Back to Journals » Infection and Drug Resistance » Volume 13
Clinical Role of Serum Interleukin-17A in the Prediction of Refractory Mycoplasma pneumoniae Pneumonia in Children
Authors Zhao J , Ji X, Wang Y, Wang X
Received 26 November 2019
Accepted for publication 22 February 2020
Published 12 March 2020 Volume 2020:13 Pages 835—843
DOI https://doi.org/10.2147/IDR.S240034
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eric Nulens
Jiuling Zhao, 1–3 Xin Ji, 4 Yushui Wang, 1 Xin Wang 1
1Department of Pediatrics, Tianjin Nankai Hospital, Tianjin, People’s Republic of China; 2Department of Pediatrics, Nankai Hospital Affiliated to Nankai University, Tianjin, People’s Republic of China; 3Nankai Clinical School, Tianjin Medical University, Tianjin, People’s Republic of China; 4School of Medical English and Health Communication, Tianjin Medical University, Tianjin, People’s Republic of China
Correspondence: Jiuling Zhao
Department of Pediatrics, Tianjin Nankai Hospital, 122 Sanweilu, Nankai, Tianjin 300100, People’s Republic of China
Tel +86-22-27435137
Fax +86-22-27435001
Email [email protected]
Background: Mycoplasma pneumoniae pneumonia (MPP) is a common community-acquired pneumonia (CAP) in children, which may become refractory MPP (RMPP) to treatment.
Objective: The purpose of this study was to evaluate the clinical utility of measuring serum interleukin (IL)-17A to predict RMPP.
Patients and Methods: A retrospective clinical study at a single pediatric center included a review of the medical records of all children hospitalized for CAP between November 2015 and October 2019. The diagnosis of MPP was based on clinical presentation, chest radiography, and measurement of serum anti-Mycoplasma immunoglobulin IgM antibody titer using the microparticle agglutination method or sputum samples for Mycoplasma pneumoniae by PCR. Serum levels of IL-18 and IL-17A were determined by ELISA.
Results: Of the 625 children diagnosed with CAP, there were 154 children with MPP and without underlying diseases who were divided into a non-refractory MPP (NRMPP) group (n = 109) and a RMPP group (n = 45). The RMPP group had a higher incidence of tachypnea, cyanosis, hypoxia, segmental or lobar pneumonia, pleural effusion, and a longer period of hospitalization compared with NRMPP group (all P-values < 0.05). A serum IL-17A level above 10.8 pg/mL was a predictor for RMPP: area under the curve (AUC) 0.822; standard error (SE) 0.039; 95% confidence interval (CI) 0.746– 0.897; diagnostic sensitivity and specificity of 77.8% and 77.1%, respectively. An LDH level above 436.5 IU/L and an IL-18 level above 464.5 pg/mL were the second most useful markers for RMPP: AUC 0.775, 0.775; SE 0.038, 0.039; 95% CI 0.700– 0.850, 0.698– 0.852; sensitivity 77.8%, 82.2%; specificity 62.4%, 59.6%; respectively.
Conclusion: This preliminary study of MPP in a pediatric population has shown that measurement of serum IL-17A may be a useful marker for the predictor of RMPP.
Keywords: Mycoplasma pneumoniae, pneumonia, refractory pneumonia, interleukin-17A
Introduction
Mycoplasma pneumoniae (MP) is the most common pathogenic microbe that causes community-acquired pneumonia (CAP) in children over 5 years-of-age and is also common in CAP in children under 5 years-of-age.1 MP is present in 40% of pediatric CAP cases, with an even higher incidence during epidemic periods.1 A recent study has shown that MP may be the cause of CAP in between 4% and 39% of children admitted to hospital.2 In general, mild MP infection is considered to be a self-limiting disease. However, MP can also cause a severe form of pneumonia with a range of extrapulmonary manifestations. Severe Mycoplasma pneumoniae pneumonia (MPP) has a clinical presentation of lobar pneumonia, pleural effusion, with respiratory distress syndrome, and even life-threatening respiratory failure and circulatory failure.3 Extrapulmonary lesions caused by MP can involve various systems, including the skin, nervous system, and bone and joints, and include non-specific exanthema, urticaria, encephalitis, meningitis, optic neuritis, Guillain–Barré syndrome, and Stevens–Johnson syndrome.2,4-6
Macrolides, fluoroquinolones, and tetracyclines are antimicrobials used to treat adults with MPP. However, fluoroquinolones may adversely affect the development of developing cartilage, and tetracyclines may cause permanent discoloration of the teeth in young children, and so first-line drug treatment for pediatric MPP is still macrolide antibiotics.7 Over the past decade, macrolide-resistant MP (MRMP) has been identified and reported worldwide,8–11 and is particularly common in some East Asian countries and regions, with a prevalence of 50–93% in Japan,12,13 and up to 96% in some areas of China.14 In recent years, refractory Mycoplasma pneumoniae pneumonia (RMPP), as a special type of MPP, has become a focus for clinical research. However, the pathogenesis of RMPP remains unclear. What is known is that the adsorption of MP in the respiratory epithelium leads to deterioration of cilia, the direct invasion of MP is associated with a cell-mediated immune response and cytokine stimulation, and a part of the existence of MRMP.5,15,16 Steroids, intravenous immunoglobulin, and fiberoptic bronchoscopy are usually required in the treatment of RMPP.17–21 For these reasons, it would be helpful to predict which patients with MP infection are likely to develop RMPP.
It has been confirmed that serum C-reactive protein (CRP), ferritin, lactate dehydrogenase (LDH), interleukin (IL)-18, and IL-17A play important roles in the progression of many infectious, inflammatory, malignant and other diseases.22–27 Previous studies have shown a correlation between the severity of MPP and serum CRP, ferritin, LDH, and IL-18.28–30 Although serum IL-17A in MPP has seldom been reported, IL-17A in bronchoalveolar lavage fluid (BALF) has been shown to play critical roles in the occurrence and development of pulmonary diseases, including acute lung injury31 and MPP.32
The purpose of this study was to compare the clinical features and laboratory findings of RMPP and non-refractory MPP (NRMPP) in children hospitalized for CAP and to explore the role of serum markers, including IL-17A, as predictive markers for RMPP.
Patients and Methods
Patients
In this retrospective study, we reviewed the electronic medical records of all children admitted to the Department of Pediatrics, Tianjin Nankai Hospital, China, between November 2015 and October 2019, with the diagnosis of community-acquired pneumonia (CAP).
Data were retrieved for each patient to include the history of the presenting illness, physical examination findings, clinical laboratory findings, chest radiographs, and family medical history.
The study protocol was approved by the Medical Ethics Committee of Tianjin Nankai Hospital (Approved No. of ethic committee: NKYY_YX_IRB_2018_043_01). The ethics committee waived the need for written informed consent provided by participants due to the retrospective nature of the study, because all patient data were analyzed in anonymity, and no additional informed consent was required. The whole process of our research is in full compliance with the principles of the Declaration of Helsinki.
Diagnostic Inclusion Criteria of MPP and RMPP
The diagnosis of Mycoplasma pneumoniae pneumonia (MPP) was made based on the following definitions: (1) patients who met the diagnostic criteria for CAP, with symptoms of respiratory tract infection and a chest radiograph showing manifestations of pneumonia, with or without pleural effusion; and (2) a fourfold or greater increase in serum anti-Mycoplasma pneumoniae immunoglobulin (Ig) M antibody titer as measured in the symptomatic recovery phase (rather than the acute phase), or positive sputum samples by polymerase chain reaction (PCR) test for Mycoplasma pneumoniae (MP).33
The standard test for the detection of anti-MP IgM antibody titers was the microparticle agglutination method using a commercial kit (Serodia-Myco II, Fujirebio, Tokyo, Japan). Quantitative PCR of MP DNA was used with a commercial nucleic acid quantitative detection kit (Daan Gene Co., Guangzhou, China).
The diagnosis of refractory Mycoplasma pneumoniae pneumonia (RMPP) was made based on the following criteria: (1) patients who met the MPP diagnostic criteria, as above; (2) patients with prolonged fever and aggravation of radiological manifestations despite appropriate antibiotic treatment for 7 days or more.34
Exclusion Criteria
To exclude other respiratory pathogens, we performed microbiological testing from nasal and throat swabs. All subjects were tested for influenza A, influenza B, human metapneumovirus, adenovirus, respiratory syncytial virus, parainfluenza 1, parainfluenza 2, and parainfluenza 3 using a commercial D3 FastPoint L-DFA Respiratory Virus Identification Kit (Diagnostic Hybrids, Inc., Athens, OH, USA). Throat swab cultures were used to exclude respiratory bacterial pathogens. And all children with MP were given skin tests of purified protein derivative of Tuberculin (TB-PPD) to exclude Mycobacterium tuberculosis infection. Patients with underlying diseases such as metabolic diseases, allergic diseases, or congenital immune deficiencies were also excluded.
Clinical Laboratory Data, Serum IL-17A and IL-18 Measurements
Routine blood tests, including white blood cell (WBC) counts, differential cell counts for neutrophils (%) and lymphocytes (%), hemoglobin, platelets, serum C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), procalcitonin, ferritin, and lactate dehydrogenase (LDH) were detected using standard protocols for our institution. On admission to hospital, venous blood samples were taken from each patient and centrifuged at 1000 ×g at 20°C for 15 mins. Serum levels of IL-18 and IL-17A were determined using the Human IL-18 Elisa Kit (Absci Inc, College Park, MD, USA) and Human IL-17A Elisa Kit (Diaclone SAS, Besancon, France), respectively; test sensitivity was 20.0 pg/mL and 2.3 pg/mL, respectively.
Statistical Analysis
The statistical evaluation was performed using the Statistical Package of Social Science Software program (SPSS), version 17.0 (SPSS Inc, Chicago, IL, USA). Data distribution in all groups was determined by the Kolmogorov–Smirnov test. The Student’s t-test, expressed as mean ± standard deviation (SD), was used to assess data that followed a normal distribution, and the Mann–Whitney U-test, with results expressed as median and interquartile range (25th–75th percentiles), was used to assess non-normally distributed data. The Chi-squared (χ2) test or Fisher’s exact test was used to assess the categorical variables. Binary logistic regression analysis was used to evaluate the correlations between IL-17A and other inflammatory biomarkers. Receiver operating characteristic (ROC) curve analysis was used to evaluate the value of inflammatory biomarkers in predicting and diagnosing RMPP. A two-tailed test was used for all P-values, and a P-value < 0.05 was considered statistically significant.
Results
There were 625 children who were hospitalized with a diagnosis of community-acquired pneumonia (CAP), including cases of viral pneumonia (n = 122), bacterial pneumonia (n = 189), Mycoplasma pneumoniae pneumonia (MPP) (n = 176); there were cases of other pathogens, mixed infection, and unclear pathogens (n = 138).
Of the 176 cases of MPP, there were 154 cases (87.5%, 154/176) included in this study, with no other detected underlying diseases; Of these patients, 122 cases were diagnosed according to paired serum anti-Mycoplasma IgM antibody titers (fourfold or greater higher at the symptomatic recovery phase than acute phase), and 32 cases were diagnosed according to positive sputum samples by PCR. These 154 children with MPP were divided into two groups: the non-refractory group (NRMPP) (n = 109) and a refractory group (RMPP) (n = 45). The study flow chart is shown in Figure 1.
Comparison of Clinical Characteristics Between the NRMPP Group and the RMPP Group
As shown in Table 1, there was no significant difference in mean age, sex, or peak body temperature between the two groups, NRMPP and RMPP (all P-values > 0.05). The incidence of tachypnea, cyanosis and hypoxia were significantly greater in the RMPP group than in the NRMPP group (all P-values < 0.05). The mean duration of hospitalization was longer in the RMPP compared with the NRMPP group (P < 0.05). In the classification of chest images, the incidence of segmental or lobar pneumonia and pleural effusion were greater in the RMPP group compared with the NRMPP group (P < 0.05).
 |
Table 1 Comparison of Clinical Characteristics Between NRMPP Group and RMPP Group |
Comparison of Laboratory Findings Between the NRMPP Group and the RMPP Group
As shown in Table 2, there were no significant differences in the white blood cell (WBC) counts, the percentage of neutrophils or lymphocytes, or in hemoglobin, platelets, erythrocyte sedimentation rate (ESR), or procalcitonin between the two groups (all P-values >0.05). Serum CRP, ferritin, LDH, IL-18and IL-17A levels were significantly higher in the RMPP group compared with the NRMPP group (all P-values < 0.05).
 |
Table 2 Comparison of Laboratory Findings Between NRMPP Group and RMPP Group |
Correlation Between Serum IL-17A and Other Biomarkers in All Children with MPP on Hospital Admission
The correlation between hospital admission serum levels of IL-17A and the four other biomarkers showing a significant difference between the NRMPP group and the RMPP group, CRP, ferritin, LDH, and IL-18 were analyzed by binary logistic regression analysis (Figure 2). With the exception of CRP (r2 = 0.024, P = 0.053), levels of serum ferritin, LDH and IL-18 were correlated with the level of serum IL-17A (r2-values of 0.094, 0.122, 0.143; P-values of 0.001, 0.000, 0.000, respectively). However, the values of the coefficient of determination (r2) were very low. The level of serum IL-17A was also affected by other unknown factors not studied in this research.
Predictive Value of Serum IL-17A in Children with RMPP
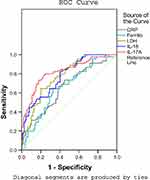
To evaluate the predictive value of serum IL-17A level for RMPP, we examined the receiver operating characteristic (ROC) curve for the biomarkers CRP, ferritin, LDH, IL-18, and IL-17A. For each biomarker, the ROC curve was significantly different between the NRMPP group and the RMPP group. The results are shown in Figure 3 and Table 3.
 |
Table 3 ROC Curve of Inflammatory Markers in Children with RMPP |
An IL-17A level above 10.8 pg/mL was a predictor for RMPP, with an area under the curve (AUC) of 0.822, standard error (SE) of 0.039, and 95% confidence interval (CI) of 0.746–0.897. The diagnostic sensitivity and specificity were 77.8% and 77.1%, respectively. An LDH level of 436.5 IU/L and an IL-18 level of 464.5 pg/mL were the second most useful biomarkers: AUC 0.775, 0.775; SE 0.038, 0.039; 95% CI 0.700–0.850, 0.698–0.852; sensitivity 77.8%, 82.2%; specificity 62.4%, 59.6%; respectively.
Discussion
This preliminary retrospective study from a single center has shown that for children admitted to hospital with community-acquired pneumonia (CAP) and a diagnosis of Mycoplasma pneumoniae pneumonia (MPP), measurement of serum interleukin (IL)-17A may be a useful marker for the development of RMPP. Although previous studies have shown a correlation between the severity of MPP and serum C-reactive protein (CRP), ferritin, lactate dehydrogenase (LDH), and IL-18,28–30 we believe that this is the first study to show that serum IL-17A in children may be a predictive marker for RMPP.
In this study, the incidence of tachypnea, cyanosis, and hypoxia was higher in children with RMPP compared with children with NRMPP. Segmental or lobar pneumonia and pleural effusion occurred at a higher incidence in patients with RMPP compared with patients with NRMPP. Patients with RMPP had a longer duration of hospitalization than those with NRMPP. However, there was no significant difference in peak body temperature between patients with RMPP and patients with NRMPP.
CRP is an acute-phase protein synthesized by hepatocytes and is one of the most widely used biomarkers of inflammation, but it is non-specific and is usually seen at very low levels in the blood of healthy people. CRP levels can increase for short periods of time in patients with acute infection, myocardial infarction, inflammation, or trauma.35 Agnello et al36 reported that CRP can predict the severity of CAP in children. In addition, a meta-analysis demonstrated that there were correlations between serum levels of CRP and pneumonia in infants.37 It has also been reported that serum CRP can be used as a biomarker for evaluating the efficacy of macrolides in macrolide-resistant MPP.28 In our study, there was no correlation between levels of CRP and IL-17A. However, CRP levels were significantly increased in the RMPP group compared with the NRMPP group.
Serum ferritin is not only an iron storage protein but also an important inflammatory biomarker.38 Studies have shown that serum ferritin levels may correlate with the severity of liver disease, acute and chronic inflammatory diseases, infections, malignancy, renal failure, and metabolic syndrome.39 In the pulmonary system, raised serum ferritin is considered to indicate the activation of alveolar macrophages and to reflect pulmonary inflammation and tissue damage.40 Kawamata et al29 reported that serum ferritin levels were positively correlated with the severity of pediatric MPP, and could be a useful indicator for the initiation of corticosteroid therapy. In our study, we found significantly increased serum ferritin levels in children in the RMPP group compared with children in the NRMPP group.
LDH is a metabolic enzyme involved in anaerobic glycolysis, is present in the cytoplasm of all cells of the body, and is composed of five isoforms: LDH-1, LDH-2, LDH-3, LDH-4, and LDH-5.41 LDH-3 is the most abundant isoform in lung tissue. Lu et al42 reported that serum LDH could be used as a biomarker for predicting RMPP and evaluating whether to initiate corticosteroid therapy during the early stages of patient hospitalization. Inamura et al43 reported that a serum LDH level of ≥410 IU/L could be an appropriate level for the initiation of steroid therapy. The findings of this study indicated that levels of LDH were significantly greater in patients in the RMPP group compared with patients in the NRMPP group, and a serum LDH level above 436.5 IU/L was predictive for RMPP.
IL-18 is a pro-inflammatory cytokine and a member of the IL-1 family and can combine with IL-12 to induce IFN-γ production by T-cells, activate natural killer (NK) cells, and participate in both innate and acquired immunity.27,44 Previous studies have shown a positive association between serum IL-18 levels and the severity of MPP in adults.44 Choi et al30 reported that levels of LDH and IL-18 were positively correlated with MPP in children, and recommended that serum IL-18 could be used as a biomarker for steroid therapy. The findings of this study showed that serum levels of IL-18 were significantly increased in patients in the RMPP group compared with patients in the NRMPP group, and a serum IL-18 level above 454.5 pg/mL was predictive for RMPP.
IL-17A is a member of the IL-17 family and is secreted from many types of innate and adaptive immune cells, with Th17 cells being the major cellular source of IL-17. It plays a crucial role in the induction of proinflammatory cytokines, chemokines, metalloproteases, and other mediators of the immune system.45–48 In addition, IL-17A also plays an important role in the progression of pulmonary diseases. Ding et al49 reported that IL-17A can facilitate pulmonary inflammation and that it may play an important role in the process of pulmonary fibrosis. Li et al31 reported that IL-17 expression was associated with the severity of acute respiratory distress syndrome (ARDS) and that blockade of IL-17 could inhibit pulmonary inflammation and reduce the severity of acute lung injury in a mouse model. Yang et al32 reported IL-17A in BALF may be a good predictor of the severity of MPP in children. In our study, we found significantly increased serum IL-17A levels in children with RMPP compared with children with NRMPP. Of all the serum markers studied, a serum IL-17A level above 10.8 pg/mL was the most significant predictor for RMPP.
It seems to be a certain correlation between IL-17A and IL-18. Despite the ability of IL-17 to mediate inflammatory pathology, it also plays a protective role in host defense against bacterial, mycobacterial and fungal pathogens.50 IL-18 is a cytokine that stimulates various cell types and has pleiotropic functions. Besides allergic inflammation, IL-18 plays an important role in host defense against various infectious microorganisms because it strongly enhances the induction of IFN-γ, nitric oxide, and reactive oxygen species in phagocytes.51 However, the relationships between IL-17A and IL-18 were rarely reported. Wynn et al52 reported that IL-17A is an effector of IL-18-mediated injury in neonatal sepsis, and the IL-18/IL-1R1/IL-17A axis exists in human neonates with sepsis. In our research, a positive correlation was found between the level of serum IL-18 and serum IL-17A, with a low r2-value of 0.143 and a P-value of 0.000, which also suggested that a certain relationship may exist between them. Moreover, further studies are still needed to explore the interaction between IL-18 and IL-17A.
Limitations
This study has several limitations. This was a retrospective study, performed in a single center. The patient sample size of the RMPP group was small and included 45 patients. Some patients may have been co-infected with other pathogens that were not detected during their hospital admission and may have been included in this study. In this retrospective study, blood sampling for serum analysis was not controlled for collection at uniform time points. These factors from this preliminary retrospective study that may have resulted in some study bias should be controlled by future larger, multicenter, prospective studies.
Conclusion
In conclusion, this preliminary study of MPP in a pediatric population has shown that measurement of serum IL-17A can be a useful marker for the development of refractory disease. Further controlled longitudinal studies are recommended to evaluate the clinical role of serum IL-17A in the prediction of RMPP in children.
Acknowledgments
The authors thank staffs in the Tianjin Nankai Hospital for their assistance in implementing this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–845. doi:10.1056/NEJMoa1405870
2. Gao LW, Yin J, Hu YH, et al. The epidemiology of paediatric Mycoplasma pneumoniae pneumonia in North China: 2006 to 2016. Epidemiol Infect. 2019;147:e192. doi:10.1017/S0950268819000839
3. Meyer Sauteur PM, Unger WWJ, Nadal D, et al. Infection with and carriage of Mycoplasma pneumoniae in children. Front Microbiol. 2016;7:329. doi:10.3389/fmicb.2016.00329
4. Narita M. Classification of extrapulmonary manifestations due to Mycoplasma pneumoniae infection on the basis of possible pathogenesis. Front Microbiol. 2016;7:23. doi:10.3389/fmicb.2016.00023
5. Parrott GL, Kinjo T, Fujita J. A compendium for Mycoplasma pneumoniae. Front Microbiol. 2016;7:513. doi:10.3389/fmicb.2016.00513
6. Olson D, Watkins LK, Demirjian A, et al. Outbreak of Mycoplasma pneumoniae-associated Stevens-Johnson syndrome. Pediatrics. 2015;136(2):e386–e394. doi:10.1542/peds.2015-0278
7. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17(4):697–728. doi:10.1128/CMR.17.4.697-728.2004
8. Zheng X, Lee S, Selvarangan R, et al. Macrolide-resistant Mycoplasma pneumoniae, United States. Emerg Infect Dis. 2015;21(8):1470–1472. doi:10.3201/eid2108.150273
9. Eshaghi A, Memari N, Tang P, et al. Macrolide-resistant Mycoplasma pneumoniae in humans, Ontario, Canada, 2010–2011. Emerg Infect Dis. 2013;19(9). doi:10.3201/eid1909.121466
10. Brown RJ, Macfarlane-Smith L, Phillips S, et al. Detection of macrolide resistant Mycoplasma pneumoniae in England, September 2014 to September 2015. Euro Surveill. 2015;20(48):30078. doi:10.2807/1560-7917.ES.2015.20.48.30078
11. Tashiro M, Fushimi K, Kawano K, et al. Comparison of efficacy of antimicrobial agents among hospitalized patients with Mycoplasma pneumoniae pneumonia in Japan during large epidemics of macrolide-resistant M. pneumoniae infections: a nationwide observational study. Clin Infect Dis. 2017;65(11):1837–1842. doi:10.1093/cid/cix695
12. Kawai Y, Miyashita N, Kubo M, et al. Nationwide surveillance of macrolide-resistant Mycoplasma pneumoniae infection in pediatric patients. Antimicrob Agents Chemother. 2013;57(8):4046–4049. doi:10.1128/AAC.00663-13
13. Yamazaki T, Kenri T. Epidemiology of Mycoplasma pneumoniae infections in Japan and therapeutic strategies for Macrolide-resistant M. pneumoniae. Front Microbiol. 2016;7:693. doi:10.3389/fmicb.2016.00693
14. Zhao F, Liu G, Wu J, et al. Surveillance of macrolide-resistant Mycoplasma pneumoniae in Beijing, China, from 2008 to 2012. Antimicrob Agents Chemother. 2013;57(3):1521–1523. doi:10.1128/AAC.02060-12
15. Nakane D, Kenri T, Matsuo L, et al. Systematic structural analyses of attachment organelle in Mycoplasma pneumoniae. PLoS Pathog. 2015;11(12):e1005299. doi:10.1371/journal.ppat.1005299
16. Shimizu T. Inflammation-inducing factors of Mycoplasma pneumoniae. Front Microbiol. 2016;7:414. doi:10.3389/fmicb.2016.00414
17. Okumura T, Kawada JI, Tanaka M, et al. Comparison of high-dose and low-dose corticosteroid therapy for refractory Mycoplasma pneumoniae pneumonia in children. J Infect Chemother. 2019;25(5):346–350. doi:10.1016/j.jiac.2019.01.003
18. Luo Z, Luo J, Liu E, et al. Effects of prednisolone on refractory Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol. 2014;49(4):377–380. doi:10.1002/ppul.22752
19. Miyashita N, Kawai Y, Inamura N, et al. Setting a standard for the initiation of steroid therapy in refractory or severe Mycoplasma pneumoniae pneumonia in adolescents and adults. J Infect Chemother. 2015;21(3):153–160. doi:10.1016/j.jiac.2014.10.008
20. Youn YS, Lee SC, Rhim JW, et al. Early additional immune-modulators for Mycoplasma pneumoniae pneumonia in children: an observation study. Infect Chemother. 2014;46(4):239–2247. doi:10.3947/ic.2014.46.4.239
21. Zhang Y, Chen Y, Chen Z, et al. Effects of bronchoalveolar lavage on refractory Mycoplasma pneumoniae pneumonia. Respir Care. 2014;59(9):1433–1439. doi:10.4187/respcare.03032
22. Gallego M, Pomares X, Capilla S, et al. C-reactive protein in outpatients with acute exacerbation of COPD: its relationship with microbial etiology and severity. Int J Chron Obstruct Pulmon Dis. 2016;11:2633–2640. doi:10.2147/COPD.S117129
23. Shrotriya S, Walsh D, Bennani-baiti N, et al. C-Reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: a systematic review. PLoS One. 2015;10(12):e0143080. doi:10.1371/journal.pone.0143080
24. Alkhateeb AA, Connor JR. The significance of ferritin in cancer: anti-oxidation, inflammation and tumorigenesis. Biochim Biophys Acta. 2013;1836(2):245–254. doi:10.1016/j.bbcan.2013.07.002
25. Shen J, Chen Z, Zhuang Q, et al. Prognostic value of serum lactate dehydrogenase in renal cell carcinoma: a systematic review and meta-analysis. PLoS One. 2016;11(11):e0166482. doi:10.1371/journal.pone.0166482
26. Duman A, Akoz A, Kapci M, et al. Prognostic value of neglected biomarker in sepsis patients with the old and new criteria: predictive role of lactate dehydrogenase. Am J Emerg Med. 2016;34(11):2167–2171. doi:10.1016/j.ajem.2016.06.012
27. Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984–1010. doi:10.1016/j.jaci.2016.06.033
28. Seo YH, Kim JS, Seo SC, et al. Predictive value of C-reactive protein in response to macrolides in children with macrolide-resistant Mycoplasma pneumoniae pneumonia. Korean J Pediatr. 2014;57(4):186–192. doi:10.3345/kjp.2014.57.4.186
29. Kawamata R, Yokoyama K, Sato M, et al. Utility of serum ferritin and lactate dehydrogenase as surrogate markers for steroid therapy for Mycoplasma pneumoniae pneumonia. J Infect Chemother. 2015;21(11):783–789. doi:10.1016/j.jiac.2015.07.009
30. Choi YJ, Jeon JH, Oh JW. Critical combination of initial markers for predicting refractory Mycoplasma pneumoniae pneumonia in children: a case control study. Respir Res. 2019;20(1):193. doi:10.1186/s12931-019-1152-5
31. Li Q, Gu Y, Tu Q, et al. Blockade of Interleukin-17 restrains the development of acute lung injury. Scand J Immunol. 2016;83(3):203–211. doi:10.1111/sji.12408
32. Yang M, Meng F, Wang K, et al. Interleukin 17A as a good predictor of the severity of Mycoplasma pneumoniae pneumonia in children. Sci Rep. 2017;7(1):12934. doi:10.1038/s41598-017-13292-5
33. Harris M, Clark J, Coote N, et al. British thoracic society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(Suppl 2):ii1–ii23. doi:10.1136/thoraxjnl-2011-200598
34. Tamura A, Matsubara K, Tanaka T, et al. Methylprednisolone pulse therapy for refractory Mycoplasma pneumoniae pneumonia in children. J Infect. 2008;57(3):223–228. doi:10.1016/j.jinf.2008.06.012
35. Liu W, Peng L, Hua S. Clinical significance of dynamic monitoring of blood lactic acid, oxygenation index and C-reactive protein levels in patients with severe pneumonia. Exp Ther Med. 2015;10(5):1824–1828. doi:10.3892/etm.2015.2770
36. Agnello L, Bellia C, Di Gangi M, et al. Utility of serum procalcitonin and C-reactive protein in severity assessment of community-acquired pneumonia in children. Clin Biochem. 2016;49(1–2):47–50. doi:10.1016/j.clinbiochem.2015.09.008
37. Xiao X, Xue L, Sheng HL, et al. Correlation between serum levels of C-reactive protein and infant pneumonia: a meta-analysis. Exp Ther Med. 2015;9(6):2331–2338. doi:10.3892/etm.2015.2417
38. Recalcati S, Invernizzi P, Arosio P, et al. New functions for an iron storage protein: the role of ferritin in immunity and autoimmunity. J Autoimmun. 2008;30(1–2):84–89. doi:10.1016/j.jaut.2007.11.003
39. Koperdanova M, Cullis JO. Interpreting raised serum ferritin levels. BMJ. 2015;351:h3692. doi:10.1136/bmj.h3692
40. Isoda K, Takeuchi T, Kotani T, et al. Pre-treatment ferritin level and alveolar-arterial oxygen gradient can predict mortality rate due to acute/subacute interstitial pneumonia in dermatomyositis treated by cyclosporine a/glucocorticosteroid combination therapy: a case control study. PLoS One. 2014;9(2):e89610. doi:10.1371/journal.pone.0089610
41. Liu TY, Lee WJ, Tsai CM, et al. Serum lactate dehydrogenase isoenzymes 4 plus 5 is a better biomarker than total lactate dehydrogenase for refractory Mycoplasma pneumoniae pneumonia in children. Pediatr Neonatol. 2018;59(5):501–506. doi:10.1016/j.pedneo.2017.12.008
42. Lu A, Wang C, Zhang X, et al. Lactate dehydrogenase as a biomarker for prediction of refractory Mycoplasma pneumoniae pneumonia in children. Respir Care. 2015;60(10):1469–1475. doi:10.4187/respcare.03920
43. Inamura N, Miyashita N, Hasegawa S, et al. Management of refractory Mycoplasma pneumoniae pneumonia: utility of measuring serum lactate dehydrogenase level. J Infect Chemother. 2014;20(4):270–273. doi:10.1016/j.jiac.2014.01.001
44. Tanaka H, Narita M, Teramoto S, et al. Role of interleukin-18 and T-helper type 1 cytokines in the development of Mycoplasma pneumoniae pneumonia in adults. Chest. 2002;121(5):1493–1497. doi:10.1378/chest.121.5.1493
45. Kirkham BW, Kavanaugh A, Reich K. Interleukin-17A: a unique pathway in immune-mediated diseases: psoriasis, psoriatic arthritis and rheumatoid arthritis. Immunology. 2014;141(2):133–142. doi:10.1111/imm.12142
46. Sun C, Kono H, Furuya S, et al. Interleukin-17A plays a pivotal role in chemically induced hepatocellular carcinoma in mice. Dig Dis Sci. 2016;61(2):474–488. doi:10.1007/s10620-015-3888-1
47. Wang Y, Wu H, Wu X, et al. Interleukin 17A promotes gastric cancer invasiveness via NF-κB mediated matrix metalloproteinases 2 and 9 expression. PLoS One. 2014;9(6):e96678. doi:10.1371/journal.pone.0096678
48. Wei L, Wang H, Yang F, et al. Interleukin-17 potently increases non-small cell lung cancer growth. Mol Med Rep. 2016;13(2):1673–1680. doi:10.3892/mmr.2015.4694
49. Ding W, Zhang XY, Pan M, et al. Interleukin-17A promotes the formation of inflammation in the lung tissues of rats with pulmonary fibrosis. Exp Ther Med. 2015;10(2):491–497. doi:10.3892/etm.2015.2564
50. Chen K, Kolls JK. Interleukin-17A (IL17A). Gene. 2017;614:8–14. doi:10.1016/j.gene.2017.01.016
51. Yasuda K, Nakanishi K, Tsutsui H. Interleukin-18 in health and disease. Int J Mol Sci. 2019;20(3):649. doi:10.3390/ijms20030649
52. Wynn JL, Wilson CS, Hawiger J, et al. Targeting IL-17A attenuates neonatal sepsis mortality induced by IL-18. Proc Natl Acad Sci USA. 2016;113(19):E2627–E2635. doi:10.1073/pnas.1515793113
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.