Back to Journals » Neuropsychiatric Disease and Treatment » Volume 11
Cerebrospinal fluid biomarkers for Alzheimer’s disease: the role of apolipoprotein E genotype, age, and sex
Authors Mehrabian S, Alexopoulos P, Ortner M, Traykov L, Grimmer T, Kurz A, Förstl H, Bickel H, Diehl-Schmid J
Received 26 August 2015
Accepted for publication 30 September 2015
Published 17 December 2015 Volume 2015:11 Pages 3105—3110
DOI https://doi.org/10.2147/NDT.S95018
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Shima Mehrabian,1 Panagiotis Alexopoulos,2,3 Marion Ortner,2 Latchezar Traykov,1 Timo Grimmer,2 Alexander Kurz,2 Hans Förstl,2 Horst Bickel,2 Janine Diehl-Schmid2
1Department of Neurology, University Hospital “Alexandrovska”, Sofia, Bulgaria; 2Department of Psychiatry, Technische Universität München, Munich, Germany; 3Department of Psychiatry, University of Patras, Patras, Greece
Introduction: Cerebrospinal fluid (CSF) biomarkers improve the diagnostic accuracy for Alzheimer’s disease (AD), even at the predementia stage of the disease. The ε4-allele of apolipoprotein E (ApoE ε4), female sex, and older age are well-known risk factors for AD. It is unclear how these risk factors affect the CSF biomarkers in patients with AD.
Aim: The objective of this study was to investigate the associations of ApoE ε4, sex, and age with CSF biomarker levels in a unicenter sample of patients with AD that includes a high proportion of patients with early-onset AD (EOAD).
Methods: The CSF levels of amyloid-β 1-42 (Aβ1-42) and total-tau of 117 subjects with mild to moderate AD (55 late-onset AD and 62 EOAD) were assessed. All subjects underwent ApoE genotyping, clinical evaluation, comprehensive neuropsychological assessments, and neuroimaging. Associations between CSF biomarker levels, ApoE ε4 allele frequency, age, and sex were evaluated.
Results: In the whole patient sample and in the late-onset AD subgroup ε4 homozygous subjects had significantly lower CSF Aβ1-42 levels compared with ε4 heterozygous subjects and ε4 noncarriers. This association was not detected in the EOAD group. Age group, sex, and severity of cognitive decline did not have a significant impact on CSF Aβ1-42 levels. No significant associations were found between ApoE ε4 allele frequency and CSF total-tau levels.
Conclusion: ApoE ε4 allele is associated with a reduction of CSF Aβ1-42 levels. This result is consistent with the findings of several previous studies. In the subgroup of patients with EOAD this association was not replicated. Larger studies are necessary to further investigate associations between ApoE ε4 allele frequency and CSF biomarker levels in patients with EOAD.
Keywords: Alzheimer’s disease, biomarkers, CSF, Aβ1-42, ApoE, LOAD, EOAD, tau
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia worldwide, affecting an increasing number of people each year due to population aging. Recently published diagnostic algorithms for AD enable its diagnosis independently of its symptoms, based on biomarkers, for instance on cerebrospinal fluid (CSF) amyloid-β 1-42 (Aβ1-42) and tau proteins, which improve the diagnostic accuracy for AD. CSF levels of total-tau (t-tau) are typically elevated in AD and are associated with neuronal and axonal damage.1 CSF levels of Aβ1-42 are reduced in AD and reflect the higher amyloid plaque burden in the brain.2,3 Consistent with histopathological findings, CSF chemistry studies have identified that reductions of Aβ1-42 occurs several years before symptom onset. At 90% specificity, Aβ1-42 discriminates AD from cognitively healthy persons with 85% sensitivity.4 Furthermore, Aβ1-42 has a high positive predictive value for the conversion from mild cognitive impairment to dementia in AD.5
The ε4 allele of the apolipoprotein E (ApoE ε4) gene is the strongest genetic risk factor for sporadic AD known to date.6 Cell culture and animal models have identified potential pathogenic mechanisms of ApoE which are related to amyloid-β production and clearance, to tau hyperphosphorylation, and to synaptic function.7 Several studies have reported that ApoE ε4 carrier have lower CSF Aβ1-42 levels than noncarriers, ie,8,9 some studies have described an association between sex and ApoE with women having a higher ε4-associated risk of developing AD than men10 but the effects of sex on CSF biomarker levels are still under debate. Although several studies indicate that not only familial but also sporadic early-onset AD (EOAD) might slightly differ from late-onset AD (LOAD) with regard to amyloid-β production and clearance pathways,11,12 studies on the possible different aspects of ApoE on CSF biomarker levels in EOAD and LOAD are scarce. We hypothesized that the effect of ApoE on CSF biomarker levels might differ between EOAD and LOAD and that sex might have additional impact on the effect of ApoE.
Therefore, the aim of the present study was to examine the associations of ApoE ε4 allele frequency, genotype, age, and sex with the CSF levels of Aβ1-42 and t-tau in a monocentric, memory-clinic based patient with AD sample that includes a high proportion of patients with EOAD.
Materials and methods
Study subjects
The research project has been approved by the institutional review board of the medical faculty, Technische Universität München, Munich, Germany.
The data of 117 German patients with mild to moderate probable AD according to the National Institute of Neurological and Communication Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association diagnostic criteria13 were identified in a pre-existing database that contains the data of patients which had been diagnosed with AD at an outpatient memory clinic between 2005 and 2011.
The patient data were included in the present study only, if a detailed, standardized neuropsychological assessment had been conducted, as well as structural and functional cerebral imaging, if a lumbar puncture had been performed with CSF biomarker analysis, if the patient’s ApoE genotype was available, and if the patients had provided written informed consent for including their data in research projects.
Clinical diagnosis was based on information gathered from neurological and neuropsychiatric examination, and informant interviews. All patients underwent a thorough medical screening including laboratory tests (serum chemistry, blood count, thyroid stimulating hormone, vitamin B12, and folate levels), and a neuropsychological evaluation using the German version of the Consortium to Establish a Registry in Alzheimer’s Disease Neuropsychological Battery14 which incorporates the Mini Mental State Examination (MMSE).15 Severity of dementia was estimated using the Clinical Dementia Rating Scale.16 All patients had got either cranial computed tomography or magnetic resonance imaging. In 71 patients, 18F-fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET) scans had been performed the results of which were consistent with AD in any case.
CSF analysis was performed within routine clinical testing in patients with cognitive impairment. The CSF samples were analyzed sample by sample, using commercially available enzyme-linked immunosorbent assays (INNOTEST, Innogenetics, Ghent, Belgium) to determine the levels of t-tau, and Aβ1-42. ApoE genotyping was performed using a standard PCR.
Statistical analysis
Descriptive statistics for variables are expressed as mean and standard deviation. The χ2-test was used to evaluate the differences of family history between patient groups (male/female and EOAD/LOAD). Fisher’s exact test was used to evaluate the differences of ApoE allele frequencies between groups. Comparisons involving three groups were performed using analysis of covariance (ANCOVA). Post hoc comparisons were performed using Bonferroni analysis. P-values are two sided and subject to a local significance level of <0.05. Possible effects of severity of disease as measured by MMSE, and sex were included as a covariate in ANCOVA models.
Results
The data of 117 patients with AD were included in the study. Patient characteristics are summarized in Table 1. In all, 49% of the patients were male, 53% were patients with EOAD, defined as having an age of disease onset below 65 years. CSF biomarker levels, family history for dementia, and ApoE allele frequency are also provided in Table 1.
Female patients had got a significantly lower MMSE score than male patients but did not differ from the male patients in any other variable. No significant differences were detected between patients with EOAD and LOAD. In particular, CSF levels of Aβ1-42 and t-tau did not significantly differ between male and female patients as well as patients with EOAD and LOAD. Likewise, the distribution of the six ApoE isoforms was similar in female and male patients as well as patients with EOAD and LOAD. In the total AD group, 60% of the patients carried at least one ApoE ε4 allele and 18% were homozygous ε4-carriers.
The effect of ApoE gene polymorphism on CSF biomarker levels
Table 2 shows the mean levels of CSF biomarkers according to ApoE genotype in the whole group of patients and in the subgroups of patients with EOAD and LOAD, respectively.
ε4 homozygous subjects in the total group of patients and in the LOAD group but not in the EOAD group had significant lower CSF Aβ1-42 levels compared with ε4 heterozygous subjects and ε4 noncarriers (whole group P=0.002; LOAD P=0.003). The difference between ε4 heterozygous subjects and ε4 noncarriers was not statistically significant. The associations remained significant after adjusting for sex and MMSE score.
CSF levels of t-tau did not demonstrate significant differences with regard to the presence of one or two ε4 alleles neither among the whole group nor among the EOAD and LOAD subgroups. Unlike in the whole group and in patients with LOAD, in the EOAD group the t-tau levels did not show an increase from ε4 noncarriers over homozygous to heterozygous carriers.
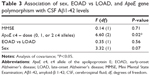
A univariate ANCOVA (Table 3) showed that in the total group of patients only the ApoE ε4 dose was significantly associated with CSF Aβ1-42 levels. Sex as well as MMSE score and age group (EOAD vs LOAD) did not have a significant effect.
Discussion
The main finding of this monocentric, memory-clinic based study that included 117 patients with AD (53% EOAD, 47% LOAD) is the significant association between ApoE ε4 allele frequency and CSF Aβ1-42 levels. Homozygous ε4-subjects had significantly lower CSF Aβ1-42 levels compared to heterozygous ε4-carriers and ε4 noncarriers, suggesting a dose-dependent effect of ApoE ε4 on CSF Aβ1-42 levels. Neither sex nor age or severity of dementia as measured by MMSE was significantly associated with CSF Aβ1-42 levels. No significant association was detected between ApoE ε4 and CSF t-tau-levels.
As early as 1993, genetic analyses identified ApoE ε4 as the major risk factor for AD.6 Since then, ApoE has been established as the most important susceptibility gene for late-onset sporadic AD.17 The APoE ε4 isoform appears to enhance amyloid-β production and cerebral plaque deposition and seems to alleviate the clearance of amyloid-β (for a detailed review, see Yu et al18). In line with these findings, several studies have found a dose effect of ApoE ε4 on CSF Aβ1-42 levels not only in AD patients but also in subjects with mild cognitive impairment and cognitively healthy controls: low CSF levels of Aβ1-42, a marker of amyloid-β plaque load, are linked to the presence of ApoE ε4. For example, Vemuri et al found a clear ApoE ε4 dose effect on CSF Aβ1-42 levels in 98 patients with AD from an Alzheimer’s Disease Neuroimaging Initiative (ADNI) study.9 A recent multicenter study by Lautner et al which included 309 patients with AD (mean age 77 years) showed that ApoE ε4 carriers had lower CSF Aβ1-42 levels compared to noncarriers in a dose-dependent manner.8
It is still under debate whether the association of low CSF Aβ1-42 levels with the presence of ApoE ε4 should be considered for the definition of cutoff levels of CSF Aβ1-42 when using them as a biomarker for AD. Some studies suggest that the ApoE genotype should be taken into account; others, however, conclude that the cutoff level for CSF Aβ1-42 should be the same for all ApoE genotypes.19
It is important to highlight, that thus far, large studies have overlooked the association between ApoE polymorphism and CSF biomarker levels focusing only on LOAD. This is surprising because the pathophysiology, particularly with regard to amyloid-β production and clearance, appears to differ between LOAD and (not only familial but also) sporadic EOAD.11,12,20 Therefore, this study is the first to examine the association of ApoE ε4 with CSF biomarker levels specifically in patients with EOAD and find that in EOAD – in contrast to LOAD and the whole patient group – the ApoE ε4 allele frequency was not significantly associated with CSF Aβ1-42 levels. However, given the relatively small sample of patients with EOAD in the present study, this finding needs to be investigated further in larger studies.
ApoE ε4 is neurotoxic and stimulates tau phosphorylation, leading to neurofibrillary tangles. It appears to affect tau neuropathological changes in AD brains and in animal models.18 Human studies of the relationship between ApoE ε4 and cerebral tau, however, showed contradictory results.21 Nonetheless, several studies found that ApoE ε4 carriers have higher CSF t-tau levels than noncarriers.9 In the present study, significant effects of the ApoE ε4 dose on CSF t-tau levels were not detected.
Our findings reveal that neither sex nor age alone appeared to influence CSF levels of Aβ1-42 and t-tau. While it is well established that, alike ApoE ε4, female sex and older age are risk factors for AD, the study results are inconclusive with regard to the effect of female sex or higher age on CSF biomarker levels. For example, an ADNI study of 144 AD patients showed that women and ApoE ε4 carriers experience higher rates of cognitive decline; however, in this study no significant effects of sex on CSF levels of Aβ1-42 and t-tau were detected.22 On the contrary, a meta-analysis reported an interaction between sex and ApoE with women having a higher ε4-associated risk of AD than men.10
In a recent large study of 2,375 Swedish AD subjects, logistic regression models revealed that female sex increased the risk of having pathologic CSF biomarker levels (decreased Aβ1-42, increased t-tau and phospho-tau). In this model, age had no effect on the likelihood of pathologic biomarkers. ApoE genotypes were not taken into account in this study.23
In all 60% of the patients in our study carried at least one ApoE ε4 allele. Approximately 18% were homozygous ε4-carriers. The reasons for the high proportion of ε4 carriers in our sample can only be speculated. The patients included in the study had extensive diagnosis including detailed neuropsychology, CSF analysis, structural imaging in all and 61% of the patients had FDG-PET-imaging. Therefore, the probability of misdiagnoses is extremely low and thus a “pure” Alzheimer cohort might explain higher ApoE ε4 rates than usual.
The present study has some limitations: first and most important, the patient sample is relatively small. Statistical power is limited with respect to analyses of CSF biomarkers in relation to a small sample of patients that homozygous ApoE ε4 carriers. The unicenter design with patients from one specialized memory clinic may limit the generalization of the results to the whole population of patients with AD. However, this study reflects the real-life practice in a specialized memory clinic. Furthermore, a unicenter study allows the use of harmonized procedures for the measurement of CSF biomarkers, avoiding assay-related preanalytical and analytical factors between laboratory variability. The strengths of the study lie in its detailed cognitive assessment protocol and careful diagnostic ascertainment. The diagnoses were performed by a trained team of psychiatrists and neuropsychologists highly specialized in cognitive disorders who used all available clinical and technical data, including FDG-PET scans in 61% of patients. Last but not least, the study included a high proportion of patients with EOAD.
Conclusion
ApoE ε4 allele is associated with a reduction of CSF Aβ1-42 levels. This result is consistent with the findings of several previous studies. In the subgroup of patients with EOAD this association was not replicated. Larger studies are necessary to further investigate associations between ApoE ε4 allele frequency and CSF biomarker levels in patients with EOAD.
Acknowledgments
We would like to thank Ms Gloria Benson for thorough language editing of the manuscript and Ms Tamara Eisele for technical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
Ahmed RM, Paterson RW, Warren JD, et al. Biomarkers in dementia: clinical utility and new directions. J Neurol Neurosurg Psychiatry. 2014;85(12):1426–1434. | ||
Shaw LM, Vanderstichele H, Knapik-Czajka M, et al; Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. | ||
Tosun D, Schuff N, Truran-Sacrey D, et al; Alzheimer’s Disease Neuroimaging Initiative. Relations between brain tissue loss, CSF biomarkers, and the ApoE genetic profile: a longitudinal MRI study. Neurobiol Aging. 2010;31(8):1340–1354. | ||
Blennow K. CSF biomarkers for mild cognitive impairment. J Intern Med. 2004;256(3):224–234. | ||
Tondelli M, Bedin R, Chiari A, et al. Role of cerebrospinal fluid biomarkers to predict conversion to dementia in patients with mild cognitive impairment: a clinical cohort study. Clin Chem Lab Med. 2015;53(3):453–460. | ||
Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. | ||
Huang Y. A beta-independent roles of apolipoprotein E4 in the pathogenesis of Alzheimer’s disease. Trends Mol Med. 2010;16(6): 287–294. | ||
Lautner R, Palmqvist S, Mattsson N, et al; Alzheimer’s Disease Neuroimaging Initiative. Apolipoprotein E genotype and the diagnostic accuracy of cerebrospinal fluid biomarkers for Alzheimer disease. JAMA Psychiatry. 2014;71(10):1183–1191. | ||
Vemuri P, Wiste HJ, Weigand SD, et al. Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol. 2010;67(3):308–316. | ||
Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. | ||
Bao XQ, Li N, Wang T, et al. FLZ alleviates the memory deficits in transgenic mouse model of Alzheimer’s disease via decreasing beta-amyloid production and tau hyperphosphorylation. PLoS One. 2013; 8(11):e78033. | ||
Choo IH, Lee DY, Kim JW, et al. Relationship of amyloid-β burden with age-at-onset in Alzheimer disease. Am J Geriatr Psychiatry. 2011; 19(7):627–634. | ||
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. | ||
Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. Consortium to establish a registry for Alzheimer’s disease (CERAD) clinical and neuropsychological assessment of Alzheimer’s disease. Psychopharmacol Bull. 1988;24(4):641–652. | ||
Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. | ||
Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. | ||
Genin E, Hannequin D, Wallon D, et al. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry. 2011;16(9):903–907. | ||
Yu JT, Tan L, Hardy J. Apolipoprotein E in Alzheimer’s disease: an update. Annu Rev Neurosci. 2014;37:79–100. | ||
Dumurgier J, Laplanche JL, Mouton-Liger F, et al. The screening of Alzheimer’s patients with CSF biomarkers, modulates the distribution of APOE genotype: impact on clinical trials. J Neurol. 2014;261(6):1187–1195. | ||
Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12(7):856–861. | ||
Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63(3):287–303. | ||
Holland D, Desikan RS, Dale AM, McEvoy LK. Higher rates of decline for women and apolipoprotein E epsilon4 carriers. Alzheimer’s Disease Neuroimaging Initiative. AJNR Am J Neuroradiol. 2013; 34(12):2287–2293. | ||
Rosén C, Farahmand B, Skillbäck T, et al. Benchmarking biomarker-based criteria for Alzheimer’s disease: data from the Swedish Dementia Registry, SveDem. Alzheimers Dement. Epub 2015 Jun 1. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.