Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Congenital Ichthyosis: A Practical Clinical Guide on Current Treatments and Future Perspectives
Received 1 March 2023
Accepted for publication 29 August 2023
Published 11 September 2023 Volume 2023:16 Pages 2473—2479
DOI https://doi.org/10.2147/CCID.S388608
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Evelyn Lilly,1 Christopher G Bunick2
1Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, 02114, USA; 2Department of Dermatology and Program in Translational Biomedicine, Yale School of Medicine, New Haven, CT, 06520, USA
Correspondence: Christopher G Bunick, Department of Dermatology and Program in Translational Biomedicine, Yale School of Medicine, 333 Cedar Street, LCI 501, PO Box 208059, New Haven, CT, 06520-8059, USA, Tel +1-203-785-4092, Fax +1-203-785-7637, Email [email protected] Evelyn Lilly, Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 50 Staniford Street, Ste 200, Boston, MA, 02114, USA, Tel +1-617-643-8618, Fax +1-617-724-2745, Email [email protected]
Abstract: Congenital ichthyoses are a group of hereditary disorders of keratinization that are challenging to treat. Affected individuals suffer not only from thickening of the skin but also associated complications such as growth restriction, hearing and eye complications, infections, and thermodysregulation. This clinical review provides a practical roadmap to the longitudinal care of patients with ichthyosis with both general and age- and disease-specific recommendations. The allure of pathogenesis-based and targeted treatments for these monogenetic severe but orphan conditions shines bright as dermatological therapies enter a new era.
Keywords: cutaneous disease, genetic mutation, skin care, clinical management, topical and oral therapy, JAK and TYK2 inhibitors, retinoids
Introduction
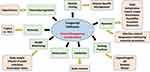
Congenital ichthyoses are a group of diseases which all have some degree of hyperkeratosis, or skin thickening. The genetic etiologies are diverse, but as many current treatments aim to address the symptoms, we will discuss the diseases together. Following the overall treatment recommendations, the major categories of which are highlighted in Figure 1, we will elaborate on disease-specific modifications or treatments if available (see Table 1 for summary). Since there are no approved treatments for any ichthyosis type, work to validate the use of topical retinoids, biologics, and anti-inflammatory small molecules in ichthyosis will be discussed in the final section.
 |
Table 1 Treatment Recommendations for Ichthyosis Type by Age Group |
General Considerations
Babies with congenital ichthyosis (CI) can be born with a variety of clinical appearances: seemingly normal skin, erosions, hyperkeratosis, and/or erythema. Given the rapidity and decreased cost of genetic testing, the standard of care as soon as pathology is detected is genetic testing in order to allow for timely disease-specific management. For neonates with epidermolytic ichthyosis (EI), collodion membranes, and especially harlequin ichthyosis (HI), neonatal intensive care with close dermatology consultation and ongoing monitoring and management is critical. The relatively high surface area to body mass of babies makes the increased transepidermal water loss (TEWL) particularly problematic in the neonatal period. Monitoring for and treatment of dehydration, sufficient caloric intake, and electrolyte imbalance is key. Humidified incubators and ointments can help minimize the impact of the abnormal skin barrier. Both the erosions of EI and the hyperkeratosis of non-bullous congenital ichthyosis predispose to skin infections, necessitating a high suspicion for and low threshold to treat bacterial and fungal skin infections to prevent sepsis. The tight collodion membrane and the thick plates of HI can physically restrict rib cage expansion, such that HI patients have shallow breaths and potential respiratory failure. Additionally, the decreased lung expansion and risk of aspiration of amniotic fluid with scale increase risk of pneumonia. Similarly, the tightness of the hyperkeratosis can constrict digits and distal limbs leading to ischemia; ointments, topical retinoids, oral retinoids, and surgery may be needed.1
In early childhood, continued monitoring, via body weight, of the effects of increased transepidermal water loss is important. Many children with ichthyosis are small for their age. Ensuring adequate water, caloric, protein, and iron intake can help minimize the effects of their erythroderma. Screening for vitamin D deficiency, especially among patients with more severe ichthyosis, darker skin types, and in the winter and spring, is recommended as the prevalence of vitamin D deficiency among patients with congenital ichthyosis was found to be higher than in the general baseline population.2
In childhood and adulthood, most congenital ichthyoses benefit from an individually honed topical skin care regimen that might include some of the following components.
Bathing
Daily bathing is helpful to hydrate and cleanse the skin. Baths are generally more helpful and recommended, although showers are often utilized due to time limitations. Hypoallergenic soap-free cleansers focused on the folds of the skin are best to minimize excessive drying of the skin.
Bathing in alkaline seawater (pH 8.1) or baking soda for 30–60 minutes at least once weekly can facilitate scale removal.3 Some patients also find colloidal oatmeal and salt additives in their bath and hydrotherapy with bubbles helpful to minimize scale, especially when combined with water pressure and massage.4 Dilute bleach baths, as often employed in atopic dermatitis to reduce Staphylococcus aureus infection, decrease microbial burden and its downstream consequences (eg, odor, bacterial infections, pustules, etc.). If a patient with CI has recurrent skin infections, bleach baths two to three times a week are often recommended, but the bleach bath should be followed by a quick shower to rinse off the dilute bleach from the skin. Gram-negative bacterial and recurrent fungal infections might respond better to dilute acetic acid (eg, vinegar) soaks, but too high of a concentration can cause stinging if the skin has any fissures.
Scale Removal
After long baths (30 minutes or more), scale may be removed with gentle physical exfoliation with a soft wash cloth or from the scalp with a fine toothed comb. Over-the-counter chemical keratolytics (eg, emollients containing urea, lactic acid, salicylic acid, glycolic acid, etc.) are frequently tried, but caution must be exercised in babies and children, who might find these products cause intolerable stinging and burning; rarely in babies salicylate toxicity could be observed. Compounded topical N-acetylcysteine is sometimes used, especially in pediatric populations, but reports of irritation, odor of the product, and compounding costs can be problematic.5
Moisturizers
Humectants (eg, glycerin and propylene glycol) and emollients (eg, petrolatum) improve xerosis and pruritus, reduce inflammation, and help repair the skin barrier. In addition to commercially available products, many patients with ichthyosis have personal formulations that they have found to work well for their skin type, and participation in patient support groups such as the Foundation for Ichthyosis & Related Skin Types, Inc., allow for sharing of these specificities.
Retinoids
Topical and oral retinoids are used for moderate or severe hyperkeratosis because of their role in promoting epidermal differentiation and barrier turnover.6 Current limitations for topical retinoids are cost and availability given its off-label use for ichthyosis, but ongoing clinical trials are attempting to minimize these barriers to patient care. Tazarotene cream is typically used because the first- and second-generation topical retinoids approved for acne (eg, tretinoin and adapalene) have not been shown to be helpful in ichthyosis and/or are too irritating. If tazarotene is too irritating, especially in anatomic sites with more sensitive skin, it can be applied less frequently or diluted with petrolatum or applied onto skin pretreated with a thin layer of petrolatum.
Oral retinoids dramatically help the most severely affected, but there are intricacies in management, especially as it pertains to certain types of ichthyosis, which will be discussed in the following section. Acitretin is generally not used in individuals of childbearing potential as pregnancy is only safe 3 years after the last dose; however, in the United States isotretinoin requires monthly counseling, attestations, and pregnancy tests for individuals of childbearing potential via the iPledge Risk Evaluation and Mitigation Strategy program. Cheilitis, xerosis, and skin irritation (especially if desquamation is excessive) are common side effects. Laboratory monitoring (at minimum alanine transaminase, aspartate transaminase, and triglyceride and cholesterol levels) is generally performed at baseline, maximum dose, and then twice yearly.7 Routine ophthalmic evaluation is recommended considering the mucosal dryness induced by systemic retinoids. Given the long-term use of systemic retinoids for CI, musculoskeletal side effects such as hyperostosis and premature epiphyseal closure have been reported, although both were diagnosed in patients treated with high doses and for long durations of retinoid exposure.8 Current recommendations are to perform growth assessments in children and baseline and follow-up DEXA scans every 3–5 years in adults on long-term systemic retinoid therapy.6
Disease-Specific Considerations
Oral retinoids for EI must be dosed at a very low level (eg, 0.25 mg/kg/day); higher doses can worsen the erosions. On the other side of the continuum, patients with severe lamellar ichthyosis (LI) often need at least 1 mg/kg/day of systemic retinoids, and after decades of use, might suffer from long-term consequences such as diffuse skeletal hyperostosis. Babies with HI also need systemic retinoids, but notably, they need acitretin 1mg/kg/day early to help shed the keratotic plates of skin to minimize the constrictive effects of the skin on breathing function.9
Both the erosions and hyperkeratosis of epidermolytic ichthyoses are often exacerbated by friction, making reasonable minimization of friction helpful. For non-bullous congenital ichthyoses, while scale removal is often helpful and desired, one does not want to remove too much scale, as the skin then becomes more tender and irritated.
For LI, the scale can occlude the eccrine glands, such that affected individuals experience hypohidrosis and more problematically thermodysregulation.10 If the hypohidrosis is mild or moderate, such that only specific activities cause thermodysregulation, cooling externally with spray bottles of water and/or use of cooling vests may be sufficient treatment, especially in children if systemic retinoids are otherwise not desired. Children with LI and more narrow external auditory canals can easily have their ears blocked with scale accumulation such that it is uncomfortable and can even cause conductive hearing loss. Many undergo routine professional ear debridement every 3–4 months. Routine hearing evaluations in young children should be considered for those with significant scale build up in the external auditory canals. The hyperkeratosis often causes ectropion and can also cause meibomian gland dysfunction; these conditions predispose individuals with lamellar ichthyosis to complications of keratoconjunctivitis sicca, such as corneal abrasions and ulcers. If the eyelids cannot completely close, nightly application of ophthalmic ointment is recommended to restore the tear film. Tazarotene cream applied to the eyelids also helps treat ectropion.11
Due to the abnormal keratinization of the congenital ichthyoses, patients–especially those with HI, EI, Netherton syndrome (NS), and KID syndrome–may experience secondary skin infections such as tinea corporis, candidiasis, viral infections, or scabies. Diagnosis may be more difficult due to the underlying baseline hyperkeratotic or inflamed skin. Having a high degree of suspicion and appreciating the patient’s perceptions of change in symptoms are important factors in early diagnosis.
For NS and ichthyosis vulgaris (IV), patients are more prone to developing atopic dermatitis and other cutaneous rashes, such as ichthyosis linearis circumflexa. These conditions can be treated using topical steroids, topical calcineurin inhibitors, narrow band UVB phototherapy, and dupilumab.12 Psoralen-UVA therapy is not recommended as patients with NS already have an increased risk of skin cancers, particularly non-melanoma, for which they should be screened regularly.
As understanding of the genetic causes and pathophysiology of congenital ichthyoses has expanded, pathogenesis-based therapies have been attempted with mixed success. These treatments that are being considered for ichthyosis fall into four categories: 1) Enzyme replacement therapy, 2) Immunomodulatory biologics, 3) Small-molecule protein inhibitors, and 4) Gene therapy.13 Cases of congenital hemidysplasia with ichthyosiform erythroderma and limb defects (CHILD syndrome) have had the skin changes improved with the application of a compounded topical statin.14,15 Individuals with NS that have associated primary immunodeficiency may experience cutaneous improvement with immunoglobulin replacement therapy.16 After finding that adults with epidermolytic ichthyosis, NS, lamellar ichthyosis, or congenital ichthyosiform erythroderma with at least moderate erythroderma had IL-17A immune skewing, a cohort of 20 subjects were treated with secukinumab with heterogeneous results.17 Four patients with NS experienced clinical improvement on secukinumab.18 Individual case reports have also demonstrated clinical improvement of erythroderma, pruritus, and frequency of cutaneous infections in a patient with congenital ichthyosiform erythroderma (due to CYP4F22 mutations) and atopic dermatitis with dupilumab and guselkumab.19
CARD14 associated papulosquamous eruption (CAPE) is caused by mutations in CARD14, which activates the nuclear factor-kappa B pathway, and responds preferentially to ustekinumab.20 Zileuton, which blocks leukotriene formation, helps some patients with Sjogren-Larsson (causing deficiency in microsomal fatty aldehyde dehydrogenase, affecting leukotriene metabolism) who have pruritus.21
Future Considerations
In dermatology in 2022–2023 Janus kinase (JAK) inhibitors have emerged, with multiple agents now approved for the treatment of atopic dermatitis (abrocitinib, ruxolitinib, and upadacitinib), alopecia areata (baricitinib, ritlecitinib), vitiligo (ruxolitinib), and plaque psoriasis (deucravacitinib). Systemically, these small-molecule JAK inhibitors show potential for a role in treating subtypes of congenital ichthyosis. While the aforementioned agents have focused selectivity for JAK 1, 2, and/or 3 or TYK2, it is the pan-JAK inhibitor tofacitinib that has been investigated in the treatment of HI to date. Studies showed that HI patients have upregulated STAT1 signaling, and consequently nitric oxide synthase (NOS2). When blocking STAT1/NOS2 signaling in an HI living skin equivalent 3D model using tofacitinib, the HI phenotype was improved.22 This finding is supported by investigations showing that multiple congenital ichthyosis subtypes harbor elevated JAK/STAT signaling.23
With accessibility of the skin and monogenetic causes, ichthyosis has always been an obvious choice for gene therapy. Unfortunately, the few completed clinical trials have been disappointing, but they provided a framework for future potential gene therapy options. Three adults with lamellar type ARCI had subtle improvement after treatment with TG1 delivered by a replication-defective herpes simplex virus type 1 vector.24 Similarly, epithelial sheets with keratinocytes expressing SPINK5 transduced by a lentiviral vector only provided temporary improvement.25
The lure of these pathogenesis-based treatments is great, but by definition, each subtype of ichthyosis requires a different treatment. Therefore, focus must be on improving and getting approval for treatments that work for many types of ichthyosis. Generalized hyperkeratosis, scaling, and dryness experienced by congenital ichthyosis patients are commonly treated using topical and/or oral retinoids; however, this use is currently off-label. Off-label retinoid use presents potential problems, including access to affordable medication in appropriate quantities (if topical) or risk of systemic and dose-limiting toxicities with the use of oral retinoids. While it has historically been difficult to formulate isotretinoin in a vehicle that can be effectively delivered topically, TMB-001 is a new topical isotretinoin ointment that uses a proprietary polyethylene glycol delivery technology to treat congenital ichthyosis by providing hydration, lubrication and scale reduction. In phase 2a26 and phase 2b27 clinical studies, TMB-001 proved to be safe and effective in patients with recessive X-linked and autosomal recessive lamellar congenital ichthyosis. Remarkably, 100% of per-protocol patients receiving TMB-001 0.05% ointment achieved the primary endpoints of (i) 50% or greater reduction in scaling as assessed by the Visual Index for Ichthyosis Severity (VIIS)28 and (ii) 2-grade or more improvement in the Investigator Global Assessment (IGA) Likert scale. A Phase 3 trial with TMB-001 0.05% ointment is ongoing, and this medication represents a promising new therapy that could provide congenital ichthyosis patients an effective on-label topical retinoid option.
Conclusion
The treatment of congenital ichthyosis is at an interesting juncture. Treatments span the spectrum of traditional dermatology (eg, baking soda and bleach baths, emollients, etc.) to cutting edge (eg, pathophysiologic-based gene therapy). Whether through new topical or systemic agents, advances are being made in the treatment of congenital ichthyosis. These breakthroughs will provide dermatologists and other healthcare providers more therapeutic options than ever before for the care of this special group of patients.
Funding
This work was supported by the US National Institutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) under Award Number R01 AR079428 (to CGB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure
CGB is a principal investigator in Timber’s TMB-001 phase 3 clinical trial, same as for the phase 2a and phase 2b trials. CGB reports personal fees from AbbVie, personal fees from Arcutis, grants from Almirall, personal fees from LEO Pharma, personal fees from Pfizer, personal fees from Eli Lilly, personal fees from UCB, personal fees from Novartis, personal fees from Sanofi-Regeneron, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Tontchev G, Silverberg NB, Shlasko E, Henry C, Roberts JL, Roth MZ. Techniques for toddlers: linear band incision for harlequin ichthyosis with associated compartment syndrome. Pediatr Dermatol. 2014;31(5):625–629. PMID: 25187390. doi:10.1111/pde.12446
2. Frascari F, Drefus I, Rodriguez L, et al. Prevalence and risk factors of vitamin D deficiency in inherited ichthyosis: a French prospective observational study performed in a reference center. Orphanet J Rare Dis. 2014;9:127. doi:10.1186/s13023-014-0127-3
3. Milstone LM. Scaly skin and bath pH: rediscovering baking soda. J Am Acad Dermatol. 2010;62(5):885–886. PMID: 20398816. doi:10.1016/j.jaad.2009.04.011
4. Bodemer C, Bourrat E, Mazereeuw-Hautier J, et al. Short- and medium-term efficacy of specific hydrotherapy in inherited ichthyosis. Br J Dermatol. 2011;165(5):1087–1094. PMID: 21729027. doi:10.1111/j.1365-2133.2011.10510.x
5. Kaplan L, Castelo-Soccio L. Topical N-acetylcysteine in ichthyosis: experience in 18 patients. Pediatr Dermatol. 2018;35(4):528–530. PMID: 29582451. doi:10.1111/pde.13489
6. Zaenglein AL, Levy ML, Stefanko NS, et al. Consensus recommendations for the use of retinoids in ichthyosis and other disorders of cornification in children and adolescents. Pediatr Dermatol. 2021;38(1):164–180. doi:10.1111/pde.14408
7. Katugampola RP, Finlay AY. Oral retinoid therapy for disorders of keratinization: single-centre retrospective 25 years’ experience on 23 patients. Br J Dermatol. 2006;154:267–276. doi:10.1111/j.1365-2133.2005.06906.x
8. DiGiovanna JJ. Isotretinoin effects on bone. J Am Acad Dermatol. 2001;45(5):S176–S182. doi:10.1067/mjd.2001.113721
9. Singh S, Bhura M, Maheshwari A, Kumar A, Singh CP, Pandey SS. Successful treatment of harlequin ichthyosis with Acitretin. Int J Dermatol. 2001;40(7):472–473. PMID: 11679007. doi:10.1046/j.1365-4362.2001.01173.x
10. Haenssle HA, Finkenrath A, Hausser I, et al. Effective treatment of severe thermodysregulation by oral retinoids in a patient with recessive congenital lamellar ichthyosis. Clin Exp Dermatol. 2008;33(5):578–581. PMID: 18355358. doi:10.1111/j.1365-2230.2008.02709.x
11. Craiglow BG, Choate KA, Milstone LM. Topical tazarotene for the treatment of ectropion in ichthyosis. JAMA Dermatol. 2013;149(5):598–600. PMID: 23677086; PMCID: PMC3809825. doi:10.1001/jamadermatol.2013.239
12. Aktas M, Salman A, Apti Sengun O, et al. Netherton syndrome: temporary response to dupilumab. Pediatr Dermatol. 2020;37(6):1210–1211. PMID: 32951242. doi:10.1111/pde.14362
13. Joosten MDW, Clabbers JMK, Jonca N, Mazereeuw-Hautier J, Gostynski AH. New developments in the molecular treatment of ichthyosis: review of the literature. Orphanet J Rare Diseases. 2022;17:269. doi:10.1186/s13023-022-02430-6
14. Paller AS, van Steensel MA, Rodriguez-Martín M, et al. Pathogenesis-based therapy reverses cutaneous abnormalities in an inherited disorder of distal cholesterol metabolism. J Invest Dermatol. 2011;131(11):2242–2248. PMID: 21753784; PMCID: PMC3193573. doi:10.1038/jid.2011.189
15. Kallis P, Bisbee E, Garganta C, Schoch JJ. Rapid improvement of skin lesions in CHILD syndrome with topical 5% simvastatin ointment. Pediatr Dermatol. 2022;39(1):151–152. PMID: 34787337. doi:10.1111/pde.14865
16. Zelieskova M, Banovina P, Kozar M, Kozarova A, Nudzajova Z, Jesenak M. A novel SPINK5 mutation and successful subcutaneous immunoglobulin replacement therapy in a child with Netherton syndrome. Pediatr Dermatol. 2020;37(6):1202–1204. PMID: 32767583. doi:10.1111/pde.14318
17. Lefferdink R, Rangel SM, Chima M, et al. Secukinumab responses vary across the spectrum of congenital ichthyosis in adults. Arch Dermatol Res. 2023;315(2):305–315. PMID: 35218370. doi:10.1007/s00403-022-02325-3
18. Luchsinger I, Knopfel N, Theiler M, et al. Secukinumab therapy for Netherton syndrome. JAMA Dermatol. 2020;156:907–911. doi:10.1001/jamadermatol.2020.1019
19. Steinhoff M, Al-Marri F, Al Chalabi R, Gieler U, Buddenkotte J. Recalcitrant erythrodermic ichthyosis with atopic dermatitis successfully treated with Dupilumab in combination with Guselkumab. Skin Health Dis. 2021;2(1):e87. PMID: 35665208; PMCID: PMC9060106. doi:10.1002/ski2.87
20. Eytan O, Sarig O, Sprecher E, van Steensel MA. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171(2):420–422. PMID: 24641799. doi:10.1111/bjd.12952
21. Fuijkschot J, Seyger MM, Bastiaans DE, Wevers RA, Roeleveld N, Willemsen MA. Zileuton for pruritus in Sjögren-Larsson syndrome: a randomized double-blind placebo-controlled crossover trial. Acta Derm Venereol. 2016;96(2):255–256. PMID: 26123322. doi:10.2340/00015555-2195
22. Enjalbert F, Dewan P, Caley MP, et al. 3D model of harlequin ichthyosis reveals inflammatory therapeutic targets. J Clin Invest. 2020;130(9):4798–4810. doi:10.1172/JCI132987
23. Ho M, Nguyen H-N, Hoang MV, et al. Altered skin microbiome, inflammation, and JAK/STAT signaling in Southeast Asian ichthyosis patients. medRxiv. 2022;2022:2022–2008.
24. Parry TJ, Zhang P, MajumdarA, et al.Preclinical Evaluation of a Modified Herpes Simplex Virus Type 1 Vector Encoding Human TGM1 for the Treatment of Autosomal Recessive Congenital Ichthyosis. J Invest Dermatol. 2021;141(4):874–882.e6. doi:10.1016/j.jid.2020.07.035
25. Di WL, Lwin SM, Petrova A, et al. Generation and clinical application of gene-modified autologous epidermal sheets in Netherton syndrome: lessons learned from a Phase 1 trial. Hum Gene Ther. 2019;30(9):1067–1078. doi:10.1089/hum.2019.049
26. Paller AS, Browning J, Parish LC, Bunick CG, Rome Z, Bhatia N. Safety, tolerability, and efficacy of a novel topical isotretinoin formulation for the treatment of X-linked or lamellar congenital ichthyosis: results from a phase 2a proof-of-concept study. J Am Acad Dermatol. 2022;87(5):1189–1191. PMID: 35271936. doi:10.1016/j.jaad.2022.02.060
27. Teng JMC, Bunick CG, Guenthner S, et al. The CONTROL study: a randomized, double-blind vehicle-controlled phase 2b study of novel topical isotretinoin formulation demonstrates improvement in recessive X-linked and autosomal recessive lamellar congenital ichthyosis. J Am Acad Dermatol. 2022;87(6):1455–1458. PMID: 35872261. doi:10.1016/j.jaad.2022.07.028
28. Marukian NV, Deng Y, Gan G, et al. Establishing and validating an ichthyosis severity index. J Invest Dermatol. 2017;137(9):1834–1841. PMID: 28596001. doi:10.1016/j.jid.2017.04.037
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.