disease.11 Actually, the cirrhosis-dictated health burden has proven to be amplified by its considerable impact on HRQoL, arising from a spectrum of physical, psychological, and social insults caused by cirrhosis and corresponding treatment. There are two mainstays for assessing HRQoL in the realm of hepatology: disease-specific instruments (chronic liver disease questionnaire etc.) and generic preference-based instruments. The EuroQol Group 5 Dimension (EQ-5D) is a representative tool with definite simplicity, validity, and reliability. Finally, EQ-5D is advocated and recommended by the health technology assessment national agency, which is beneficial in close connection with participants’ survival status, sufficient sensitivity to detect deteriorated health status, and usage to facilitate healthcare decision-making.12
Previous studies have used EQ-5D to evaluate HRQoL in various liver diseases;13–16 however, there is a lack of an outcome-based threshold to stratify patients with cirrhosis into distinct risk groups. Identification of such threshold may shed light on comparison between different diseases regarding relative impact, accurate appraisal of influencing HRQoL domain and precision medicine tailored to individual patient’s needs.17 Without the aforementioned information, it is impractical to provide prompt and effective intervention for this vulnerable population as well as direct treatment and management. In this study, we aimed to (1) elucidate the optimal threshold pertinent to the EQ-5D utility value for risk stratification and (2) elaborate on the most pronounced domain in the EQ-5D survey linked to inferior outcomes.
Materials and Methods
Study Population
The current study population comprised patients with cirrhosis hospitalized at the Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital from 2018 to 2022. The inclusion criteria were as follows: (1) age ≥18 years, (2) diagnosis of cirrhosis according to clinical features plus at least one of histopathology, radiology, endoscopy, or non-invasive elastography findings, and (3) informed consent for participation. The exclusive criteria were as follows: (1) concomitant acute-on-chronic liver failure (ACLF), (2) intra- or extra-hepatic malignancies, (3) serious hepatic encephalopathy (West Haven grade ≥2), and (4) denial of regular follow-up (Figure S1). ACLF definition adheres to the guideline constructed by the Asian Pacific Association for the Study of the Liver, incorporating coagulation abnormalities (prothrombin time-international normalized ratio (PT-INR) ≥1.5) and jaundice (total bilirubin ≥85µmol/L) complicated by physical examination-determined ascites and/or hepatic encephalopathy within 4 weeks in the context of chronic liver disease or cirrhosis.18 Acute decompensation covers the presence of any of the following events or combined: ascites, which is clinically evident determined by radiological examination or physical examination in terms of the International Ascites Club classification,19 grading of the hepatic encephalopathy in terms of the West Haven Criteria and esophagogastric variceal bleeding determined on endoscopy.20,21 The present study protocol was reviewed and approved by the ethics committee of Tianjin Medical University General Hospital (IRB-YX-004-01). All investigating procedures were conducted in accordance with the Declaration of Helsinki, and written informed consent was obtained from all participants included in the study.
Data Collection
For each participant, the following clinical features and biochemical parameters were retrieved: demographics (ie, age, sex), body mass index (BMI), cirrhosis etiology (ie, chronic viral infection, alcohol-associated liver disease, autoimmune liver disease, cholestatic liver disease, MASLD and cryptogenic reasons), cirrhosis-related complications (ie, esophagogastric variceal bleeding, ascites, hepatic encephalopathy), laboratory data (ie, sodium, potassium, albumin, alanine aminotransferase, alkaline phosphatase (ALP), total bilirubin, platelet, hemoglobin, creatinine and PT-INR). In this sense, several scoring systems specific to liver disease severity (ie, Child-Turcotte-Pugh (CTP) class/score, Model for End-stage Liver Disease (MELD) score and MELD-sodium (MELD-Na) score) were calculated.
Outcomes
In this study, we defined a composite inferior outcome covering 1-year all-cause mortality or readmission. This setting was built on account of retrieving sufficient numbers of events for appropriate statistical analysis and its intimate connection with the healthcare burden, in addition to health resource utilization in the context of cirrhosis. This selection conforms to previous report; avoidance of unplanned hospitalization definitely leverages cost benefits on any healthcare system.22
Quality of Life Instrument
To evaluate HRQoL, we used the EQ-5D-3L, which belonged to a well-built tool estimating quality-of-life characterized by generic and preference-based features (Figure S2).23 This survey was conducted within 48 hours of index hospitalization. The EQ-5D items consist of five domains: mobility, self-care, usual activities, pain, and anxiety/depression. The three response levels/categories indicated no problems, some problems, or extreme problems in each domain. A total of 243 possible combinations were constructed to shape the individual health state. Accordingly, a unique health state can be linked to a preference-weighted score, namely utility value, based on direct utility elicitation from general population samples. Moreover, the EQ-5D utility value derives from a country-dictated level, previously constructed by applying the trade-off method, and represents the whole preference of a particular country. In our study, we retrieved the utility value calculated from the representative Japanese-specific population setting owing to high comparability between these two East Asian countries.24 The utility values ranged from 0 to 1.00, with 0 equivalent to death and 1.00 equivalent to perfect health; a negative utility value corresponded to worse health state relative to the death.
Optimal Threshold Estimation
The X-tile project was employed to build a single global estimation of each possible modality to categorize the study population into high- and low-level expressions pertaining to predefined biomarkers. Moreover, X-tile manipulation can leverage a robust statistical analysis by dividing the single cohort into primary and validation subsettings to retrieve the best P value estimation when the aforesaid manipulation is practically unavailable.25
Statistical Analysis
SPSS 23.0 software or R 3.3.2 package was used for statistical analysis. The median was regarded as the central tendency parameter (interquartile range) for continuous variables, and proportions for categorical variables. Inter-group comparisons were performed using the Mann–Whitney U-test for continuous variables, and χ2 or Fisher’s exact test for categorical variables. Variables with statistical significance in the univariate Cox regression analysis were included in multivariate models. Six prediction models were established incorporating EQ-5D utility value dichotomization or each domain in the EQ-5D.26 The non-linear relationship between EQ-5D utility values and the likelihood of unfavorable outcome was demonstrated using a restricted cubic spline (RCS) with three knots at the 10th, 50th, and 90th percentiles.27 Survival analysis was performed using the Kaplan–Meier graph alongside Log rank test. A two-tailed P value <0.05 was considered as statistically significant.
Results
HRQoL data from 420 patients with cirrhosis were included in this study (Table 1). The median age of the study population was 64 (57, 69) years, of which 50% were male (n = 210). The major cirrhosis-related complications included esophagogastric variceal bleeding in 70.7% (n = 297) and ascites (n = 250) in 59.5% patients. Looking into liver disease severity, the majority of hospital inpatients were ranked as CTP class B&C (n = 279, 66.4%), while the median MELD score and MELD-Na score were 8.5 (5.0, 11.5) and 9.7 (6.1, 12.9), respectively. The median EQ-5D utility value was 0.77 (0.65, 1.00) and 59.8% (n = 251) reported impairment in minimal one EQ-5D domain. During the follow-up period, we recorded 179 episodes concerning all-cause mortality or readmission (42.6%).
 |
Table 1 Hospital Cirrhotic Inpatients’ Baseline Characteristics (n = 420) |
Next, stratification using the X-tile project revealed an optimal threshold of 0.59 in relation to the composite inferior outcome (Figure S3). As depicted in Table 1, patients with EQ-5D utility value ≤0.59 had lower levels of BMI, higher proportions of ascites, lower levels of sodium, higher levels of ALP, higher levels of platelet, higher levels of creatinine as compared to those with utility value >0.59.
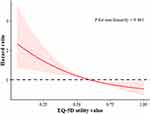
In the univariate analysis, the significant risk factors associated with 1-year all-cause mortality or readmission were CTP score, serum sodium, ALP, albumin, total bilirubin, creatinine, and EQ-5D utility value (Table 2). Subsequently, multivariate Cox regression models were constructed based on significant findings with regard to univariate analysis incorporating the EQ-5D utility value or one of the five EQ-5D domains (exclusion of overlapped parameters within the CTP score). By adjusting for CTP score, serum sodium, ALP, and creatinine, compromised HRQoL indicative of EQ-5D utility value ≤0.59 (HR: 2.18, 95% confidence interval (CI): 1.53, 3.10, P <0.001) remained independently associated with the composite inferior outcome. This tendency was also maintained for the other two models by incorporating respective EQ-5D domains, that is, mobility (HR: 2.16, 95% CI: 1.37, 3.41, P = 0.001) and usual activities (HR: 1.78, 95% CI: 1.12, 2.80, P = 0.015) (Figure 1). To ascertain the validity of our findings, we applied RCS analysis to illuminate a linear relationship between continuous EQ-5D utility values and the hazard ratio of indicative composite inferior outcome (P = 0.461 for non-linearity, Figure 2).
 |
Table 2 Univariate and Multivariate Cox’s Regression Analyses for 1-Year All-Cause Mortality or Readmission |
Finally, the survival analysis using the Kaplan–Meier graph demonstrated that there was a significant difference in the survival curves between patients with an EQ-5D utility value ≤0.59 versus >0.59 (Log rank test: P < 0.001, Figure 3). The median survival time was significantly shorter among patients with cirrhosis and compromised HRQoL (182 days vs 365 days).
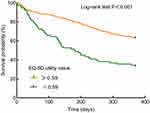 |
Figure 3 Survival curves in relation to 1-year all-cause mortality stratified by EQ-5D utility value. Abbreviation: EQ-5D, EuroQol Group 5 Dimension. |
Discussion
In the present era of patient-reported outcome metrics, there is increasing interest in clarifying the potential role of HRQoL as an endpoint in clinical trials. However, concerns have been raised regarding the appropriate construct to assess the quality of life and the resultant optimal threshold for identifying patients at high risk surrounding distinct dire outcomes. By analyzing a large sample of 420 hospital patients whose HRQoL was measured according to a generic preference tool EQ-5D utility value, this study indicated that a utility value ≤0.59 can identify a subset of inpatients with cirrhosis experiencing both compromised HRQoL and mortality/readmission risk. Moreover, deteriorated mobility and usual activities within the EQ-5D domains were representative defects related to inferior outcomes in the context of cirrhosis. Collectively, our preliminary findings, to some extent, allow for the designation of clinical trial beneficials and development of interventive targets when considering EQ-5D-defined HRQoL.
It is highlighted that patients with cirrhosis, particularly those at advanced stages, may struggle in daily lives owing to high mortality, a considerable burden of physical/psychological deficits and unpredictable disease trajectories.28 On the other hand, dilemmas do exist since conventional therapies are solely directed towards managing decompensating insults (ie, hepatic encephalopathy and variceal bleeding), despite many people with cirrhosis experiencing multiple distressing symptoms that dramatically degrade their HRQoL, physical and social functioning.10,29 More recently, the American Association for the Study of Liver Diseases has released its expert consensus to operationalize symptom-focused clinical trial design, aiming at advancement in innovative health care and improvement in better quality of life of this population.30 In that programmatic paper, one core aspect to be addressed covers the selection and implementation of standard metrics. Moreover, the selected validated tool/instrument is expected to detect minimally clinically important differences and facilitate tracing improvements over time. Notably, it is crucial for investigators who intend to instigate clinical trials exploring disease-modifying interventions to consider the use of comparable measures across populations and studies.31 Intriguingly, the construct framework of EQ-5D questionnaire appears to meet these needs, as discussed below.
First, both disease-specific and generic instruments have been recommended to achieve holistic assessment of participants’ HRQoL.32,33 We ascertain that multi-dimensional disease-specific quality of life surveys have advantages in terms of sensitivity to detect symptomatic perturbations, but they fail to compare the health status of the general population or patients with other pathological entities, liver- or non-liver-associated. On the contrary, the generic preference-based EQ-5D questionnaire adopted in the present study can foster making healthcare decisions and weighting intervention benefits advocated by reputative institution, by getting insights into cirrhosis-dictated HRQoL impact relative to other populations.12 Second, we herein opt to apply the EQ-5D-3L instead of other generic metrics (eg, SF-36) because it is sufficient shortness as a complement to other routinely reported data within the execution of clinical studies such as biochemical parameters, clinical scoring scales, and treatment efficacy data. As a matter of fact, this aspect holds clinical relevance and importance in the collection of massive data, especially when these programs are conducted by investigators/practitioners. It is noteworthy that 91% of cirrhosis providers complained about competing demands for time during heavy work load.34 For instance, although SF-36 was capable of identifying more fine variations concerning HRQoL due to its granular nature, EQ-5D could determine differences influencing participants’ lives.35 Last, a report clearly stated that EQ-5D can be used to measure and monitor altering health status in the context of chronic liver disease.36 Zoe et al showed the EQ-5D utility index remained static during a follow-up of 12 months following diagnosis. Responsiveness, which refers to the ability to detect variations within and between participants over time coinciding with changes in status, is pivotal.37
To the best of our knowledge, this is the first study to investigate the optimal threshold of EQ-5D utility value to stratify mortality/readmission risk among hospital patients with cirrhosis; thus, no comparative data are found in the realm of hepatology. A literature search has revealed similar findings in other populations and disease backgrounds. Berg et al reported that every increase of 0.1 in EQ-5D scale was indicative of 24% decrease in one-year mortality risk in a cardiology setting.38 Another study conducted in patients undergoing cardiac resynchronization therapy also revealed that each 10% increase in the visual analogue scale of EQ-5D corresponded to an 8% decreased all-cause mortality risk.39 In addition, Pan et al showed that the EQ-5D-3L index score was significantly lower in dead community-dwelling older people, and physical-related problems (ie, mobility, self-care, and usual activities) could serve as better predictors of mortality than psychological defects (ie, depression/anxiety and pain).40 Accordingly, our results demonstrated that EQ-5D utility value and two domains in relation to physical performance were independently associated with higher mortality/readmission risk to different magnitudes. We speculate that these reflect the facts that physical-based domains based on self-report scan capture underestimated health conditions and provide a comprehensive illustration of physical health, closely linked to poor outcomes.41 Furthermore, these defects are sensitive biomarkers for disease progression.42 Regarding cirrhosis, the advancement and aggravation of this entity is always accompanied by the onset of various phenotypic manifestations related to malnutrition and physical inactivity like frailty and sarcopenia.43 Indeed, plenty of studies have elaborated on the connections between declining HRQoL and nutritional or body composition abnormalities by others and us among cirrhosis.44–46 Last, the magnitude of detriments to physical health is not in alignment with those to mental health, leading to limited predictive capability concerning baseline mental domains.40,47
The results of this study should be interpreted with caution due to these limitations. First, although the study population was sizable, the single-center nature of the study design may have limited its generalizability. Further prospective studies on multi-center data are warranted to verify the validity of our preliminary findings. However, the adoption of a generic instrument EQ-5D alongside distilling the best classification threshold may handle this flaw to a lesser extent. In other words, our proposed utility value ≤0.59 is capable of comparing the relative impact between distinct disease and population settings. Second, we lacked data regarding the longitudinal responsiveness of the EQ-5D utility value, whose temporal changes may serve as more profound issues adherent to the improvement or deterioration of disease. Finally, selection bias can be anticipated, given the recruitment criteria, to exclude patients with overt hepatic encephalopathy.
Conclusion
In conclusion, a low EQ-5D-defined utility value can be used to identify patients with cirrhosis who exhibit compromised HRQoL and mortality/readmission risk. It is imperative to effectively manage declining HRQoL by applying temporal measurements and monitoring changes in utility value, considering the intimate connection between poor HRQoL and inferior outcomes. Interventive approaches appear to focus primarily on improving mobility and the usual activities. Moreover, future investigations are supposed to testify the effectiveness and robustness of our proposed threshold of 0.59.
Abbreviations
EQ-5D, EuroQol Group 5 Dimension; HRQoL, health-related quality of life; ACLF, acute-on-chronic liver failure; RCS, restricted cubic spline; DALY, disability-adjusted life year; MASLD, metabolic dysfunction-associated steatotic liver disease; PT-INR, prothrombin time-international normalized ratio; BMI, body mass index; ALP, alkaline phosphatase; CTP, Child-Turcotte-Pugh; MELD, Model for End-stage Liver Disease; HR, hazard ratio; CI, confidence interval.
Data Sharing Statement
The datasets generated and/or analyzed during this study are available from the corresponding author (Chao Sun) upon reasonable request.
Acknowledgments
We thank all nurses who participated in this study and Dr Kui Jiang for technical support. Yangyang Hui, Han Wang and Gaoyue Guo contributed equally to this work and share first authorship.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that there is no conflict of interest.
References
1. Sheena BS, Hiebert L, Han H.; Collaborators GBDHB. Global, regional, and national burden of hepatitis B, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Gastroenterol Hepatol. 2022;7(9):796–829. doi:10.1016/S2468-1253(22)00124-8
2. Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. doi:10.1016/S0140-6736(20)30925-9
3. Jepsen P, Younossi ZM. The global burden of cirrhosis: a review of disability-adjusted life-years lost and unmet needs. J Hepatol. 2021;75 Suppl 1:S3–S13. doi:10.1016/j.jhep.2020.11.042
4. Wang X, Luo JN, Wu XY, Zhang QX, Wu B. Study of liver cirrhosis over twenty consecutive years in adults in Southern China. World J Hepatol. 2023;15(12):1294–1306. doi:10.4254/wjh.v15.i12.1294
5. Xiong J, Wang J, Huang J, Sun W, Wang J, Chen D. Non-alcoholic steatohepatitis-related liver cirrhosis is increasing in China: a ten-year retrospective study. Clinics. 2015;70(8):563–568. doi:10.6061/clinics/2015(08)06
6. Wang X, Liu H, Qi J, et al. Trends of mortality in end-stage liver disease - China. China CDC Wkly. 2023;5(30):657–663. doi:10.46234/ccdcw2023.128
7. Enomoto H, Ueno Y, Hiasa Y, et al. Transition in the etiology of liver cirrhosis in Japan: a nationwide survey. J Gastroenterol. 2020;55(3):353–362. doi:10.1007/s00535-019-01645-y
8. Desai AP, Mohan P, Nokes B, et al. Increasing economic burden in hospitalized patients with cirrhosis: analysis of a national database. Clin Transl Gastroenterol. 2019;10(7):e00062. doi:10.14309/ctg.0000000000000062
9. Younossi ZM, Boparai N, Price LL, Kiwi ML, McCormick M, Guyatt G. Health-related quality of life in chronic liver disease: the impact of type and severity of disease. Am J Gastroenterol. 2001;96(7):2199–2205. doi:10.1111/j.1572-0241.2001.03956.x
10. Tapper EB, Kanwal F, Asrani SK, et al. Patient-reported outcomes in cirrhosis: a scoping review of the literature. Hepatology. 2018;67(6):2375–2383. doi:10.1002/hep.29756
11. Orr JG, Homer T, Ternent L, et al. Health related quality of life in people with advanced chronic liver disease. J Hepatol. 2014;61(5):1158–1165. doi:10.1016/j.jhep.2014.06.034
12. Brazier J, Ratcliffe J, Salomon JA, Tsuchiya A. Measuring and Valuing Health Benefits for Economic Evaluation. Oxford University Press; 2017:348.
13. Kimbell B, Murray SA, Byrne H, et al. Palliative care for people with advanced liver disease: a feasibility trial of a supportive care liver nurse specialist. Palliat Med. 2018;32(5):919–929. doi:10.1177/0269216318760441
14. Wu X, Hong J, Zhou J, et al. Health-related quality of life improves after entecavir treatment in patients with compensated HBV cirrhosis. Hepatol Int. 2021;15(6):1318–1327. doi:10.1007/s12072-021-10240-4
15. Younossi Z, Aggarwal P, Shrestha I, et al. The burden of non-alcoholic steatohepatitis: a systematic review of health-related quality of life and patient-reported outcomes. JHEP Rep. 2022;4(9):100525. doi:10.1016/j.jhepr.2022.100525
16. Skladany L, Liska D, Liptakova E, Tapajcikova T, Vnencakova J, Koller T. Comparison of the quality of life of patients with liver cirrhosis before and during the COVID-19 lockdown in Slovakia. Sci Rep. 2023;13(1):2463. doi:10.1038/s41598-023-29510-2
17. Rajpurohit S, Musunuri B, Mohan PB, Bhat G, Shetty S. Factors affecting and promoting health-related quality of life in patients with liver cirrhosis: an underestimated domain in patient care. J Clin Exp Hepatol. 2024;14(1):101264. doi:10.1016/j.jceh.2023.07.417
18. Sarin SK, Kedarisetty CK, Abbas Z, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8(4):453–471. doi:10.1007/s12072-014-9580-2
19. Moore KP. The management of ascites in cirrhosis: report on the consensus conference of the international ascites club. Hepatology. 2003;38(1):258–266. doi:10.1053/jhep.2003.50315
20. American Association for the Study of Liver D. European Association for the Study of the l. hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol. 2014;61(3):642–659. doi:10.1016/j.jhep.2014.05.042
21. Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65(1):310–335. doi:10.1002/hep.28906
22. Kok B, Whitlock R, Ferguson T, et al. Health-related quality of life: a rapid predictor of hospitalization in patients with cirrhosis. Am J Gastroenterol Apr. 2020;115(4):575–583. doi:10.14309/ajg.0000000000000545
23. EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi:10.1016/0168-8510(90)90421-9
24. Tsuchiya A, Ikeda S, Ikegami N, et al. Estimating an EQ-5D population value set: the case of Japan. Health Econ. 2002;11(4):341–353. doi:10.1002/hec.673
25. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi:10.1158/1078-0432.CCR-04-0713
26. Belayachi J, El Khattate A, Bizrane M, Madani N, Abouqal R. Pre-admission quality of life as predictor of outcome after acute care: the role of emotional well-being. QJM. 2018;111(2):111–115. doi:10.1093/qjmed/hcx209
27. Xu X, Zhu Y, Li S, Xia D. Dietary intake of anthocyanidins and renal cancer risk: a prospective study. Cancers. 2023;15(5):1406. doi:10.3390/cancers15051406
28. Rogal SS, Hansen L, Patel A, et al. AASLD practice guidance: palliative care and symptom-based management in decompensated cirrhosis. Hepatology. 2022;76(3):819–853. doi:10.1002/hep.32378
29. Foster C, Baki J, Nikirk S, Williams S, Parikh ND, Tapper EB. Comprehensive health-state utilities in contemporary patients with cirrhosis. Hepatol Commun. 2020;4(6):852–858. doi:10.1002/hep4.1512
30. Patel AA, Tapper EB, Kanwal F, et al. Targets and study design for symptom-focused trials aimed at patients with cirrhosis: an expert consensus. Hepatol Commun. 2023;7(6):doi:10.1097/HC9.0000000000000135
31. Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an nih roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3–S11. doi:10.1097/01.mlr.0000258615.42478.55
32. Mells GF, Pells G, Newton JL, et al. Impact of primary biliary cirrhosis on perceived quality of life: the UK-PBC national study. Hepatology. 2013;58(1):273–283. doi:10.1002/hep.26365
33. Newton JL, Bhala N, Burt J, Jones DE. Characterisation of the associations and impact of symptoms in primary biliary cirrhosis using a disease specific quality of life measure. J Hepatol. 2006;44(4):776–783. doi:10.1016/j.jhep.2005.12.012
34. Ufere NN, Donlan J, Waldman L, et al. Barriers to use of palliative care and advance care planning discussions for patients with end-stage liver disease. Clin Gastroenterol Hepatol. 2019;17(12):2592–2599. doi:10.1016/j.cgh.2019.03.022
35. Brazier J, Jones N, Kind P. Testing the validity of the Euroqol and comparing it with the SF-36 health survey questionnaire. Qual Life Res. 1993;2(3):169–180. doi:10.1007/BF00435221
36. Zoe T, Jane C, Rebecca H, Joe W, Guha IN, Morling JR. Health related quality of life in individuals at high risk of chronic liver disease: impact of a community diagnostic pathway. Public Health Pract. 2020;1:100033. doi:10.1016/j.puhip.2020.100033
37. Health USDo, Human Services FDACfDE, Research. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. doi:10.1186/1477-7525-4-79
38. Berg SK, Thorup CB, Borregaard B, et al. Patient-reported outcomes are independent predictors of one-year mortality and cardiac events across cardiac diagnoses: findings from the national DenHeart survey. Eur J Prev Cardiol Apr. 2019;26(6):624–637. doi:10.1177/2047487318769766
39. Nagy KV, Merkely B, Rosero S, et al. Quality of life predicting long-term outcomes in cardiac resynchronization therapy patients. Europace. 2019;21(12):1865–1875. doi:10.1093/europace/euz262
40. Pan CW, Liu RJ, Yang XJ, et al. Could the EQ-5D-3L predict all-cause mortality in older Chinese? Evidence from a 5-year longitudinal study in eastern China. Qual Life Res. 2021;30(10):2887–2894. doi:10.1007/s11136-021-02883-5
41. Phyo AZZ, Freak-Poli R, Craig H, et al. Quality of life and mortality in the general population: a systematic review and meta-analysis. BMC Public Health. 2020;20(1):1596. doi:10.1186/s12889-020-09639-9
42. Cooper R, Kuh D, Hardy R, Mortality Review G, Falcon THAS. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi:10.1136/bmj.c4467
43. Tandon P, Montano-Loza AJ, Lai JC, Dasarathy S, Merli M. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol. 2021;75(Suppl 1):S147–S162. doi:10.1016/j.jhep.2021.01.025
44. Hui Y, Cui B, Wang X, et al. The relationship between patient-reported health-related quality of life and malnutrition risk in cirrhosis: an observational cohort study. Br J Nutr. 2023;130(5):860–867. doi:10.1017/S0007114522003841
45. Shanavas N, Devadas K, Nahaz N, Varghese J, Cyriac R, Mathew D. Association of sarcopenia with health related quality of life in cirrhotics. J Assoc Physicians India. 2021;69(11):11–12.
46. Sugiyama Y, Ishizu Y, Ando Y, et al. Obesity and myosteatosis: the two characteristics of dynapenia in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2021;33(1S Suppl 1):e916–e921. doi:10.1097/MEG.0000000000002303
47. Masel MC, Ostir GV, Ottenbacher KJ. Frailty, mortality, and health-related quality of life in older Mexican Americans. J Am Geriatr Soc. 2010;58(11):2149–2153. doi:10.1111/j.1532-5415.2010.03146.x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.