Back to Journals » Neuropsychiatric Disease and Treatment » Volume 10
Cerebral palsy in Al-Quseir City, Egypt: prevalence, subtypes, and risk factors
Authors El-Tallawy H, Farghaly W , Shehata G, Rageh T, Metwally N, Badry R, Sayed M, Abd El Hamed M, Abdelwarith A, Kandil M
Received 22 December 2013
Accepted for publication 13 February 2014
Published 8 July 2014 Volume 2014:10 Pages 1267—1272
DOI https://doi.org/10.2147/NDT.S59599
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Hamdy N El-Tallawy,1 Wafaa MA Farghaly,1 Ghaydaa A Shehata,1 Tarek A Rageh,1 Nabil A Metwally,2 Reda Badry,1 Mohamed AM Sayed,3 Mohamed Abd El Hamed,1 Ahmed Abd-Elwarth,2 Mahmoud R Kandil1
1Department of Neurology, Assiut University, 2Department of Neurology, El Azhr University, Assiut Branch, Assuit, 3Department of Neurology, Sohag University, Solag, Egypt
Abstract: Cerebral palsy (CP) is the most frequent cause of motor handicap. The present door-to-door survey was conducted in Al-Quseir City, Egypt, to investigate the epidemiology of CP. All inhabitants were screened by three neurologists. Medical and neurological examinations were performed for all residents and suspected cases of CP were confirmed by meticulous neurological assessment, brain magnetic resonance imaging, electroencephalography, and testing with the Stanford-Binet Intelligence Scale. Forty-six of 12,788 children aged ≤18 years were found to have CP, yielding a childhood prevalence of 3.6 (95% confidence interval 1.48–2.59) per 1,000 live births. Five adults (aged 19–40 years) among 13,056 inhabitants had CP, giving an adult prevalence of 0.4 (95% confidence interval 0.04–0.72) per 1,000. The risk factors for CP identified in this study were premature birth, low birth weight, neonatal jaundice, neonatal seizures, and recurrent abortion in mothers of children with CP.
Keywords: cerebral palsy, prevalence, subtypes, risk factors, Egypt
Introduction
Cerebral palsy (CP) is essentially a permanent disorder affecting movement and posture, causing limitations in activity due to nonprogressive disturbances that occur in the developing fetal or immature infant brain.1 CP is one of the most common causes of physical disability in childhood, with a reported prevalence of approximately 1.5–3 per 1,000.2–4 El-Tallawy et al reported that 52 of 25,540 children in Al-Karga District, Egypt, had CP, giving a prevalence of 2.04 (95% confidence interval 1.48–2.59) per 1,000 live births.5 The subtypes and severity of CP vary between studies, as does the proportion of patients with associated impairments, and this variation is likely to be due to differences in the diagnostic criteria and classification used.6
Surveillance of Cerebral Palsy in Europe has agreed on a definition of CP, and has suggested a revised classification of subtypes that may be less dependent on subjective judgment.7 Although the precise number of adults with cerebral palsy is not known,8 it was estimated in 1990 that 90% of children with cerebral palsy survived until the age of 20 years.9
CP is associated with prenatal, perinatal, and neonatal risk factors.10,11 Premature birth is recognized as the main risk factor for CP, while perinatal asphyxia accounts for less than 10%–20% of cases.12 The aim of this study was to determine the prevalence and subtypes of CP and risk factors for the disease among children and adults in El-Quseir City, located in the Red Sea Governorate in Egypt.
Materials and methods
Study design
The study took the form of a door-to-door survey (every household included) among the population of Al-Quseir City, and was approved by the ethics committee of Assiut University. Informed written consent was obtained from the Health Institute of the Red Sea Governorate and from the responsible person in each patient’s family.
Case ascertainment and classification
CP is divided into spastic, dyskinetic, and ataxic subtypes.7 The spastic subtype is further divided into a hemiplegic or unilateral type (limb involvement on one side of the body) and a bilateral type (limb involvement on both sides of the body). In this study, the spastic bilateral type was further subdivided into quadriplegia and diplegia.5
Participants and recruitment
All individuals who had been living in Al-Quseir City for at least 6 months at the time of interview were included as a sampling frame to identify the potentially eligible population of children and adults with CP in the defined area. Screening of all households was done using a simple standardized Arabic screening questionnaire designed specifically for this study to identify neurological disorders including CP, as described previously.5 CP, according to Andersen et al7 comprises a group of permanent and nonprogressive disorders of movement and posture caused by a lesion, damage, or dysfunction of the central nervous, originating early in life. In the second stage of the study, suspected positive cases of CP were invited to attend Al-Quseir Hospital, where they underwent meticulous medical and neurological evaluation using a specifically prepared sheet.
Interictal electroencephalography and computed tomography head scans or cranial magnetic resonance imaging (MRI) findings were evaluated in all subjects with CP. Cranial computed tomography was performed with 1 cm thick slices using a Radix Turbo scanner (Hitachi, Tokyo, Japan), brain MRI was done with T1-weighted and T2-weighted axial and coronal images and T1-weighted sagittal images using a Picker 1 Tesla Vista HPQ (Picker Corporation, New York, NY, USA).
Associated impairment
Cognitive development was assessed using the standardized, validated Arabic version13 of the Stanford Binet Intelligence Scale (Fourth Edition).14 Total intelligence quotient (IQ) was classified according to Melika13 as: mentally retarded (IQ ≤67), slow learner (IQ 68–78), below average intelligence (IQ 79–88), average intelligence (IQ 89–110), above average intelligence (IQ 111–120), high intelligence (IQ 121–131), and genius (IQ ≥132). Stanford Binet Intelligence Scale testing was done in only 24 of 51 patients (47.05%). IQ assessment was not done in 15 children who were under the age of 4 years, in five who refused to take the test, and in a further seven who were deaf mute.
Epilepsy was diagnosed on clinical grounds and on electroencephalographic changes. The diagnosis was confirmed according to Commission of Classification and Terminology of the International League against Epilepsy.15 Active epilepsy is considered to exist when two or more unprovoked seizures have occurred during the previous year.5,7
Risk factors
A structured questionnaire was completed on direct interview with the responsible personnel in each family. Perinatal data were obtained, including toxemia of pregnancy, toxins, and maternal exposure to radiation, medication, or trauma during pregnancy.5,12,16–19 A detailed pregnancy history was taken and any complications at delivery were noted. Complications in the neonatal period, such as premature labor, low birth weight, and neonatal jaundice, seizures, or cyanosis, were also taken in consideration. In addition, data on any history of previous abortion, family history of similar conditions, parental consanguinity, and parental age at delivery were collected for children with CP. We then applied the same questionnaire in a control group comprising 180 healthy children matched for age, sex, educational level, and socioeconomic status from the same population.
Sample size
In total, 33,285 residents of Al-Quseir City were screened. 12,788 were children (≤18 years of age), resident in the area of the study; all were included in this study. 13,056 were adult (19–40 years) inhabitants. No case was recorded in the age group of more than 40 years (7,441).
Study area
The Red Sea Governorate is the largest governorate in Egypt. All its cities lie directly on the Red Sea. The study area of Al Quseir City is one of largest cities of the Red Sea Governorate, which has a land surface area about 119,000 km2.20
Statistical analysis
SPSS version 16 (SPSS Inc., Chicago, IL, USA) was used for the data analysis. Prevalence rates are presented with exact binomial 95% confidence intervals. The chi-squared test, independent-samples t-test, and one-way analysis of variance followed by the post hoc test (least significant difference) were used to analyze differences in proportions between groups. A P-value <0.05 was considered to indicate a statistically significant difference.
Results
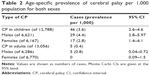
Detailed demographic data on CP, MRI findings, subtype of CP, and IQ are shown in Table 1. The lifetime prevalence of CP per 1,000 live births is shown in Table 2. Of the 12,788 children aged ≤18 years, 46 were diagnosed to have CP, yielding an age-specific prevalence rate of 3.6 per 1,000 live births. Five of the 13,056 adults were diagnosed to have CP, yielding an age-specific prevalence rate of 0.4 per 1,000. In our study, there were no adult cases aged 40 years or older. Prematurity was the most significant risk factor for CP when compared with the control group; this and other risk factors are shown in Table 3. The prevalence of CP in this study is compared with that in other studies worldwide in Table 4.
  | Table 4 Worldwide prevalence of cerebral palsy |
Discussion
CP is a complex disability syndrome that primarily involves impaired control of movement, and is frequently accompanied by lifelong neurological and psychosocial problems.2 In this study, the prevalence of CP in children was 3.6 per 1,000, which is higher than that recorded in Al-Kharga, Egypt,5 and in other studies worldwide.1,5,7,21–24 The high prevalence of CP in our study area may be attributed to several factors. First, an improved survival rate of preterm and low birth weight infants has been reported.25–27 Second, inclusion of mild CP cases in our study may have elevated the prevalence of CP, whereas other studies have focused only on “disabled” children. Third, a lack of neonatal intensive care facilities may have had an impact on local CP rates over time, but this influence is difficult to assess. Fourth, the prevalence of CP seems to have fluctuated over time, more than can be explained by simple random variation. This variation may be related to the level of health care and mortality rates in the study areas.28
In our study, the prevalence of CP in adults was 0.4 per 1,000. Evans et al9 have reported a 90% survival rate in children with CP who were followed until the age of 20 years, but the precise number of adults with CP is not known.8 According to our results, the prevalence of CP in children (3.6 per 1,000 live births) was higher than that in adults (0.4 per 1,000 live births). Unfortunately, there are no databases or reports in Egypt from which to obtain information on the number of children dying of probable CP annually, and the most serious cases are likely to die just after birth. In addition, increased survival rate of children with CP in recent years can be attributed to eating a well balanced diet. Children with profound mental retardation and multiple physical disabilities (including CP) did not survive to adulthood so easily in the past.8 Further, adults with a disability may have left to look for work or seek other services outside the study area, so could not be included in our prevalence data.
Diplegia and quadriplegia, considered to be the most severe form of motor impairment arising from CP, was the most common subtype in our study (72.5%). That was followed by mixed type (23.5%), and ataxic type (3.9%). The proportion of children with various subtypes of CP in this study was similar to the proportions reported by other researchers.5–7,29
Active epilepsy was found in 47.1% and mental retardation in 37.7% of subjects with CP in this study. These high rates could be explained by the fact that children with bilateral CP might suffer from extensive brain injury, including to the cortex, deep white matter, and central nuclei, and therefore are more liable to have mental retardation and epilepsy.7,30
Whether the insult producing CP acts during the peripartum period is a matter of speculation.31 In this study, prematurity, low birth weight, recurrent abortion, neonatal seizures, and jaundice were significantly more common in the CP group than in the control group. This finding is consistent with other reports of significantly higher rates of prematurity and low birth weight in patients with CP.18,31–33
The prevalence of CP among preterm children has risen due to the effect of improved neonatal intensive care management during recent years, leading to increasing survival of children born extremely preterm.34 Genetic factors are believed to play an important role in prematurity and CP generally.23 Possible causes of CP related to early (premature) birth involve development of the brain. Babies born too early are at risk for intraventricular hemorrhage, ie, bleeding in the brain. Periventricular leukomalacia, which reflects injury to the white matter of the brain, is also more likely in babies born prematurely than in those born at term. Both intraventricular hemorrhage and periventricular leukomalacia increase the risk of CP.12,19 In addition, children born preterm have a combination of antenatal and perinatal risk factors, and possible combination of interdependent risk factors were observed more often.32 Therefore, early intervention programs such as the massage intervention developed by Guzzetta et al35 based on manipulation of the extrauterine environment, have been used in preterm infants with the aim of improving development and functional outcomes.
Jaundice is caused by excessive bilirubin in the blood. Normally, bilirubin is filtered by the liver. However, it takes a few days for the neonatal liver to start doing this effectively, so it is not uncommon for infants to have jaundice for a few days after birth. In most cases, jaundice can be treated successfully with phototherapy and have no lasting health effects. However, in rare cases, severe untreated jaundice can damage brain cells.36 Prolonged jaundice may be responsible for CP.36 Meconium staining of the amniotic fluid is likely to indicate intrapartum hypoxia, and is more common in CP cases. We also found that meconium staining of amniotic fluid was more frequent in children with CP. Jaundice was also significantly more common in CP cases, and given that jaundice is treatable, early treatment is advised to prevent CP. A neonatal seizure history was more common in children with CP in this study, again consistent with previous research.33,37 Recurrent previous abortion was more common in mothers of CP cases than in those of controls. Recurrent previous abortion is considered to be a risk factor for CP and may increase the risk of preterm birth. This was in accordance with the finding of reviews in this century.38,39 A review by Rooney and Calhoun38 which included 49 meta-analyses, found that previous abortion increases the risk of preterm birth, and Thorp et al39 also found a higher risk of premature birth in women with prior abortion.
Conclusion
The prevalence of CP in children (46/12,788) was 3.6 (95% confidence interval 2.6–4.6) per 1,000. The prevalence of CP in adults (five per 13,056) was 0.4 (95% confidence interval 0.04–0.72) per 1,000. The most common subtype was spastic. Significant risk factors of CP were prematurity and low birth weight, neonatal seizures, jaundice, and recurrent abortion in mothers of children with CP.
Disclosure
The authors report no conflicts of interest in this work.
References
Park MS, Kim SJ, Chung CY, Kwon DG, Choi IH, Lee KM. Prevalence and lifetime healthcare cost of cerebral palsy in South Korea. Health Policy. 2011;100(2–3):234–238. | ||
McCullough N, Parkes J, Kerr C, McDowell BC. The health of children and young people with cerebral palsy: a longitudinal, population-based study. Int J Nurs Stud. 2013;50(6):747–756. | ||
Blair E, Watson L. Epidemiology of cerebral palsy. Semin Fetal Neonatal Med. 2006;11(2):117–125. | ||
Odding E, Roebroeck ME, Stam HJ. The epidemiology of cerebral palsy: incidence, impairments and risk factors. Disabil Rehabil. 2006;28(4):183–191. | ||
El-Tallawy HN, Farghaly WM, Shehata GA, Metwally NA, Rageh TA, Abo-Elfetoh N. Epidemiology of cerebral palsy in El-Kharga District-New Valley (Egypt). Brain Dev. 2011;33(5):406–411. | ||
Himmelmann K, Beckung E, Hagberg G, Uvebrant P. Gross and fine motor function and accompanying impairments in cerebral palsy. Dev Med Child Neurol. 2006;48(6):417–423. | ||
Andersen GL, Irgens LM, Haagaas I, Skranes JS, Meberg AE, Vik T. Cerebral palsy in Norway: prevalence, subtypes and severity. Eur J Paediatr Neurol. 2008;12(1):4–13. | ||
Rapp CE Jr, Torres MM. The adult with cerebral palsy. Arch Fam Med. 2000;9(5):466–472. | ||
Evans PM, Evans SJ, Alberman E. Cerebral palsy: why we must plan for survival. Arch Dis Child. 1990;65(12):1329–1333. | ||
Cans C, Surman G, McManus V, Coghlan D, Hensey O, Johnson A. Cerebral palsy registries. Semin Pediatr Neurol. 2004;11(1):18–23. | ||
Stromberg B, Dahlquist G, Ericson A, Finnstrom O, Koster M, Stjernqvist K. Neurological sequelae in children born after in-vitro fertilisation: a population-based study. Lancet. 2002;359(9305):461–465. | ||
Drougia A, Giapros V, Krallis N, et al. Incidence and risk factors for cerebral palsy in infants with perinatal problems: a 15-year review. Early Hum Dev. 2007;83(8):541–547. | ||
Melika LK. The Stanford Binet Intelligence Scale. 4th ed. In: Arabic Examiner’s Handbook. Egypt, Cairo: Dar El Maref Publishing; 1998. | ||
Delany EA, Hopkins TF. The Stanford Binet Intelligence Scale. 4th edition. In: Examiner’s Handbook. Chicago, IL, USA: The Riverside Publishing Co; 1986. | ||
[No authors listed]. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Commission of Classification and Terminology of the International League against Epilepsy. Epilepsia. 1981;22(4):489–501. | ||
Himpens E, Oostra A, Franki I, Vansteelandt S, Vanhaesebrouck P, den Broeck CV. Predictability of cerebral palsy in a high-risk NICU population. Early Hum Dev. 2011;86(7):413–417. | ||
Himpens E, Oostra A, Franki I, Van Maele G, Vanhaesebrouck P, Van den Broeck C. Predictability of cerebral palsy and its characteristics through neonatal cranial ultrasound in a high-risk NICU population. Eur J Pediatr. 2011;169(10):1213–1219. | ||
Taylor CL, de Groot J, Blair EM, Stanley FJ. The risk of cerebral palsy in survivors of multiple pregnancies with cofetal loss or death. Am J Obstet Gynecol. 2009;201(1):e41–e46. | ||
Skrablin S, Maurac I, Banovic V, Bosnjak-Nadj K. Perinatal factors associated with the neurologic impairment of children born preterm. Int J Gynaecol Obstet. 2008;102(1):12–18. | ||
Gaber SM, Aly SM, Masood KA. Center of information and decision on making, Red Sea Governorate achievement index. Red Sea Governorate, Egypt: 2010. Arabic. | ||
Limperopoulos C, Majnemer A, Shevell MI, et al. Predictors of developmental disabilities after open heart surgery in young children with congenital heart defects. J Pediatr. 2002;141(1):51–58. | ||
Surveillance of Cerebral Palsy in Europe. Prevalence and characteristics of children with cerebral palsy in Europe. Dev Med Child Neurol. 2002;44:633–640. | ||
Stanley F, Blair E, Alberman E. Cerebral Palsies: Epidemiology and Causal Pathways. London, UK: MacKeith Press; 2000. | ||
Al-Rajeh S, Bademosi O, Awada A, et al. Community survey of neurological disorders in Saudi Arabia: results of the pilot study in Agrabiah. Ann Saudi Med. 1995;15(1):32–35. | ||
Vincer MJ, Allen AC, Joseph KS, Stinson DA, Scott H, Wood E. Increasing prevalence of cerebral palsy among very preterm infants: a population-based study. Pediatrics. 2006;118(6):e1621–e1626. | ||
Topp M, Uldall P, Langhoff-Roos J. Trend in cerebral palsy birth prevalence in eastern Denmark: birth-year period 1979–86. Paediatr Perinat Epidemiol. 1997;11(4):451–460. | ||
Bhushan V, Paneth N, Kiely JL. Impact of improved survival of very low birth weight infants on recent secular trends in the prevalence of cerebral palsy. Pediatrics. 1993;91(6):1094–1100. | ||
Cans C, Cruz J, Mermet M. Epidemiology of cerebral palsy. Paediatr Child Health. 2008;18(9):393–398. | ||
Chan HS, Lau PH, Fong KH, Poon D, Lam CC. Neuroimpairment, activity limitation, and participation restriction among children with cerebral palsy in Hong Kong. Hong Kong Med J. 2005;11(5):342–350. | ||
Jaseja H. Cerebral palsy: interictal epileptiform discharges and cognitive impairment. Clin Neurol Neurosurg. 2007;109(7):549–552. | ||
Spinillo A, Capuzzo E, Orcesi S, Stronati M, Di Mario M, Fazzi E. Antenatal and delivery risk factors simultaneously associated with neonatal death and cerebral palsy in preterm infants. Early Hum Dev. 1997;48(1–2):81–91. | ||
Stoknes M, Andersen GL, Elkamil AI, et al. The effects of multiple pre- and perinatal risk factors on the occurrence of cerebral palsy. A Norwegian register based study. Eur J Paediatr Neurol. 2012;16(1):56–63. | ||
Ozturk A, Demirci F, Yavuz T, et al. Antenatal and delivery risk factors and prevalence of cerebral palsy in Duzce (Turkey). Brain Dev. 2007;29(1):39–42. | ||
Hagberg B, Hagberg G, Beckung E, Uvebrant P. Changing panorama of cerebral palsy in Sweden. VIII. Prevalence and origin in the birth year period 1991–94. Acta Paediatr. 2001;90(3):271–277. | ||
Guzzetta A, D’Acunto MG, Carotenuto M, et al. The effects of preterm infant massage on brain electrical activity. Dev Med Child Neurol. 2011;53 Suppl 4:46–51. | ||
Reddihough DS, Collins KJ. The epidemiology and causes of cerebral palsy. Aust J Physiother. 2003;49(1):7–12. | ||
Nelson K, Leviton A. How much of neonatal encephalopathy is due to birth asphyxia? Am J Dis Child. 1991;145(11):1325–1331. | ||
Rooney B, Calhoun BC, Roche L. Does induced abortion account for racial disparity in preterm births, and violate the Nuremberg Code? J Am Phys Surg. 2008;13(4):102–104. | ||
Thorp JM Jr, Hartmann KE, Shadigian E. Long-term physical and psychological health consequences of induced abortion: review of the evidence. Obstet Gynecol Surv. 2003;58(1):67–79. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.