Back to Journals » Pragmatic and Observational Research » Volume 14
Avelumab for Advanced Merkel Cell Carcinoma: Global Real-World Data on Patient Response and Survival
Authors Lohray R, Verma KK, Wang LL, Haynes D , Lewis DJ
Received 31 July 2023
Accepted for publication 10 November 2023
Published 16 November 2023 Volume 2023:14 Pages 149—154
DOI https://doi.org/10.2147/POR.S398151
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor David Price
Rishabh Lohray,1 Kritin K Verma,2 Leo L Wang,3 Dylan Haynes,3 Daniel J Lewis3
1Baylor College of Medicine, Houston, TX, USA; 2Texas Tech University Health Sciences Center, School of Medicine, Lubbock, TX, USA; 3Department of Dermatology, University of Pennsylvania, Philadelphia, PA, USA
Correspondence: Daniel J Lewis, Penn Dermatology Oncology Center, Perelman School of Medicine at the University of Pennsylvania, 3400 Civic Center Boulevard, Suite 1-330S, Philadelphia, PA, 19104, USA, Tel +1 215-360-0909, Fax +1 215-662-4132, Email [email protected]
Introduction: Avelumab is a programmed cell death-ligand 1 (PD-L1) inhibitor approved by the Food and Drug Administration for advanced Merkel cell carcinoma (MCC). Studies conducted in real-world settings have shed light on its effectiveness and safety in clinical settings.
Areas Covered: Real-world studies on avelumab for MCC from North and South America, Europe, and Asia have been presented in this review. Most studies are on patients over age 70 and have a male-predominant sex ratio. Overall response rates range from 29.1% to 72.1%, (disease control rate: 60.0– 72.7%; complete response rate: 15.8%– 37.2%; partial rate: 18.2– 42.1%; stable disease: 7.1– 30.9%; progressive disease: 7.1– 40.0%) and median progression free survival ranges from 8.1 to 24.1 months depending on the population studied. Immunosuppressed patients appear to benefit from avelumab as well, with response rates equivalent to the general population. Patients receiving avelumab as a first-line agent tend to have better outcomes than those using it as a second-line therapy. Fatigue, infusion-related reactions, and dyspnea were some of the most common adverse events identified in real-world studies. Autoimmune hepatitis and thyroiditis were also observed.
Conclusion: The use of avelumab as a safe and effective treatment option for advanced MCC is supported by real-world data, although additional study is required to assess long-term efficacy and safety outcomes.
Keywords: Merkel cell carcinoma, avelumab, real-world studies, immune-checkpoint inhibitors, tumor response, adverse events
Introduction
Merkel cell carcinoma (MCC) is a rare, life-threatening, aggressive neuroendocrine tumor of the skin that is associated with immunocompromised states, advanced age, ultraviolet (UV) exposure, and human polyomavirus exposure.1 It is the second-most common cause of skin cancer-related death in the world after melanoma. MCC classically presents as an erythematous nodule in a sun-exposed area but can be non-specific and notoriously difficult to identify. A third of the patients may present with nodal or metastatic disease at presentation.2 Skin biopsy, imaging, sentinel lymph node biopsy, and polyomavirus antibody status may be considered for staging and prognostication. Depending on the stage, a combination of surgical excision with negative margins, radiation therapy, or, immunotherapy may be used. Prior to the approval of immunotherapy for MCC, traditional chemotherapy was primarily used for palliation due to low durability of response.2 MCC can have a disease-specific mortality of up to 44.6% and an all-cause mortality of up to 51.9% over a median follow-up duration of 30.2 months.2
The JAVELIN Merkel 200 trial showed that avelumab, a programmed cell death ligand-1 inhibitor (PD-L1), can be used as a first- or second-line agent in the treatment of advanced MCC. The PD-L1 ligand functions as an immune checkpoint by inhibiting programmed cell death protein 1 receptors (PD-1) on T lymphocytes, preventing the immune system from inappropriately attacking the body.3 However, tumor cells maladapt PD-L1 to bind to PD-1 and inhibit T-cell immunosurveillance of tumors.3
Avelumab is a human IgG monoclonal antibody that inhibits PD-L1, restoring T-cell activity and allowing them to target and destroy tumor cells. In 2017, the US Food and Drug Administration made avelumab the first approved drug for advanced MCC. It was followed by the approval of pembrolizumab in 2018, another immune-checkpoint inhibitor (ICI). Since then, multiple studies have sought to demonstrate the effectiveness and safety profile of avelumab in a clinical setting. This paper aims to summarize the current literature on real-world outcomes associated with avelumab use in advanced MCC.
Methods
Real-world studies were defined as studies outside of clinical trials, specifically the JAVELIN trial in 2018. This includes global data from expanded access programs (EAPs) and retrospective studies. A search for English language articles published with the keywords, “real-world”, “avelumab”, or “Merkel cell carcinoma” was made on PubMed, Embase, and Scopus databases. Studies investigating response and survival were reviewed. Eight studies met our search criteria and have been included in this review.
Demographics
The incidence of MCC has been rising in the United States, thought primarily to be driven by the growth of the elderly population.4 According to the Surveillance, Epidemiology, and End Results (SEER) database, the age-adjusted incidence of MCC from 2012–2016 was 2.5-times higher than that from 1987–1991.5 Similarly, Europe, China, and Australia have all seen rising incidences over the past several years.6–10 While incidence data in Latin America and parts of Asia are scarce, multiple studies have shown that fair-skinned individuals have twice the risk when compared to the general population.7,11–14 Current studies have examined a wide range of populations, including the US, Latin America, the Netherlands, Italy, Israel, and the Middle East. A significant portion of these studies are supported by expanded access programs (EAPs). EAPs allow for the compassionate use of avelumab in patients in whom multiple chemotherapies have failed, surgery is not an option, and clinical trials are not accessible (secondary to immunosuppressed status).11
The incidence of MCC peaks in the eighth decade of life likely due to higher cumulative UV radiation exposure and immunosenescence, both of which are risk factors for MCC.15,16 The median age range in real-world studies ranges from 67.1 to 78 years. Most studies have a higher male-to-female ratio, with 55–86% of the cohort being male, consistent with reports that MCC is more common in males.10,17,18 Unlike the JAVELIN trial, most of these studies include patients of Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0–3 and those with immunosuppressed status. Immunosuppressed patients such as solid-organ transplant recipients, patients with HIV, and those with hematologic malignancies are at a higher risk of developing MCC, hence their inclusion is vital.19,20 Patient demographics seen in real-world studies are described in Table 1. 3,9,11–13,21–23
 |
Table 1 Patient Demographics in Real-World Studies |
Although MCC is reported to occur predominantly on the head and neck, an analysis of 54 advanced MCC patients conducted in the Netherlands, indicated that tumors occurred on the trunk, head and neck, and extremities 15%, 24%, and 46% of the time, respectively.21,24 In another study, primary tumor locations were lower limbs, lungs, and viscera, which metastasized to the lymph nodes 38%, 52%, and 68% of the time, respectively.22
Dosing Regimen
The 2023 National Comprehensive Cancer Network (NCCN) guidelines recommend ICIs such as avelumab, pembrolizumab, and nivolumab as first-line agents, only for disseminated disease (advanced MCC).1 In most real-world studies, advanced MCC was treated with intravenous (IV) avelumab 10 mg/kg every two weeks until one of the following endpoints occurred: complete response, progressive disease clinically or radiographically, significant clinical deterioration, or intolerable adverse events occurred.9,11,13,21 Cowey et al documented the median time from diagnosis to imitation of therapy as 6 weeks.3 Overall, this regimen is similar to that used in the JAVELIN trial.25,26
Some studies also described the use of premedication to lessen infusion-related reactions. Infusions of acetaminophen 1000 mg IV and clemastine 2 mg, a first-generation H1 antagonist, 2 mg IV were administered as a premedication during the first three cycles.11,21
Tumor Response and Survival
Tumor responses from real-world studies are summarized in Table 2.3,9,11–13,21–23 The median duration of treatment in current literature ranges from 7.9–13.5 months.12,22 The median real-world progression-free survival rate varies from 8.1–24.1 months, and the median real-world overall survival rate (OS) ranges from 20.2–30.7 months, depending on the stage of disease and use of concomitant therapies.3,13,21,22 Data from EAP studies are limited by variations in physician reporting and discretion and country-specific reporting restrictions.23
 |
Table 2 Patient Response Seen in Real-World Studies |
While the JAVELIN trial excluded immunocompromised patients, some real-world studies did not. Immunosuppression is a major risk factor for MCC and its inclusion in real-world studies is warranted.10 Walker et al’s global EAP study found clinical benefit in immunocompromised patients (Overall Response Rate (ORR): 37.5%, Disease Control Rate (DCR): 68.8%, Complete Response (CR): 18.8%, Partial Response (PR):18.8%, Stable Disease (SD): 31.3%, Progressive Disease (PD): 31.3%) similar to the overall cohort (ORR: 46.7%, DCR:71.2%, CR: 22.9%, PR:23.8%, SD: 24.6%, PD:28.8%).23 Response duration was also similar in both groups.23 In a study conducted by Averbach et al, which included a cohort of 62 patients, 22% of the participants were immunocompromised: two kidney transplant recipients, nine patients with myeloproliferative disorders, and three patients with iatrogenic immunosuppression. Interestingly, the study revealed no difference in outcomes between the immunocompromised and immunocompetent patients, with an overall response rate of 59.0%.13 Hence, an immunocompromised state does not appear to limit the efficacy of avelumab in MCC according to real-world studies.
Another consideration when evaluating tumor response is the sequence in which avelumab is administered. Parts A and B of the JAVELIN trial evaluated outcomes with avelumab as a second- and first-line agent, respectively.25,26 As a second-line agent, avelumab was associated with an ORR of 33.0% and CR of 11.4%, with a response duration of at least 2 years in 67.0% of the cohort. As a first-line agent, avelumab had an ORR of 39.7% and a CR of 16.4%, with a response duration of at least 2 years in 30.2% of the cohort.25,26 In real-world studies, Bhatia et al demonstrated an ORR of 72.1%.22 The ORR also varied based on whether avelumab was utilized first-line (75.3%) or second-line (64.7%).22 The median progression free survival (PFS) was 24.4 months.22 Levy et al did not show any significant differences in PFS and OS between first-line and second-line groups in their study; however, they reported that first-line was associated with a higher CR when compared to second-line treatment with avelumab (28.0% vs 14.0%).21 In summary, the data support better outcomes associated with avelumab when used as a first-line agent, suggesting tumors in patients without prior lines of therapy may be more receptive to PD-L1 inhibition. Alternatively, patients who have failed chemotherapy and thus using avelumab as a second-line agent may have more aggressive disease.21
Adverse Events
In the JAVELIN trial, fatigue (23.7%), infusion-related reactions (18.4%), and nausea (15.8%) were the most common adverse events.25,26 In addition, 33% of patients experienced ≥ grade 3 adverse events, with the most common being autoimmune hepatitis (3.7%), elevated liver enzymes (2.6%), and infusion-related reactions (2.6%).25,26 Grade 3 toxicity in real-world studies was noted in 11% of the patients, with no instances of grade 4 or grade 5 toxicity.21 In EAP studies, some adverse events may be unreported due to reporting variations among physicians.11
Ten to thirty percent of patients experience immune-related adverse events (irAEs) associated with ICIs such as avelumab.27,28 ICIs can result in irAEs such as autoimmune thyroiditis, and hypophysitis.22 Hypothyroidism (0.6%) was the irAE that was most frequently reported.11 One study found that treatment-related adverse effects (TRAEs), including with infusion-related reactions, (2.4%), pyrexia (2.1%), dyspnea (0.9%), and chills (0.9%) were the most common adverse events.11 Adverse events led to treatment discontinuation in 6–11.1% of patients in real-world cohorts.3–13
Rarer side effects such as acute kidney injury, anemia, abdominal pain, ileus, and cellulitis were among the serious adverse events documented in the JAVELIN trial. Myasthenia gravis was observed in one study.9 In extremely rare cases, avelumab may also result in hyperprogression of metastatic MCC as demonstrated by a case report.29 In another case report, a patient with no prior history of neurological disorders, developed diplopia after the fourth dose of avelumab for advanced MCC. On re-initiation of avelumab 7 months later, he developed demyelinating polyneuropathy and cranial neuropathy refractory to immunosuppressive treatments.30 A list of the commonly identified AEs in real-world studies is provided in Table 3.3,9,11–13,21–23
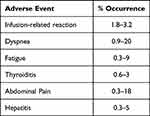 |
Table 3 Adverse Events Recorded in Real-World Studies |
Finally, a multicenter Phase II trial by Nghiem et al noted pembrolizumab, used as a first-line agent, was associated with an ORR of 58% (CR: 30% and PR: 28%) in advanced MCC.31 This response rate was lower than that reported by Bhatia et al with avelumab, where the ORR for avelumab as a first-line agent was 75.3%.22 Glutsch et al report that in a cohort of 14 patients with avelumab-resistant advanced MCC, a combination of ipilimumab and nivolumab resulted in an ORR of 50%.32 Additionally, a systematic review and Bayesian network meta-analysis of ICIs in solid-organ cancers revealed that avelumab was linked to a significantly lower OS compared to nivolumab and pembrolizumab (HR 1.37, 95% CI 1.05–1.78 and HR 1.33, 95% CI 1.02–1.73, respectively).33 Nivolumab significantly outperformed avelumab in terms of progression-free survival (PFS) (HR 1.60, 95% CI 1.03–2.51) as well.33 In terms of safety and tolerability, avelumab was associated with the most favorable safety profile with the least incidence of ≥ grade 3 TRAEs in patients with solid organ tumors.33 However, adverse events associated with avelumab and pembrolizumab seem to be similar in patients with advanced MCC.22,31 Head-to-head comparisons of the safety and efficacy of avelumab and pembrolizumab for advanced MCC in real-world studies are yet to be performed.
Conclusion
Avelumab has demonstrated notable efficacy and a tolerable safety profile for advanced MCC in a variety of real-world clinical settings. The use of avelumab as an alternative to chemotherapy is supported by high response rates, durable responses, and prolonged survival across numerous investigations, including the JAVELIN trial. Real-world data further support its efficacy and safety across a variety of groups and settings. These findings add to the growing body of evidence that supports the use of ICIs in the treatment of MCC, although more study is needed to examine their long-term efficacy and safety profile.
Disclosure
The authors report no conflicts of interest in this work.
References
1. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Merkel cell carcinoma; 2023. Available from: https://merkelcell.org/wp-content/uploads/2022/04/NCCN-Guidelines-for-Merkel-Cell-Carcinoma-v1.2023.pdf.
2. Harvey JA, Mirza SA, Erwin PJ, Chan AW, Murad MH, Brewer JD. Recurrence and mortality rates with different treatment approaches of Merkel cell carcinoma: a systematic review and meta-analysis. Int J Dermatol. 2022;61(6):687–697. doi:10.1111/ijd.15753
3. Cowey CL, Liu FX, Kim R, et al. Real-world clinical outcomes with first-line avelumab in locally advanced/metastatic Merkel cell carcinoma in the USA: SPEAR-Merkel. Future Oncol. 2021;17(18):2339–2350. doi:10.2217/fon-2020-1250
4. Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78(3):457–463.e2. doi:10.1016/j.jaad.2017.10.028
5. Sergi MC, Lauricella E, Porta C, Tucci M, Cives M. An update on Merkel cell carcinoma. Biochim Biophys Acta Rev Cancer. 2023;1878(3):188880. doi:10.1016/j.bbcan.2023.188880
6. Kieny A, Cribier B, Meyer N, Velten M, Jégu J, Lipsker D. Epidemiology of Merkel cell carcinoma. A population-based study from 1985 to 2013, in northeastern of France. Int J Cancer. 2019;144(4):741–745. doi:10.1002/ijc.31860
7. Youlden DR, Soyer HP, Youl PH, Fritschi L, Baade PD. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993–2010. JAMA Dermatol. 2014;150(8):864–872. doi:10.1001/jamadermatol.2014.124
8. Song PI, Liang H, Wei WQ, Jiang YQ, Smith JS, Qiao YL. The clinical profile of Merkel cell carcinoma in mainland China. Int J Dermatol. 2012;51(9):1054–1059. doi:10.1111/j.1365-4632.2011.05251.x
9. Grignani G, Chiarion Sileni V, Pinto C, et al. Avelumab treatment in Italian patients with metastatic Merkel cell carcinoma: experience from an expanded access program. J Transl Med. 2021;19(1):70. doi:10.1186/s12967-021-02730-8
10. Lewis DJ, Sobanko JF, Etzkorn JR, et al. Merkel Cell Carcinoma. Dermatol Clin. 2023;41(1):101–115. doi:10.1016/j.det.2022.07.015
11. Ascierto PA, Orlova K, Grignani G, et al. Avelumab expanded access program in metastatic Merkel cell carcinoma: efficacy and safety findings from patients in Europe and the Middle East. Int J Cancer. 2021;149(11):1926–1934. doi:10.1002/ijc.33746
12. Munhoz RR, Cayol F, Corrales L, et al. Merkel cell carcinoma in Latin America: a contribution from an expanded access program for avelumab to address issues from experts’ recommendations. Cancer Immunol Immunother. 2021;70(4):1031–1036. doi:10.1007/s00262-020-02756-9
13. Averbuch I, Stoff R, Miodovnik M, et al. Avelumab for the treatment of locally advanced or metastatic Merkel cell carcinoma-A multicenter real-world experience in Israel. Cancer Med. 2023;12(11):12065–12070. doi:10.1002/cam4.5890
14. Robertson JP, Liang ES, Martin RC. Epidemiology of Merkel cell carcinoma in New Zealand: a population-based study. Br J Dermatol. 2015;173(3):835–837. doi:10.1111/bjd.13782
15. Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120(4):435–446. doi:10.1111/j.1365-2567.2007.02555.x
16. Moll R, Löwe A, Laufer J, Franke WW. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol. 1992;140(2):427–447.
17. Rastrelli M, Ferrazzi B, Cavallin F, et al. Prognostic factors in Merkel cell carcinoma: a retrospective single-center study in 90 patients. Cancers (Basel). 2018;10(10):350. doi:10.3390/cancers10100350
18. Tarantola TI, Vallow LA, Halyard MY, et al. Prognostic factors in Merkel cell carcinoma: analysis of 240 cases. J Am Acad Dermatol. 2013;68(3):425–432. doi:10.1016/j.jaad.2012.09.036
19. Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133(3):642–646. doi:10.1038/jid.2012.388
20. Wang LL, Lin SK, Stull CM, et al. Cutaneous Oncology in the Immunosuppressed. Dermatol Clin. 2023;41(1):141–162. doi:10.1016/j.det.2022.07.012
21. Levy S, Aarts MJB, Eskens FALM, et al. Avelumab for advanced Merkel cell carcinoma in the Netherlands: a real-world cohort. J Immunother Cancer. 2020;8(2):e001076. doi:10.1136/jitc-2020-001076
22. Bhatia S, Nghiem P, Veeranki SP, et al. Real-world clinical outcomes with avelumab in patients with Merkel cell carcinoma treated in the USA: a multicenter chart review study. J Immunother Cancer. 2022;10(8):e004904. doi:10.1136/jitc-2022-004904
23. Walker JW, Lebbé C, Grignani G, et al. Efficacy and safety of avelumab treatment in patients with metastatic Merkel cell carcinoma: experience from a global expanded access program. J Immunother Cancer. 2020;8(1):e000313. doi:10.1136/jitc-2019-000313
24. Lewis DJ, Fathy RA, Nugent S, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: rates and predictors of compliance with the National Comprehensive Cancer Network guidelines. J Am Acad Dermatol. 2023;88(2):448–450. doi:10.1016/j.jaad.2022.05.054
25. D’Angelo SP, Lebbé C, Mortier L, et al. First-line avelumab in a cohort of 116 patients with metastatic Merkel cell carcinoma (JAVELIN Merkel 200): primary and biomarker analyses of a phase II study. J Immunother Cancer. 2021;9(7):e002646. doi:10.1136/jitc-2021-002646
26. Kaufman HL, Russell JS, Hamid O, et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a Phase 2 clinical trial. J Immunother Cancer. 2018;6(1):7. doi:10.1186/s40425-017-0310-x
27. Kamińska-Winciorek G, Cybulska-Stopa B, Lugowska I, Ziobro M, Rutkowski P. Principles of prophylactic and therapeutic management of skin toxicity during treatment with checkpoint inhibitors. Postepy Dermatol Alergol. 2019;36(4):382–391. doi:10.5114/ada.2018.80272
28. Hanania HL, Lewis DJ. Systematic review of programmed cell death-1 inhibitor therapy for advanced-stage cutaneous squamous cell carcinoma in solid-organ transplant recipients. J Dermatolog Treat. 2022;33(8):3119–3126. doi:10.1080/09546634.2022.2118516
29. Pirker R, Fink A, Stella A, Stifter L, Posch C. Hyperprogression of Merkel cell carcinoma after avelumab treatment. J Eur Acad Dermatol Venereol. 2023;37(5):e675–e677. doi:10.1111/jdv.18887
30. Bilić H, Sitaš B, Hančević M, Habek M, Simetić L, Bilić E. Severe demyelinating polyneuropathy and cranial neuropathy during avelumab treatment of metastatic Merkel cell carcinoma. Clin Neuropharmacol. 2021;44(5):193–195. doi:10.1097/WNF.0000000000000464
31. Nghiem P, Bhatia S, Lipson EJ, et al. Three-year survival, correlates and salvage therapies in patients receiving first-line pembrolizumab for advanced Merkel cell carcinoma. J Immunother Cancer. 2021;9(4):e002478. doi:10.1136/jitc-2021-002478
32. Glutsch V, Schummer P, Kneitz H, et al. Ipilimumab plus nivolumab in avelumab-refractory Merkel cell carcinoma: a multicenter study of the prospective skin cancer registry ADOREG. J Immunother Cancer. 2022;10(11):e005930. doi:10.1136/jitc-2022-005930
33. Al-Showbaki L, Nadler MB, Desnoyers A, Almugbel FA, Cescon DW, Amir E. Network meta-analysis comparing efficacy, safety and tolerability of anti-PD-1/PD-L1 antibodies in solid cancers. J Cancer. 2021;12(14):4372–4378. doi:10.7150/jca.57413
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
