Back to Journals » Journal of Pain Research » Volume 17
Clinical Effectiveness of Transversus Abdominis Plane (TAP) Block versus Local Anesthesia Wound Infiltration for Postoperative Pain Relief After Laparoscopic Appendicectomy in Children: A Study Protocol for a Multicenter Double-Blind Randomized Controlled Phase III Trial
Authors Bloy G, Jurine A, Chaussy Y, Auber F, Guinot PG , Bouhemad B, Francois M, Vettoretti L, Pili-Floury S, Nguyen M, Besch G
Received 6 December 2023
Accepted for publication 11 April 2024
Published 24 April 2024 Volume 2024:17 Pages 1547—1553
DOI https://doi.org/10.2147/JPR.S453661
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jinlei Li
Geoffrey Bloy,1 Amelie Jurine,1 Yann Chaussy,2 Frederic Auber,2 Pierre-Gregoire Guinot,3 Belaid Bouhemad,3 Michel Francois,4 Lucie Vettoretti,1 Sebastien Pili-Floury,5 Maxime Nguyen,3 Guillaume Besch5
1CHU Besançon, Département d’Anesthésie Réanimation Chirurgicale, Besançon, F-25000, France; 2Université de Franche-Comté, CHU Besançon, SINERGIES, Service de Chirurgie Pédiatrique, Besançon, F-25000, France; 3University of Burgundy and Franche-Comté, Dijon University Hospital, INSERM LNC UMR1231, FCS Bourgogne Franche-Comté LipSTIC LabEx, Dijon, F-21000, France; 4Université de Bourgogne, CHU Dijon, Service de Chirurgie Pédiatrique, Dijon, F-21000, France; 5Université de Franche-Comté, CHU Besançon, SINERGIES, Département d’Anesthésie Réanimation Chirurgicale, Besançon, F-25000, France
Correspondence: Guillaume Besch, Département d’Anesthésie Réanimation Chirurgicale, CHU Besançon, 3 bvd Alexandre Fleming, Besançon, 25030, France, Tel +3381218958, Fax +3381669331, Email [email protected]
Purpose: Postoperative pain relief after laparoscopic appendicectomy is a key determinant of early rehabilitation in children. Recent guidelines recommend performing either a transversus abdominis plane (TAP) block or local anesthesia (LA) wound infiltration as part of multimodal postoperative analgesia after appendectomy. To date, the clinical effectiveness of TAP block versus LA wound infiltration has never been compared. The hypothesis of this study is that the TAP block may provide a greater opioid-sparing effect after laparoscopic appendicectomy in children than LA wound infiltration.
Study Design and Methods: We designed a multicenter double-blind randomized controlled phase III trial and aim to include 110 children who undergo laparoscopic appendicectomy. Children are randomized to receive either TAP block (TAP group) or LA wound infiltration (infiltration group). Multimodal analgesia is standardized in the two groups using the same protocol, which includes the stepwise prescription of paracetamol, phloroglucinol, ketoprofene, and nalbuphine according to the hetero-evaluation of pain performed by the nurses who were blinded to the treatment allocated using the validated FLACC scale. The primary outcome is the total dose of nalbuphine administered within 24 hours after surgery.
Discussion: No study has specifically compared the clinical effectiveness of TAP block versus LA wound infiltration for postoperative pain relief after laparoscopic appendectomy in children. This paper describes the protocol for a randomized trial that addresses this issue. The results of this trial will be useful for editing guidelines with a higher level of evidence on this topic.
Keywords: laparoscopic appendectomy, TAP block, wound infiltration, analgesia, children
Introduction
Laparoscopic appendectomy is the most common surgical procedure performed during emergency surgeries in children. Postoperative pain relief after laparoscopic appendectomy should allow early rehabilitation to shorten hospital stay. Current European guidelines recommend postoperative multimodal analgesia, including either transversus abdominis plane (TAP) block or local anesthesia (LA) wound infiltration of the trocar site insertion.1
TAP block was reported to improve postoperative pain relief after laparoscopic appendectomy in children in several randomized controlled trials.2–5 TAP block significantly decreased postoperative opioid consumption and delayed the first analgesic intake after surgery.2,4 LA wound infiltration of the trocar site insertion was also described to provide efficient pain relief compared to laparoscopic appendectomy.6 The current European guidelines mentioned that TAP block could be more efficient than LA wound infiltration and should be considered as the first-line LA technique for postoperative pain relief after laparoscopic appendectomy in children.1 However, the level of evidence for this recommendation is weak because no study has directly compared the clinical effectiveness of TAP block versus LA wound infiltration of trocar site insertion. TAP block combined to LA wound infiltration of trocar site insertion compared to LA wound infiltration of trocar site insertion alone did not significantly decrease the proportion of children requiring more than 0.2 mg/kg of morphine after laparoscopic appendectomy.7 In an observational study conducted in adult patients, no significant difference was observed between TAP block and LA wound infiltration of trocar site insertion after either laparoscopic appendectomy or cholecystectomy.8
The purpose of the present study is to investigate, for the first time, whether TAP block could improve postoperative pain relief after laparoscopic appendectomy in children compared with LA wound infiltration at trocar site insertion.
Objectives
The primary objective is to compare the opioid-sparing effect provided by the TAP block performed by the anesthesiologist versus local anesthesia (LA) wound infiltration performed by the surgeon within 24 h after laparoscopic appendicectomy in children.
The secondary objectives are as follows: 1) to assess the opioid-sparing effect of TAP block versus LA wound infiltration within 12 h and between the 13th and 24th hours after surgery; 2) to compare the postoperative pain relief provided by TAP block versus LA wound infiltration at 1, 2, 6, 12, and 24 h after surgery; 3) to investigate whether TAP block versus LA wound infiltration could improve postoperative comfort and rehabilitation; and 4) to describe the safety of TAP block and LA wound infiltration for postoperative analgesia after laparoscopic appendicectomy in children.
Methods and Analysis
Trial Design
This study is a phase III randomized controlled double-blind multicenter trial.
Eligibility
Children who undergo laparoscopic appendicectomy under general anesthesia at the University Hospital of Besancon, France (Centre Hospitalier Universitaire (CHU) de Besançon) or the University Hospital of Dijon, France (CHU de Dijon) are eligible for inclusion. Inclusion and exclusion criteria are listed below.
Inclusion Criteria
- Signed informed consent provided by the child and by the holders of parental authority
- Laparoscopic appendicectomy under general anesthesia
- Acute appendicitis
- American Society of Anesthesiologists’ (ASA) physical status I to III
Exclusion Criteria
- Age under 3 years or over 15 years
- Body weight >50 kg
- ASA physical status IV
- Child or holder of parental authority refusal to participate
- Peritonitis (grade IV appendicitis)
- Long-term opioid treatment
- Epilepsy
- Liver failure
- Allergy to local anesthetic
- Allergy to nalbuphine
- Known or suspected coagulopathy
- Subjects without health insurance
Study Outline
Eligible patients are screened during the preoperative anesthetic assessment within the hours preceding the surgical procedure. After oral explanation of the study, written informed consent is obtained from the eligible child (written consent if aged over 6 years prior surgery; oral consent if aged under 6 years prior surgery) and from at least one holder of parental authority, according to the French law.9 When written informed consent was obtained, children included are randomized using sealed opaque envelopes according to a randomization list generated with R (R Core Team (2019). R: Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/) software. The randomization list is stratified according to the center.
Standard monitoring (General Electric Healthcare) was set up on arrival in the operating room. After induction of anesthesia, children are intubated, and tidal volume and respiratory rate are set-up to maintain the end tidal carbon dioxide between 35- and 40-mm Hg. The inspired oxygen fraction is adjusted to obtain pulse oximetry of >95%. General anesthesia is induced using propofol and maintained using sevoflurane in all children included in the study. The type of opioid administered (either sufentanil or alfentanil) and the use of neuromuscular blocking agents are left at the discretion of the physician in charge of the child considering the individual risks of aspiration and anaphylaxis. Ketamine 0.3 mg.kg−1 was administered prior to the incision.
According to the treatment allocated by randomization, children received either a bilateral TAP block (TAP group) prior surgical incision or LA wound infiltration after surgical wound closure (infiltration group) with a total dose of 0.6 mL.kg−1 of levobupivacaine 2.5 mg.mL−1.
The following surgical and anesthetic details are recorded: type and total dose of anesthetic drugs administered, stage of appendicitis (catarrhal, phlegmonous, or gangrenous), duration of surgery, total dose of LA injected, number of surgical trocars used, and number of trocar insertion points infiltrated with LA.
At the end of surgery, children are admitted to the post-anesthesia care unit (PACU) for at least one hour. Postoperative analgesia is standardized in the two groups (Figure 1) and is based on the intravenous administration of paracetamol 15 mg.kg−1 4 times per day, ketoprofene 1 mg.kg−1 three times per day, and phloroglucinol 1 mg.kg−1 three times per day. Postoperative analgesia is anticipated intraoperatively in the operating room to provide an optimal analgesic effect at emergence from general anesthesia, considering the individual pharmacokinetic profile of each analgesic drug. Thus, the first administration of paracetamol, ketoprofene and phloroglucinol is performed intraoperatively.
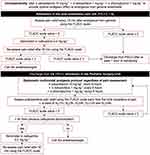 |
Figure 1 Standardized post-operative analgesia protocol used in the 2 groups. Abbreviations: iv, intravenous; PACU, post-anesthesia care unit; FLACC, Face Legs Activity Cry Consolability. |
Pain relief is assessed using the Face Legs Activity Cry Consolability (FLACC) scale at 1 h, 2 h, 6 h, 12 h and 24 h after the admission in the PACU, and each time the child complains of insufficient pain relief. The FLACC scale is a validated tool for hetero-evaluation of postoperative pain in children aged 3–15 years.10 The FLACC scale includes five criteria: facial expression, leg position and movement, type of activity, presence and intensity of crying, and consolability. A score of 0, 1, or 2 is assigned to each criterion, providing a total score ranging from 0 to 10, with 0 representing no pain. All FLACC scale values are reported in a case report form.
Nalbuphine 0.2 mg.kg−1 up to 6 times per day can be administered in cases of insufficient pain relief, defined as a FLACC scale value higher than 3. The FLACC scale is systematically reassessed within 30 min of nalbuphine administration. In case of postoperative nausea and/or vomiting, ondansetron 3 mg.kg−1 up to three times per day, can be delivered.
The type and total dose of each analgesic drug delivered within 24 hours after surgery is collected.
The study period ends at the 24th hour after the admission in the PACU.
Randomization, Allocation Concealment, and Blinding
Since laparoscopic appendicectomy is an emergent surgery, randomization is performed within the hours preceding admission to the operating room using sealed opaque envelopes. Sealed opaque envelopes were prepared by an independent investigator according to the randomization list with a constant block size of 4 (ratio 1:1), generated using R (R Core Team (2019). R: Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/) software. The independent investigator is involved in neither patient inclusion nor patient management. The randomization list is stratified according to the center. The block size is unknown to the investigator. When written informed consent is obtained, the investigators opened the first envelope available at the top of the storage box to enable inclusion and randomization. Randomly allocated treatments are immediately communicated to the investigator in charge of the patient.
Holders of parental authority, children, and outcome assessors (nurses in charge of the patient) are blinded to treatment allocation.
Study Procedure and Interventions
In the TAP group, children received an ultrasound-guided bilateral TAP block with 0.6 mL.kg−1 of levobupivacaine 2.5 mg.mL−1 immediately after the induction of general anesthesia and prior surgical incision, according to the lateral approach described by Tran et al.11 The same volume of LA is injected into both sides.
In the infiltration group, LA wound infiltration is performed following surgical wound closure. A total of 0.6 mL.kg−1 of levobupivacaine 2.5 mg.mL−1 is divided between the different trocar insertion points. The same dose is administered at each trocar-insertion point. If only one trocar is used for surgery, the total dose of 0.6 mL.kg−1 is injected in the unique insertion point.
The intraoperative analgesia protocol is left to the discretion of the physician in charge of the patient. After induction of general anesthesia, additional intraoperative administration of opioids (either sufentanil or alfentanil) can be administered if deemed necessary. No nociception monitoring device is used to guide perioperative analgesia. Postoperative analgesia is standardized in the two groups according to the protocol described in Figure 1.
Outcome Measures
The primary outcome is the total dose of nalbuphine delivered within 24 hours after surgery, expressed in mg.kg−1. Nalbuphine is administered based on the assessment of pain relief using the FLACC scale according to the protocol described in Figure 1.
Secondary outcomes are as follows: the total dose of nalbuphine delivered within 12 h and between the 13th and 24th hours after surgery; the FLACC scale values at 1 h, 2 h, 6 h, 12 h, and 24 h after surgery; the mean FLACC scale value within 24 h after surgery; the intra-individual variation of the FLACC scale value within 24 h after surgery; the time from the end of surgery to the first dose of nalbuphine; the time from the end of surgery to the first sitting up; the rate of postoperative nausea and/or vomiting; and the rate of adverse events, including hematoma at the site of LA injection.
Safety
All serious adverse events are collected and reviewed by the principal investigator of the study and reported to the trial sponsor (CHU Besancon, Besancon, France) and Pharmacovigilance Department of the University Hospital of Besancon (CHU Besancon, Besancon, France). The study insurance has been contracted to all participants by a trial sponsor (CHU Besancon, Besancon, France).
Since no off-label use of levobupivacaine is prescribed during the study and since both TAP block and LA wound infiltration are commonly used for postoperative pain relief after laparoscopic appendicectomy, a data and safety monitoring board was considered unnecessary by the trial sponsor and the principal investigator.
Sample Size Calculation
The sample size calculation was based on a retrospective analysis of data recording sheets from 81 children who underwent laparoscopic appendicectomy in the Pediatric Surgery Department of the University Hospital of Besançon, prior to the PABLO trial (data not published). The expected value of the primary outcome was, respectively, 0.26 mg.kg−1 and 0.34 mg.kg−1 in children who underwent TAP block and LA wound infiltration for postoperative pain relief with an estimated of 0.16 mg.kg−1.
Based on these results, we hypothesized that the TAP block performed by the anesthesiologist would be superior to LA wound infiltration performed by the surgeon to provide an opioid-sparing effect within 24 hours after laparoscopic appendicectomy in children. Considering a mean absolute difference of 0.08 mg.kg-1 in the TAP group, an α risk of 0.05, a β risk of 0.10, and a loss to follow-up rate of 10%, 55 children were required in each group (one-sided hypothesis).
Statistical Analysis
The normality of the distribution of continuous variables will be tested using the Shapiro–Wilk test. The TAP and the infiltration groups will be compared based on intention-to-treat analysis. Intergroup comparisons will be conducted using the Mann–Whitney U-test or Student t-test for quantitative variables, depending on the distribution of data, and using the Fisher’s exact test of the chi-square test for qualitative variables. The significance level is set at P < 0.05. No interim analysis is planned.
Monitoring
The Department of Research and Clinical Investigation of our institution will monitor all written informed consent according to the French law.9
Planning and Dissemination
Inclusions started on July 30, 2021. The initial planned duration of the trial was two years. No amendments were made to the study protocols. The University Hospital of Besancon (CHU Besancon, Besancon, France) is a trial sponsor and holder of all the data and publication rights. The results of this study will be submitted for publication in a peer-reviewed international medical journal and presented in an abstract form at national and international conferences.
Ethics Approval and Registration
This study was approved by the French Ethics Committee (Comité de Protection des Personnes Ile de France IV, Dr. Shahnaz Klouche, N°2021/15, March 24, 2021). The PABLO study was registered with ClinicalTrials.gov (Identifier: NCT04969133; principal investigator: Dr. Amélie Jurine, M.D., date of registration: July 20, 2021). This study is conducted in accordance with GCP-ICH-6 in two university-affiliated hospitals (CHU de Besancon, Besancon, France and CHU de Dijon, Dijon, France) and in accordance with the Declaration of Helsinki. Eligible patients are screened in the pediatric emergency department or pediatric surgery department during the preoperative anesthetic assessment, within hours preceding the surgical procedure. Holders of parental authority and eligible children receive all information related to the study. Informed consent is obtained by investigators from both eligible children (oral informed consent if age <6 years; written informed consent if age >6 years) and at least one holder of parental authority before inclusion in the study, according to French law.9
Acknowledgments
This work is funded by CHU Besançon, Besançon, France, and Région Bourgogne Franche-Comté, France.
Disclosure
Dr Maxime Nguyen reports grants and/or personal fees from Baxter, Pfizer, Fresenius, during the conduct of the study; personal fees from Pfizer, personal fees from Fresenius; grants from Baxter, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Vittinghoff M, Lönnqvist PA, Mossetti V, et al. Postoperative pain management in children: guidance from the pain committee of the European Society for Paediatric Anaesthesiology (ESPA Pain Management Ladder Initiative). Paediatr Anaesth. 2018;28(6):493–506. doi:10.1111/pan.13373
2. Sahin L, Sahin M, Gul R, Saricicek V, Isikay N. Ultrasound-guided transversus abdominis plane block in children: a randomised comparison with wound infiltration. Eur J Anaesthesiol. 2013;30(7):409–414. doi:10.1097/EJA.0b013e32835d2fcb
3. Abdallah FW, Laffey JG, Halpern SH, Brull R. Duration of analgesic effectiveness after the posterior and lateral transversus abdominis plane block techniques for transverse lower abdominal incisions: a meta-analysis. Br J Anaesth. 2013;111(5):721–735. doi:10.1093/bja/aet214
4. Carney J, Finnerty O, Rauf J, Curley G, McDonnell JG, Laffey JG. Ipsilateral transversus abdominis plane block provides effective analgesia after appendectomy in children: a randomized controlled trial. Anesth Analg. 2010;111(4):998–1003. doi:10.1213/ANE.0b013e3181ee7bba
5. Seyedhejazi M, Motarabbesoun S, Eslampoor Y, Taghizadieh N, Hazhir N. Appendectomy pain control by transversus abdominis plane (TAP) block in children. Anesthesiol Pain Med. 2019;9(1):e83975. doi:10.5812/aapm.83975
6. Liu Y, Seipel C, Lopez ME, et al. A retrospective study of multimodal analgesic treatment after laparoscopic appendectomy in children. Paediatr Anaesth. 2013;23(12):1187–1192. doi:10.1111/pan.12271
7. Sandeman DJ, Bennett M, Dilley AV, Perczuk A, Lim S, Kelly KJ. Ultrasound-guided transversus abdominis plane blocks for laparoscopic appendicectomy in children: a prospective randomized trial. Br J Anaesth. 2011;106(6):882–886. doi:10.1093/bja/aer069
8. Molfino S, Botteri E, Baggi P, et al. Pain control in laparoscopic surgery: a case-control study between transversus abdominis plane-block and trocar-site anesthesia. Updat Surg. 2019;71(4):717–722. doi:10.1007/s13304-018-00615-y
9. Toulouse E, Granier S, Nicolas-Robin A, et al. The French clinical research in the European Community regulation era. Anaesth Crit Care Pain Med. 2023;42(2):101192. doi:10.1016/j.accpm.2022.101192
10. Aubrun F, Nouette-Gaulain K, Fletcher D, et al. Revision of expert panel’s guidelines on postoperative pain management. Anaesth Crit Care Pain Med. 2019;38(4):405–411. doi:10.1016/j.accpm.2019.02.011
11. Tran DQ, Bravo D, Leurcharusmee P, Neal JM. Transversus abdominis plane block: a narrative review. Anesthesiology. 2019;131(5):1166–1190. doi:10.1097/ALN.0000000000002842
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
