Back to Journals » Vascular Health and Risk Management » Volume 19
Benefits of the Polypill on Medication Adherence in the Primary and Secondary Prevention of Cardiovascular Disease: A Systematic Review
Authors Lopez-Lopez JP , Gonzalez AM , Lanza P , Lopez-Jaramillo P
Received 30 May 2023
Accepted for publication 22 August 2023
Published 12 September 2023 Volume 2023:19 Pages 605—615
DOI https://doi.org/10.2147/VHRM.S421024
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Jose P Lopez-Lopez,1,2,* Ana Maria Gonzalez,1,* Paola Lanza,1 Patricio Lopez-Jaramillo1
1MASIRA Research Institute, Universidad de Santander (UDES), Bucaramanga, Santander, Colombia; 2Department of Internal Medicine. Cardiology Unit, Hospital Universitario San Ignacio, Pontificia Universidad Javeriana, Bogotá, Colombia
*These authors contributed equally to this work
Correspondence: Patricio Lopez-Jaramillo, MASIRA Research Institute, Facultad de Ciencias de la Salud, Universidad de Santander (UDES), Bloque G, piso 6, Bucaramanga, Santander, Colombia, Tel +57 315 306 8939, Email [email protected]
Background: Higher medication adherence reduces the risk of new cardiovascular events. However, there are individual and health system barriers that lead to lower adherence. The polypill has demonstrated benefits in cardiovascular morbidity and mortality mainly driven by an increase in adherence. We aim to evaluate the impact of the polypill on adherence to cardiovascular medication, its efficacy and safety in cardiovascular disease (CVD) prevention.
Methods: A systematic review following PRISMA guidelines was conducted. Databases were searched from January 2003 to December 2022. We included randomized, pragmatic, or real-world clinical trials and observational studies. The primary outcome was medication adherence, secondary outcomes were efficacy in cardiovascular disease in primary and secondary prevention and safety.
Results: From the 490 publications screened, 13 met the inclusion criteria and were incorporated into a comparative table Of those included, 70% were randomized controlled trials (RCTs) and 53.8% focused on secondary prevention. Most of the studies received a high and moderate quality rating. Self-report, pill counting and, the Morisky scale were the most frequent methods to evaluate adherence (84.6%). Compared with standard medication, the polypill improved overall medication adherence by 13%, with percentages ranging from 7.6% to 34.9%. Moreover, a potential benefit was also observed in reducing Major Adverse Cardiovascular Events (MACE), particularly in secondary prevention studies, with hazard ratios ranged between 0.43 to 0.76. Compared to standard care, the profile of side effects was similar.
Conclusion: The polypill is an effective, safe, and practical strategy to improve adherence in people at risk of CVD. Although there is a demonstrated benefit in reducing MACE, predominantly in secondary prevention, there are still gaps in its efficacy in primary prevention and reducing total mortality. Therefore, the importance of obtaining long-term results of the polypill effect and how this strategy can be implemented in real practice.
Keywords: polypill, cardiovascular disease, hypertension, dyslipidemia, major cardiovascular events
A Letter to the Editor has been published for this article.
A Response to Letter by Professor Zhu has been published for this article.
Introduction
Cardiovascular disease (CVD) is the leading cause of total and premature mortality, being ischemic heart disease the most prevalent.1 According to the Global Burden of Disease study, from 1990 to 2019, prevalent cases of total CVD doubled from an estimated 271 million to 523 million worldwide.1 Hypertension is one of the modifiable CVD risk factors with the highest population-attributable fraction for premature cardiovascular death, being responsible for 10.8 million cardiovascular deaths in 2021 followed by diabetes, non-High-density lipoprotein cholesterol (HDL-C), and smoking.2,3 The necessity to achieve better control of modifiable CVD risk factors and improve preventive measures has led to the proposal of several strategies in people with established CVD and people at high risk.4 Although the demonstrated benefits of pharmacological treatment in CVD prevention, its effect in the real world appears to be lower than observed on RCT.5 This discrepancy between efficacy trials and effectiveness trials has been largely attributed to low medication adherence, which has been exacerbated by complex drug regimens with multiple daily intakes.6 Accordingly, a meta-analysis with 376,162 participants reported that overall medication adherence in CVD prevention was 57% at 24 months of follow-up. Adherence was 50% and 66% for primary and secondary prevention studies, respectively.7 These findings are important given that evidence in real-world studies highlights the relationship between low pharmacological adherence and the number of both cardiovascular events and mortality in patients with CVD.5 Consequently, fixed-dose combination therapy has been applied in hypertension management and consistently demonstrated improvement in the control and reduction of the associated major cardiovascular events (MACE).8 In addition, the polypill, defined by the World Heart Federation as a compound of a fixed-dose combination antihypertensive therapy, a statin to reduce low-density cholesterol (LDL-C), and/or low-dose aspirin has been implemented.9 In 2003, Wald and Law initially proposed a polypill strategy that combined six medications in a single pill to control four CVD risk factors (dyslipidemia, hypertension, platelet function, and homocysteine). Polypill use showed the potential for reducing the risk of acute myocardial infarction by 88% and stroke by 80%.10 Subsequently, the effectiveness of the polypill composed of medications with cardiovascular benefits (aspirin, renin-angiotensin-aldosterone system inhibitors, and statins) has been reported in secondary prevention, leading to its approval in more than 30 countries.11 A recent meta-analysis assessing the efficacy of the polypill for the reduction of the risk of mortality and cardiovascular events in participants of RCT showed that although there was no significant difference in the primary outcome, the polypill group had better treatment adherence. These data are interesting; however, as mentioned above, these data do not necessarily reflect real-world behavior.12 Therefore, the main objective of this systematic review is to analyze the potential benefits of the polypill on medication adherence in the primary and secondary prevention of CVD with a broader view of the information by including different types of studies (both efficacy and effectiveness trials, as well as observational studies). In addition, investigated the impact of the polypill on MACE and evaluated its safety when compared with individual components.
Methods
We conducted a systematic review of the literature following PRISMA criteria.13 Initially, PubMed, MEDLINE, EMBASE and Scopus databases were selected to identify articles that covered the PICO question. We identified those that included the following terms: (((polypill) OR (polycap) OR (combination pill) OR (component combination)) AND ((medication adherence) OR (treatment adherence) OR (adherence) OR (compliance) OR (patient compliance))) AND ((cardiovascular disease) OR (cardiovascular diseases) OR (cardiovascular prevention) OR (cardiovascular events) OR (cardiovascular risk)) that had been published between January 2003 and December 2022 in the English language (search strategy in Supplementary Material S1). These search conditions obtained 487 articles and by snowball strategy 3 more articles were collected, giving a total of 490. Of these, 136 duplicates were detected by means of the Rayyan® program and 76 were deleted, giving a new total of 414 articles. Two authors (AMG and PL) with the arbitration of a third reviewer (JPLL) reviewed the titles and abstracts of the articles obtained in the research. The initial selection was made according to the inclusion criteria: randomized, pragmatic, or real-world clinical trials and observational studies that covered the PICO question. Systematic reviews, meta-analyses, review articles, and conference abstracts were excluded. Likewise, those articles that did not comply with the definition of polypill by the World Heart Federation, or the outcomes raised in the research question were excluded. After removing duplicates, 19 articles were eligible for full-text reading, 6 were excluded (1 by manual duplication, 2 by different outcome, 1 for not corresponding to the definition of polypill, and 2 for being a conference abstracts) (Figure 1). The main outcome was medication adherence, secondary outcomes were efficacy in CVD primary and secondary prevention and safety. For the safety outcomes, we assessed the studies that reported side effects. According to the design of each study, checklists were applied to assess the pertinent methodological quality: CONSORT for Clinical Trials and STROBE for observational studies.14,15 In addition, potential bias was also considered among the evaluated items. The score achieved was converted to a percentage and to assess the quality it was stratified into 3 categories: ≥80%, high quality, 79–50% moderate quality, and <49% low quality.
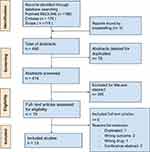 |
Figure 1 Registration format for the article selection process, based on prisma’s systematic methodology format. Note: PRISMA figure adapted from Page et al.13 Creative Commons. |
Results
Thirteen studies were included in the systematic review; of these, four (30.7%) had an observational design (two cross-sectional and two retrospective cohorts), and nine (69.2%) were randomized clinical trials (including efficacy and effectiveness trials). Regarding pharmacological adherence measurement, self-report, pill counting and the Morisky scale were the most used methods (up to 84.6%). The description of polypill components is summarized in Table 1. Of the included studies, the most used polypills were the Red Heart pill (version 1 or 2) (Dr Reddy’s Laboratories Ltd, Hyderabad, India) in 38.4% and the CNIC-FS FERRER (Ferrer Internacional, S.A, Barcelona, Spain) in 15%. Among the included studies, seven (53.8%) focused on secondary prevention, two (15.38%) on primary prevention, and the remaining four (30.7%) included both types of prevention. The results of the outcome of interest are shown in Table 2.
 |
Table 1 Formulation of the Most Used Polypills |
 |
Table 2 Medication Adherence in Studies with Polypill |
Regarding the evaluation of the quality of the articles related to the risk of bias, it was found that 38.46% and 61.53% received a high and moderate quality rating, respectively. None of the studies included obtained a rating of less than 50%. The overall average score was 77%.
Primary Objective: Medication Adherence
The main result shows an increase in medication adherence with polypill intervention and is summarized in Figure 2. In the Neptune retrospective observational study, which included 6456 adults in secondary prevention that already started pharmacological treatment, assessed the effectiveness of CNIC-Polypill (aspirin 100 mg, atorvastatin 20/40 mg, and ramipril 2.5/5/10 mg once daily) in preventing new cardiovascular events compared to three standard medications cohorts (treatment with monocomponent, equipotentials or other therapies). Additionally, its impact on adherence, defined as the days without abandoning or changing the initial treatment at least 30 days after the initial prescription, was evaluated. Patients who received the CNIC-polypill had greater continuity of therapy than the three comparative cohorts (72.1% vs 62.2%, 60.0%, and 54.2%, respectively; p < 0.001).26 Complementarily, the Secondary Prevention of Cardiovascular Disease in the Elderly (SECURE), assessed the efficacy of the Polypill AARR40 (with the same components of the CNIC-Polypill) compared with conventional management in 2499 individuals older than 65 years in secondary prevention. Using the Morisky scale, it was reported that the intervention group had greater adherence than the control group (70% vs 62.7% HR: 1.13; CI 95%: 1.06–1.20) after a mean follow-up of 6 months.27 Meanwhile, the second phase of the Fixed-Dose Combination Study Drug for Secondary Cardiovascular Prevention (FOCUS) showed that the use of the CNIC-FS-FERRER polypill (aspirin 100 mg, simvastatin 40 mg, and ramipril 2.5/5/10 mg) improved by a 22% medication adherence when compared with the use of the three drugs separately (50.8% vs 41%; p = 0.019).22 In the Aurora Study, a multicenter cohort including 366 participants with a history of CVD showed that the polypill regimen composed of aspirin, ramipril, and atorvastatin compliance was 57.7%. In contrast, those who took the pills separately had a 41.3% (p < 0.0001).23 The Evening versus Morning Polypill Utilization Study (TEMPUS) was another RCT that used the Morisky scale, reporting that adherence was 5.2% (95% CI: 1.4–9.1) higher with morning use of the polypill (Red Heart Pill 2) and 5.0% (95%-CI: 1.5–8.5) higher with evening use compared to the individual agents.20 The UMPIRE study, an RCT conducted in Europe and India with 2138 participants with established CVD or at high risk, evaluated two versions of the Red Heart Pill (Dr Reddy’s Laboratories Ltd, Red Heart Pill, Hyderabad, India); version 1 contained aspirin 75 mg, simvastatin 40 mg, lisinopril 10 mg, and atenolol 50 mg, while version 2 contained aspirin 75 mg, simvastatin 40 mg, lisinopril 10 mg, and hydrochlorothiazide 12.5 mg. The polypill group was reported to have higher adherence (86.3%) compared to the standard treatment group (64.7%) (RR: 1.33; 95% CI: 1.26–1.41).17 These findings were consistent with those found in the Kanyini GAP study (using the Red Heart Pill 1), where adherence was significantly greater in the polypill group compared to usual care (70.1% vs 46.9%) (RR: 1.49; 95% IC 1.30 −1.72).18 Similar results were observed in the IMPACT study (who also used the Red Heart Pill 1) carried out in New Zealand with 513 participants in primary and secondary prevention, where a higher proportion of adherence was reported in the polypill group (81%) compared to treatment alone (46%) (RR: 1.75; 95% IC 1.52–2.03).19 In a real-world retrospective study conducted with 104 patients with a history of stroke, an adherence (measured by the Morisky scale) of 93% in the polypill group (same components of the CNIC-Polypill) and 88% in usual management was reported.25 In contrast, the TIPS-3 study, an RCT with a factorial design that included 5713 adults with intermediate or high INTERHEART score recibing the Polycap (Cadila Pharmaceuticals, Polycap™, Ahmedabad, India) containing 40 mg of simvastatin, 100 mg of atenolol, 25 mg of hydrochlorothiazide, and 10 mg of ramipril compared with placebo showed that both arms had a similar proportion of adherence (81%) at 24-month follow-up.16 Also, the multicap study involved 100 patients with a history of acute myocardial infarction within the last 7 days and were randomized and assigned to either the Multi-cap (Hospital El Cruce, Buenos aires, Argentina, composed of aspirin 100 mg, atenolol 50 or 100 mg, ramipril 5 or 10 mg, and simvastatin 40 mg) or the control group. At 6 months, 92 (95.8%) patients were adherent to medical treatment, 98.0% in the multicap group and 93.5% in the control group (RR: 1.05; 95% IC 0.96–1.14; p = 0.347).28
 |
Figure 2 Medication adherence of polypill intervention compared to standard care. |
Secondary Objective: MACE
Primary Prevention
The TIPS-3 study,16 showed that after a follow-up of 4.6 years, the Polycap with the addition of aspirin reduced the primary composite outcome of cardiovascular death, acute myocardial infarction, stroke, heart failure and cardiac arrest by 31% compared to the control group (HR: 0.69; 95% CI: 0.50–0.97).16 When analyzing by subgroups (polypill with or without aspirin), a significant reduction in the primary outcome was also evidenced (HR: 0.53; 95% CI: 0.41–0.67 and HR 0.68; 95% CI: 0.57–0.81, respectively).16 In contrast, the UMPIRE, KAYINI and IMPACT clinical trials that included patients in both primary and secondary prevention found no significant differences regarding development of MACE; (RR: 1.45; 95% CI 0.94–2.24), (RR: 1.15; 95% CI: 0.65, 2.03) and (p = 0.73), respectively.17–19
Secondary Prevention
The Neptuno study showed that the incidence of recurrent MACE was lower in the CNIC-Polypill cohort (19.8%) compared to the cohorts treated with monocomponent (23.3%), equipotent (25.5%) and other therapies (27%) (p < 0.001).26 Additionally, in the SECURE study, the polypill intervention achieved a relative risk reduction of 24% (HR: 0.76; 95% CI: 0.60–0.90) of new MACE at three years of follow-up.27 Similarly, the PolyIran RCT involving 13,875 participants with or without a history of CVD from the Golestan cohort randomized 6838 individuals to use the polypill (Alborz Darou Pharmaceutical Company; Tehran, Iran) or care minimal. Initially, polypill number one (hydrochlorothiazide 12.5 mg, aspirin 81 mg, atorvastatin 20 mg, and enalapril 5 mg) was used. If participants developed a cough, they were switched to polypill number two, replacing enalapril with valsartan 40 mg. The study reported a reduction in CVD risk in the polypill group compared to the control group (HR: 0.66; 95% CI: 0.55–0.80). Strikingly, participants who achieved greater adherence using the polypill had an even lower CVD risk (HR: 0.43; 95% CI: 0.33–0.55), with a number needed to treat of 20.7 (17.5–26.5) to prevent one MACE.24 In contrast, the study of Ros-Castello et al showed that stroke recurrence only occurred in one patient in the usual treatment group vs none in the polypill group.25
Side Effects
The side effects most frequently documented in the studies were, in frequency order: cough (53.8%), dizziness (46%), hypotension (30.7%), musculoskeletal symptoms (23%), dyspepsia or epigastric pain (15%), a detailed description of the side effects in each study is summarized in Table S1. However, there was no statistical difference in the intergroup comparison, and the use of polypill did not represent a substantial reason for discontinuation of therapy. Additionally, regarding safety, most studies described that the number of patients with serious adverse events was not statistically different but minimal and comparable between groups.16–22,24,25,28
Discussion
In this systematic review it was observed that the polypill is a reliable and effective strategy that improves medication adherence in primary and secondary CVD prevention and has a potential impact in the reduction of MACE. In terms of safety, no statistical differences were found in adverse events or discontinuation rates due to side effects.
Organizations such as the World Heart Federation have described the barriers by which individuals in low- and middle-income countries with established CVD or at-risk lack of adequate CVD risk factors control. Barriers at the individual level include low health literacy and beliefs about the long-term benefits of medicines.6 However, CVD risk factors control depends not only on the individual but also on the availability and access to medicines each country has. The PURE study reported that in 90% of the communities studied, there was at least one drug to lower blood pressure, but the availability of more than two classes of antihypertensive drugs was less in low- and middle-income countries without ensuring access to it.29 Low adherence is also enhanced by a lack of health resources (training, medication, and equipment), entailing difficulties in monitoring, titration, or optimizing the management of CVD risk factors.30 A qualitative study conducted in Colombia explored patients’ knowledge, attitudes, behavior, and healthcare-seeking experiences concerning the detection, treatment, and control of hypertension and reported that barriers to difficult medication adherence include costs, transportation to recall medication, and unavailability of main antihypertensive drugs. Another barrier are the complex pharmacological regimens, in which multiple intakes of different groups of medications contributes to low adherence.6 Therefore, the implementation of the polypill strategy could meet the objectives of simplifying the medication regimens and increasing its availability. Both objectives are necessary to reduce the burden of CVD.
In our results, most studies showed that polypill increased medication adherence. A meta-analysis of 20 studies with a total of 376,162 individuals with or without a history of ischemic heart disease showed an adherence to pharmacological treatment of 50% and 66% in primary and secondary prevention, respectively.8 This suggests the need for measures to improve adherence that do not depend exclusively on the prescribed drug class. Additionally, a systematic review and meta-analysis of 8 RCTs, including 25,584 adults with or without established CVD, evaluated the impact of polypill use on cardiovascular outcomes, mortality, and adherence. Consistent with our results it was shown that the polypill significantly improved treatment adherence compared to usual treatment (HR: 1.31; 95% CI: 1.11–1.55).31 It should be noted that this meta-analysis was limited to RCTs, whereas our review provides data from both RCTs (including pragmatic and real-world studies) and observational studies. In addition, adherence evaluated was a secondary outcome, mentioned in only 5 of the 8 RCTs included, while our review focuses on pharmacological adherence to the polypill as the primary objective, which was mentioned in the 13 studies involved.31 Therefore, simplifying the pharmacological regimen improves adherence by the ease of prescription, overcoming physician inertia, patient acceptability, packaged delivery, and ease of taking a single pill. All these advantages of the polypill are impacting in the reduction in CV events.
The landmark meta-analysis by Law and Wald that included 354 RCTs, of which 50 studies compared drugs from two or more categories (aspirin, thiazide, β-blocker, angiotensin-converting enzyme inhibitors, statin, and folic acid) showed that the combination of three drugs at half the standard dose achieved a 63% reduction in the risk of CVD and a 46% reduction in ischemic heart disease events.32 This led to the suggestion of using combinations of two or three drugs in low doses due to their similar efficacy and low prevalence of adverse effects compared to standard treatment. Since then, multiple studies have been conducted to evaluate this concept, including The International Polycap Study (TIPS) series of studies. In 2009, the results of the Phase II TIPS-1 study, which had a factorial design and evaluated the Polycap. In 2053 adults with at least one CVD risk factors, after 8 weeks of follow-up the Polycap intervention achieved the objective of lowering LDL-C levels similarly to standard treatment.33 Subsequently, in the TIPS-2 study, the doses of the polypill were doubled. It was shown that the new version further reduced levels of LDL-C and, consequently, a greater relative reduction in cardiovascular risk.34 Moreover, in 2021, the TIPS-3 study showed the positive contribution of adding aspirin to the polypill in the primary prevention of CVD.16
Also, a meta-analysis of 3 RCTs (TIPS-3, HOPE-3, and PolyIran) with 18,162 adults at 10-year intermediate cardiovascular risk (calculated by Framingham score) reported that the polypill strategy (two antihypertensive agents plus a statin with or without aspirin) decreases the MACE compound by 48% compared to standard treatment (p <0.0001).35 When analyzing by subgroups (polypill with or without aspirin), a significant reduction in the primary outcome was also evidenced (HR: 0.53; 95% CI: 0.41–0.67 and HR 0.68; 95% CI: 0.57–0.81, respectively). Therefore, it was concluded that fixed-dose combination therapy in primary prevention substantially reduces MACE with greater reductions in those receiving aspirin.35
In contrast to our results the meta-analysis of Rao et al31 in adults with or without established CVD found no differences in secondary prevention (p = 0.538). However, this analysis had a significant degree of heterogeneity in several studies included, especially when evaluating adherence and treatment discontinuation.29 The results of the studies included in our systematic review demonstrate that the polypill strategy improves adherence to drug treatment and impacts the secondary prevention of CVD.
One of the strengths of the present review is to provide information from original articles with moderate and high quality that support the evidence of the beneficial effect of the polypill on medication adherence in the population with or at risk of CVD, as well as documentation of the safety of the intervention. Most of the articles included correspond to RCTs, which, allow us to propose a safe and effective alternative for the control of CVD risk factors. In addition, the review was performed under the methodological quality criteria defined by PRISMA.
The limitations of our study are the reduced number of articles finally included. In addition, not all the included articles reported the effect size of the primary objective. It is worth noting that heterogeneity in the type of tests used to measure pharmacological adherence can influence the results, especially those self-reporting tools that tend to overestimate the effect size of primary objective. Likewise, sample size and selection bias documented in some of the studies are also obstacles when generalizing the data. Finally, using the STROBE and CONSORT lists instead of other more suitable methods, such as the Effective Public Health Practice Project (EPHPP) or JBI tools, is a limitation for the quality assessment. Besides, the thresholds used to define high, moderate, and low quality were arbitrary. However, the results are convincing and reinforce the findings at the level of controlled studies such as observational studies.
Conclusion
The polypill is an effective, safe, and practical strategy to improve pharmacological adherence in people at risk of CVD. Although there is a demonstrated benefit in reducing MACE, predominantly in secondary prevention, there are still gaps in its efficacy in primary prevention and reducing total mortality. Therefore, the importance of obtaining long-term results of the polypill effect. The polypill strategy has the potential to break down barriers to the control of CVD risk factors, opening a new challenge for the application of these interventions in the real world and consequently in daily practice.
Acknowledgments
We thank Dr Gary O´Donovan of Universidad de Santander for helping us to revise our manuscript.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Khan MA, Hashim MJ, Mustafa H, et al. Global epidemiology of ischemic heart disease: results from the global burden of disease study. Cureus. 2020;12(7):e9349.
2. Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022;80(25):2361–2371.
3. Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795–808.
4. Whelton SP, McEvoy JW, Shaw L, et al. Association of normal systolic blood pressure level with cardiovascular disease in the absence of risk factors. JAMA Cardiol. 2020;5(9):1011–1018.
5. Chen C, Li X, Su Y, You Z, Wan R, Hong K. Adherence with cardiovascular medications and the outcomes in patients with coronary arterial disease: “Real‐world” evidence. Clin Cardiol. 2022;45(12):1220–1228.
6. Jeemon P, Severin T, Amodeo C, et al. World heart federation roadmap for hypertension - a 2021 update. Glob Heart. 2021;16(1):63.
7. Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med. 2012;125(9):882–887.e1.
8. Unger T, Borghi C, Charchar F, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357.
9. World Heart Federation. The polypill could avoid millions of premature deaths, heart attacks and strokes every year, say leading cardiology experts; 2021. Available from: https://world-heart-federation.org/news/the-polypill-could-avoid-millions-of-premature-deaths-heart-attacks-and-strokes-every-year-say-leading-cardiology-experts.
10. Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80%. BMJ. 2003;326(7404):1419.
11. Sosa-Liprandi A, Sosa-Liprandi MI, Alexanderson E, et al. Clinical impact of the polypill for cardiovascular prevention in latin america: a consensus statement of the inter-American society of cardiology. Glob Heart. 2019;14(1):3–16 e1.
12. Abushouk AI, Sayed A, Munir M, et al. Fixed-dose combination (polypill) for cardiovascular disease prevention: a meta-analysis. Am J Prev Med. 2022;63(3):440–449.
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
14. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340(mar23 1):c869.
15. Von Elm E, Altman DG, Egger M. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:7626.
16. Yusuf S, Joseph P, Dans A, et al. Polypill with or without Aspirin in Persons without Cardiovascular Disease. N Engl J Med. 2021;384(3):216–228.
17. Thom S, Poulter N, Field J, et al. Effects of a fixed-dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE randomized clinical trial. JAMA. 2013;310(9):918–929.
18. Patel A, Cass A, Peiris D, et al. A pragmatic randomized trial of a polypill-based strategy to improve use of indicated preventive treatments in people at high cardiovascular disease risk. Eur J Prev Cardiol. 2015;22(7):920–930.
19. Selak V, Elley CR, Bullen C, et al. Effect of fixed dose combination treatment on adherence and risk factor control among patients at high risk of cardiovascular disease: randomised controlled trial in primary care. BMJ. 2014;348:g3318.
20. Lafeber M, Grobbee DE, Schrover IM, et al. Comparison of a morning polypill, evening polypill and individual pills on LDL-cholesterol, ambulatory blood pressure and adherence in high-risk patients; a randomized crossover trial. Int J Cardiol. 2015;181:193–199.
21. Soliman EZ, Mendis S, Dissanayake WP, et al. A Polypill for primary prevention of cardiovascular disease: a feasibility study of the World Health Organization. Trials. 2011;12(1):5675.
22. Castellano JM, Sanz G, Penalvo JL, et al. A polypill strategy to improve adherence: results from the FOCUS project. J Am Coll Cardiol. 2014;64(20):2071–2082.
23. Cosin-Sales J, Murcia-Zaragoza JM, Pereyra-Rico HO, la Guia-Galipienso F, Hermans K, Rubio G. Evaluating patients’ satisfaction and preferences with a secondary prevention cardiovascular polypill: the Aurora Study. J Comp Eff Res. 2021;10(13):975–985.
24. Roshandel G, Khoshnia M, Poustchi H, et al. Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): a pragmatic, cluster-randomised trial. Lancet. 2019;394(10199):672–683.
25. Ros-Castello V, Natera-Villalba E, Gomez-Lopez A, et al. Use of the cardiovascular polypill in secondary prevention of cerebrovascular disease: a real-life tertiary hospital cohort study of 104 patients. Cerebrovasc Dis Extra. 2020;10(3):166–173.
26. Gonzalez-Juanatey JR, Cordero A, Castellano JM, et al. The CNIC-Polypill reduces recurrent major cardiovascular events in real-life secondary prevention patients in Spain: the NEPTUNO study. Int J Cardiol. 2022;361:116–123.
27. Castellano JM, Pocock SJ, Bhatt DL, et al. Polypill strategy in secondary cardiovascular prevention. N Engl J Med. 2022;387(11):967–977.
28. Mariani J, Rosende A, De Abreu M, et al. Multicap to improve adherence after acute coronary syndromes: results of a randomized controlled clinical trial. Ther Adv Cardiovasc Dis. 2020;14:175394472091207.
29. Attaei MW, Khatib R, McKee M, et al. Availability and affordability of blood pressure-lowering medicines and the effect on blood pressure control in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet Public Health. 2017;2(9):e411–e9.
30. Legido-Quigley H, Camacho Lopez PA, Balabanova D, et al. Patients’ Knowledge, Attitudes, Behaviour and Health Care Experiences on the Prevention, Detection, Management and Control of Hypertension in Colombia: a Qualitative Study. PLoS One. 2015;10(4):e0122112.
31. Rao S, Jamal Siddiqi T, Khan MS, et al. Association of polypill therapy with cardiovascular outcomes, mortality, and adherence: a systematic review and meta-analysis of randomized controlled trials. Prog Cardiovasc Dis. 2022;73:48–55.
32. Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326(7404):1427.
33. Indian Polycap S, Yusuf S, Pais P, et al. Effects of a polypill (Polycap) on risk factors in middle-aged individuals without cardiovascular disease (TIPS): a Phase II, double-blind, randomised trial. Lancet. 2009;373(9672):1341–1351.
34. Yusuf S, Pais P, Sigamani A, et al. Comparison of risk factor reduction and tolerability of a full-dose polypill (with potassium) versus low-dose polypill (polycap) in individuals at high risk of cardiovascular diseases: the Second Indian Polycap Study (TIPS-2) investigators. Circ Cardiovasc Qual Outcomes. 2012;5(4):463–471.
35. Joseph P, Roshandel G, Gao P, et al. Fixed-dose combination therapies with and without aspirin for primary prevention of cardiovascular disease: an individual participant data meta-analysis. Lancet. 2021;398(10306):1133–1146.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
