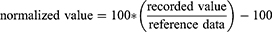Back to Journals » Journal of Pain Research » Volume 17
Contact-Heat Evoked Potentials: Insights into Pain Processing in CRPS Type I
Authors Allmendinger F , Scheuren PS, De Schoenmacker I, Brunner F , Rosner J, Curt A, Hubli M
Received 29 September 2023
Accepted for publication 23 February 2024
Published 13 March 2024 Volume 2024:17 Pages 989—1003
DOI https://doi.org/10.2147/JPR.S436645
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Alaa Abd-Elsayed
Florin Allmendinger,1 Paulina Simonne Scheuren,1– 4 Iara De Schoenmacker,1 Florian Brunner,5 Jan Rosner,1,2,6 Armin Curt,1 Michèle Hubli1
1Spinal Cord Injury Center, Balgrist University Hospital, University of Zurich, Zurich, Switzerland; 2Department of Neurology, University Hospital Bern, Inselspital, University of Bern, Bern, Switzerland; 3International Collaboration on Repair Discoveries, Vancouver, BC, Canada; 4Department of Anesthesiology, Pharmacology & Therapeutics, Faculty of Medicine, University of British Columbia, Vancouver, BC, Canada; 5Department of Physical Medicine and Rheumatology, Balgrist University Hospital, University of Zurich, Zurich, Switzerland; 6Danish Pain Research Center, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark
Correspondence: Florin Allmendinger, Spinal Cord Injury Center, Balgrist University Hospital, University of Zurich, Forchstrasse 340, Zurich, CH-8008, Switzerland, Tel +41 44 510 72 11, Email [email protected]
Purpose: The pathophysiological mechanisms underlying the development of chronic pain in complex regional pain syndrome (CRPS) are diverse and involve both peripheral and central changes in pain processing, such as sensitization of the nociceptive system. The aim of this study was to objectively distinguish the specific changes occurring at both peripheral and central levels in nociceptive processing in individuals with chronic CRPS type I.
Patients and Methods: Nineteen individuals with chronic CRPS type I and 16 age- and sex-matched healthy controls (HC) were recruited. All individuals underwent a clinical examination and pain assessment in the most painful limb, the contralateral limb, and a pain-free control area to distinguish between peripheral and central mechanisms. Contact-heat evoked potentials (CHEPs) were recorded after heat stimulation of the three different areas and amplitudes and latencies were analyzed. Additionally, quantitative sensory testing (QST) was performed in all three areas.
Results: Compared to HC, CHEP amplitudes in CRPS were only increased after stimulation of the painful area (p=0.025), while no increases were observed for the pain-free control area (p=0.14). None of the CHEP latencies were different between the two cohorts (all p> 0.23). Furthermore, individuals with CRPS showed higher pain ratings after stimulation of the painful limb compared to their contralateral limb (p=0.013). Lastly, compared to HC, mechanical (p=0.012) and thermal (p=0.046) sensitivity was higher in the painful area of the CRPS cohort.
Conclusion: This study provides neurophysiological evidence supporting an intact thermo-nociceptive pathway with signs of peripheral sensitization, such as hyperexcitable primary afferent nociceptors, in individuals with CRPS type I. This is further supported by the observation of mechanical and thermal gain of sensation only in the painful limb. Additionally, the increased CHEP amplitudes might be related to fear-induced alterations of nociceptive processing.
Keywords: sensitization, thermo-nociceptive processing, pain mechanism, pain hypersensitivity, complex regional pain syndrome, contact-heat evoked potentials
Introduction
Complex regional pain syndrome (CRPS) is a pain condition typically beginning in a single limb as a result of an inciting event, such as an injury or surgery. The pain is disproportionate in magnitude and duration to this inciting event.1–3 Mechanical hyperalgesia and dynamic allodynia in the painful limb are hallmark signs in individuals with CRPS4–6 and can spread to initially unaffected areas, eg, the contralateral limb, potentially associated with central sensitization.4,5,7–9 While sensitization of the nociceptive system is thought to play an essential role in CRPS,10,11 it remains challenging to objectively assess its presence in humans.
Contact heat evoked potentials (CHEPs) have been introduced as a promising tool for the objective assessment of nociceptive processing in a variety of peripheral12 and central13 neuropathies, and may also be valuable to investigate pathophysiological pain processing in CRPS. CHEPs assess the functional integrity of the nociceptive system upon activation of peripheral nociceptors and the transmission to cortical areas14,15 Reduced amplitudes and prolonged latencies of pain-evoked potentials have been associated with peripheral fiber loss in painful sensory polyneuropathies,16 while enhanced amplitudes could be partially explained by central sensitization and deficient descending inhibition.14 In addition, heat-evoked potentials have been shown to be enhanced by attentional focusing on the painful region in chronic pain patients17 and decreased by positive emotional modulation.18 Therefore, heat-evoked potentials open the possibility to examine altered pain processing within peripheral and central levels of the nociceptive system and in the sensory-discriminative and affective-motivational aspects of pain processing.
Several previous studies have reported increased heat-evoked potentials in individuals with fibromyalgia,19–22 a widespread chronic pain with assumed central sensitization.23 These studies suggested enhanced thermo-nociceptive processing possibly indicating the involvement of dysfunctional pain processing at central levels, including greater attention or cognitive appraisal of painful stimuli, reduced brain-stem or cortical inhibition, as well as increased afferent input due to peripheral hyperexcitability. However, the use of heat-evoked potentials in CRPS is sparse. So far, only one study applied laser-evoked potentials (LEP) to assess the function of the thermo-nociceptive system in individuals with CRPS,24 demonstrating decreased LEP amplitudes and prolonged latencies after laser stimulation of the painful limb. While Caty and colleagues postulated a dysfunction of the thermo-nociceptive system in individuals with CRPS, they leave it open whether it results from pathological mechanisms involving the peripheral and/or the central nervous system. Although absence of an unequivocal nerve lesion is a diagnostic criterion for CRPS type I,25 there is accumulating evidence for an impairment of small fiber function with reductions in intra-epidermal nerve fiber densities (IENFD).26,27 Decreased IENFD would also be in line with the findings of Caty et al, 2013,24 as the loss of A-delta fibers would explain prolonged latencies and decreased amplitudes of pain-evoked potentials.16 The complex interplay between peripheral small fiber pathology and sensitization processes along the nociceptive axis still remains unknown with regard to the underlying pathophysiology of pain in CRPS.
The objective of the present study was to disentangle peripheral from central origins of dysfunctional thermo-nociceptive processing in individuals with CRPS by assessing CHEPs not only from the painful and the contralateral limb but also from a remote, pain free, control area. We hypothesized that (1) individuals with CRPS will have decreased CHEP amplitudes and prolonged CHEP latencies after stimulation of the painful area, compared to healthy controls (HC) and (2) increased CHEP amplitudes after stimulation of the contralateral and the control limb. These results would reflect known peripheral impairments in individuals with CRPS24 and reveal widespread hyperexcitability (ie, central sensitization) of the nociceptive system.
Methods
Study Subjects
This study was part of a larger study conducted between November 2019 and April 2022, including several patient cohorts and matched HC. The presented analysis was performed on data acquired in the CRPS cohort, of which other outcomes have already been published.9,28 Nineteen individuals with chronic CRPS type I, diagnosed based on the Budapest criteria,1 were recruited at the Department of Physical Medicine and Rheumatology of the Balgrist University Hospital in Zurich, Switzerland. Individuals included in the study had to be between 18 and 80 years old, were not pregnant and showed no signs of any neurological disorder, psychiatric disorder or history of chronic pain other than CRPS-related pain.
In addition to individuals with CRPS, 16 age- and sex-matched HC were recruited. Exclusion criteria for HC were a history of neurological or psychiatric disorder, acute or chronic pain, pregnancy, as well as intake of pain medication.
Informed consent was obtained from all individuals prior to the study. The study was approved by the local ethics committee “Kantonale Ethikkommission Zürich” (EK-04/2006, PB_2016-02051) and conducted in accordance with the Declaration of Helsinki.
Study Design
The study visit consisted of a clinical examination and pain assessment, quantitative sensory testing (QST) and recording of CHEPs and pain ratings towards contact-heat stimuli, as well as the completion of psychological and pain questionnaires (Hospital Anxiety and Depression Scale (HADS)29 and Pain Catastrophizing Scale (PCS)).30 A part of the painDETECT questionnaire31 was used to assess self-reported current pain and average pain intensity over the last month on a numeric rating scale (NRS) from 0 (no pain) to 10 (worst pain imaginable). Assessments were performed in 1) the most painful limb of individuals with CRPS, 2) the contralateral limb and 3) a pain-free, remote, control area. The order in which the areas were assessed was randomized. The control area was defined as either the contralateral shoulder, if the most painful area was an upper extremity, or the contralateral hand, if the painful area was a lower extremity. In one case where the most painful area was the shoulder, no control area was assessed. The exact same three tested areas (painful, contralateral and control) were also tested in the age- and sex-matched HC.
A flowchart of the study procedure can be found in the Supplementary Material Figure S1.
Clinical Examination and Pain Assessment
Clinical examination was done by a physician specialized in CRPS (F.B.) and comprised of the assessment of signs and symptoms including sudo- and vasomotor changes, trophic changes, motor changes and neglect-like symptoms. Additionally, a semi-quantitative bedside sensory testing, including vibration detection, light touch, pinprick sensation and thermal cold and warm detection, was performed in all HC to exclude any sub-clinical sensory impairments.
Contact Heat-Evoked Potentials (CHEPs)
CHEP Stimulation Paradigm
Fifteen to 20 heat stimuli were applied to each of the three testing areas, ie the painful, the contralateral and the control area. There was a two-minutes break between the areas. Heat stimuli were applied with a 27mm diameter CHEPs thermode (PATHWAY Pain and Sensory Evaluation System, Medoc Ltd., Ramat Yishai, Israel). The thermode had a baseline temperature of 42°C and heated up to 52°C with a speed of 70°C/s.32 If the participant did not tolerate the heat stimuli, the baseline temperature was lowered to 35°C, while keeping the same destination temperature of 52°C,32 as these stimuli have previously been reported to be less painful. The interstimulus interval was set to 13–17s and the thermode was slightly shifted after each stimulus to avoid peripheral receptor fatigue.33
CHEP Recording Set-Up
The protocol for the recording of CHEPs has previously been published.34–36 Briefly, during the recording of the electroencephalographic (EEG) signals, all individuals were lying in a supine position with open eyes. We used 9mm Ag/AgCl cup electrodes filled with conductive adhesive gel (Elefix, Nihon Kohden, Tokyo, Japan) to record the EEG signals. The active electrode was placed at the vertex (Cz) with the earlobes (A1 and A2) as references. Before attaching the electrodes, recording sites were prepared with Nuprep (D.O. Weaver & Co., Aurora, Co, USA) and alcohol. EEG signals were sampled at a frequency of 2000 Hz. Signals were amplified (20000x, ALEA Solutions, Zurich, Switzerland) and bandpass filtered (0.5–30 Hz). Additionally, the electro-oculogram (EOG) was recorded via two surface electrodes (Ambu BlueSensor, Ballerup, Denmark) placed above and below one eye to detect ocular artefacts within the EEG traces. A time window of 10s (1s pre-trigger until 9s post-trigger) was recorded in a customized program based on LabView (V2.6.1. CHEP, ALEA Solutions).
Quantitative Sensory Testing (QST)
A subset of the QST battery defined by the German Network on Neuropathic Pain37 was performed in all individuals to detect potential mechanical or thermal hypersensitivities. Specifically, heat pain thresholds (HPT), mechanical pain threshold (MPT) and mechanical pain sensitivity (MPS) were assessed in the painful area, as well as in the contralateral limb and the control area by trained experimenters. An unaffected area other than the three tested areas was used for familiarization prior to testing. QST values were normalized to age, sex and body region-specific reference values of the German Research Network on Neuropathic Pain38 using the eQuiSTA software and are presented as z-scores. Raw values of the QST can be found in the Supplementary Material Tables S2a (HPT and MPT) and S2b (MPS).
Data and Statistical Analysis
Data Analysis
Recorded EEG data were visually inspected by two independent examiners and trials contaminated with ocular or other artifacts were excluded offline. For each individual and test area, the first twelve artifact-free trials were used to compute an average waveform. A customized algorithm created in RStudio software (R version 4.2.2 for Windows) was used for an automated detection of N2- and P2-peaks of CHEPs. Latencies and amplitudes detected by the algorithm underwent a final inspection by two independent examiners to ensure correct detection. CHEP latencies and amplitudes, as well as corresponding heat pain ratings were then normalized to reference data34,39 and are reported as percentage of deviation from the reference data (Equation 1).
As CHEP amplitudes and latencies are known to depend on factors including age, stimulation location and intensity,34,39,40 normalization of the data was a crucial step, enabling us to combine and analyze data from various stimulation areas (such as hand, shoulder, or foot), individuals of different age groups (eg, younger than 40 years, older than 40 years, or older than 60 years), and different stimulation intensities (eg, normal baseline at 35°C or increased baseline at 42°C). Thus, this approach allowed us to establish a common data reference space for accurate analysis and interpretation.
Statistical Analysis
The differences in age and questionnaire outcomes (ie PCS, HADS) between the two cohorts were assessed with Wilcoxon signed-rank tests. Separate linear mixed effect models (“lmer” function of the R package “lme4”) were used to assess the main effect of stimulated “area” (painful, contralateral or control) and “cohort” (CRPS or HC) on 1) N2-latencies, 2) P2-latencies, 3) N2-amplitudes, 4) P2-amplitudes, 5) N2P2-amplitudes, 6) heat pain ratings, 7) HPT z-score, 8) MPT z-score and 9) MPS z-score. The interaction “area x cohort” was included in all models and “subject-ID” was included as random effect. Planned, hypothesis-driven post-hoc multiple comparisons (R package “emmeans”) were performed on the interaction “area x cohort” in each model to detect cohort-specific differences within each tested area and area-specific differences within the two cohorts. Fulfillment of model criteria was checked with diagnostic plots (ie, quantile-quantile plots and histograms).
All statistical analyses were performed in R statistical software (R version 4.2.2 for Windows). Significance level was set to p < 0.05. P-values are reported after Bonferroni correction for multiple comparison.
Results
Demographics and Clinical Information
Nineteen individuals with chronic CRPS type I were recruited. One individual had to be excluded due to noncompliance during CHEPs recording, leaving 18 individuals with CRPS type I (16 women, 2 men) included in the study. Additionally, 16 HC (14 women, 2 men) were recruited. There were no differences in age between the two cohorts (CRPS: 45.6 ± 12.1 years (range 29–65 years); HC: 41.8 ± 13.3 years (range 23–61 years); W=171; p=0.36). Individuals with CRPS reported pain since 33.5 ± 26.3 months (range 6–96 months) with a current pain intensity of NRS 4.9 ± 2.4 and an average pain intensity over the last month of NRS 5.6 ± 2.5. Furthermore, individuals with CRPS showed higher pain catastrophizing scores (CRPS: 23.0 ± 12.3; HC: 5.9 ± 7.9; W=251; p<0.001), as well as higher anxiety (CRPS: 8.0 ± 4.1; HC: 3.6 ± 2.8; W=235.5; p=0.002) and depression scores (CRPS: 7.1 ± 5.2; HC: 1.5 ± 1.7; W=239.5; p<0.001) than HC. Additional clinical characteristics of the CRPS cohort are shown in Table 1. Overall, 15 individuals with CRPS (83%) were on pain medication, eg, non-steroidal anti-inflammatory drugs (NSAIDs), antidepressants, during the study.
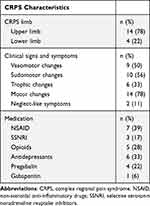 |
Table 1 Clinical Characteristics of Individuals with CRPS |
CHEPs
All numbers of included individuals with CRPS and HC and the corresponding raw values of CHEP amplitudes and latencies, as well as heat pain ratings used for further analyses can be found in Table 2.
 |
Table 2 Raw Data (Mean (± SD)) of CHEPs Characteristics (N2-Latency, P2-Latency, N2P2-Amplitude and Heat Pain Rating) |
Four individuals with CRPS could not follow the instructions due to too much pain during heat stimuli applied in the painful limb and therefore did not present with twelve artifact-free CHEPs. One individual with CRPS did not tolerate the heat stimuli in the control area. In total, two individuals with CRPS did not show a detectable evoked potential after stimulation of the painful limb. In the contralateral limb, all but one individual with CRPS presented with detectable evoked potentials.
For the HC cohort, CHEPs were detectable in all individuals after stimulation of the matched painful limb. However, three HC presented with absent CHEPs after stimulation of the contralateral area and one after stimulation of the control area.
All statistical analyses with corresponding model statistics, including F- and p-values, can be found in the Supplementary Tables S3 (model statistics), S4 and S5 (post-hoc comparisons).
Latencies
Normalized N2-latencies did not differ between individuals with CRPS and HC for all three areas: painful (CRPS: −2.15 ± 21.07%, HC: 0.82 ± 13.49%), contralateral (CRPS: −0.58 ± 19.82%, HC: −4.77 ± 10.92), and control area (CRPS: 5.41 ± 18.88%, HC: −3.68 ± 7.05%; all p>0.24) (Figure 1). N2-latencies did also not differ between the areas within each cohort (all p>0.36).
Similarly, P2-latencies did not differ between the cohorts in the painful (CRPS: 1.09 ± 14.66%, HC: 1.72 ± 11.32%), the contralateral (CRPS: 4.82 ± 18.09%, HC: −2.31 ± 11.04%), and the control area (CRPS: 8.73 ± 14.93%, HC: −0.66 ± 10.54%; all p>0.2). P2-latencies did also not differ between areas within each cohort (all p>0.09).
The lack of differences in CHEPs latencies therefore indicates a normal stimuli conductance in individuals with CRPS type I.
Amplitudes
For illustrative purpose, Figure 2 shows the average CHEP after stimulation of the painful limb from an individual with CRPS and the matched HC.
N2P2-amplitudes were increased in individuals with CRPS compared to HC after stimulation of the painful limb (CRPS: 24.89 ± 30.80%, HC: −11.19 ± 35.34%; p=0.025), but not after stimulation of the contralateral (CRPS: 6.24 ± 33.75%, HC: −1.33 ± 38.84%) or the control area (CRPS: 17.17 ± 43.53%, HC: 0.30 ± 38.42%; all p>0.14) (Figure 3). Within each cohort, N2P2-amplitudes did not differ between areas (all p>0.23).
Similar findings were found for the N2-amplitudes of CHEPs. N2-amplitudes were increased in individuals with CRPS compared to HC after stimulation of the painful limb (CRPS: 43.36 ± 58.61%, HC: −14.60 ± 49.53%; p=0.046), but not after stimulation of the other two areas (all p>0.64). In addition, individuals with CRPS presented with increased N2-amplitudes in the painful compared to the contralateral limb (−4.31 ± 65.59%; p=0.025).
In contrast, P2-amplitudes did not differ between individuals with CRPS and HC in all three areas (all p>0.07). Within each cohort, P2-amplitudes did not differ between areas (all p>0.16).
These findings suggest that the increased N2P2-amplitudes are mainly driven through an increase of the N2-peak after stimulation of the CRPS painful limb.
Heat Pain Ratings
Mean normalized heat pain ratings did not differ between CRPS and HC after stimulation of the painful (CRPS: −16.33 ± 47.59%, HC: −44.12 ± 29.85%), the contralateral (CRPS: −34.27 ± 44.14%, HC: −49.84 ± 32.27%), or the control area (CRPS: 37.32 ± 31.51%, HC: −43.35 ± 35.64%; all p>0.05). However, the pain ratings were increased after stimulation of the painful limb compared to the contralateral limb in CRPS (p=0.013) (Figure 4) but not compared to the control area (p=0.12). Pain ratings did not differ between areas within the cohort of HC (all p>0.42).
Quantitative Sensory Testing
Mechanical Pain Sensitivity (MPS)
Individuals with CRPS showed higher MPS z-scores compared to HC in the painful area (CRPS: 2.32 ± 1.33; HC: 1.19 ± 0.99; p=0.012), but not in the contralateral (CRPS: 1.51 ± 1.39, HC: 1.25 ± 0.96) or the control area (CRPS: 1.73 ± 1.81, HC: 1.54 ± 0.88; all p>0.49) (Figure 5). Furthermore, individuals with CRPS presented with increased MPS z-scores in the painful compared to the contralateral area (p=0.008) but not compared to the control area (p=0.14). In HC, the MPS did not differ between all areas (all p>0.62).
Mechanical Pain Threshold (MPT)
MPT z-scores did not differ between CPRS and HC in all three tested areas (all p>0.23). However, individuals with CRPS presented with higher MPT z-scores in the painful limb (1.72 ± 1.11) compared to the control (0.61 ± 1.24; p=0.0003), but not the contralateral limb (1.10 ± 0.94; p=0.09) (Figure 5). In HC, MPT z-scores did not differ between the three tested areas (all p=1).
These findings highlight the presence of mechanical hypersensitivity in the painful limb of individuals with CRPS.
Heat Pain Threshold (HPT)
The HPT z-scores of individuals with CRPS were higher compared to HC in the painful area (CRPS: 0.94 ± 1.32; HC: 0.15 ± 1.17; p=0.046), but not in the contralateral (CRPS: 0.42 ± 1.13; HC: 0.07 ± 1.30) or the control area (CRPS: 0.03 ± 1.0; HC: −0.41 ± 0.77; all p>0.36). Additionally, in individuals with CRPS, the HPT z-scores were higher in the painful compared to the control (p=0.024), but not the contralateral area (p=0.33) (Figure 5). In HC, HPT z-scores did not differ between the tested areas (all p>0.3).
These results suggest an additional thermal hypersensitivity in the painful limb of individuals with CPRS type I.
Discussion
The aim of this study was to disentangle peripheral from central of dysfunctional thermo-nociceptive processing in the cohort of chronic CRPS type I. The present study shows that individuals with CRPS have increased CHEP amplitudes after noxious contact-heat stimulation of their painful limb compared to HC, but not after stimulation of the contralateral or the control area. CHEP latencies, however, did not differ between CRPS and HC. These increased CHEPs were mainly driven by increased N2-peaks. Additionally, mechanical and thermal hypersensitivities were present in the painful area of individuals with CRPS. These results contrast the findings of one single previous study investigating heat-evoked potentials in CRPS,24 demonstrating decreased LEP-amplitudes and prolonged latencies after stimulation of the painful limb in individuals with CRPS compared to HC. Furthermore, we found higher heat pain ratings after stimulation of the painful, compared to the contralateral limb in individuals with CRPS. In the following paragraphs, we will discuss how these contradicting findings to the study of Caty et al, 2013,24 could be interpreted and what aspects of the peripheral and central nervous system could drive this hypersensitivity towards thermal stimuli.
Normal CHEP Latencies Indicate Intact Thermo-Nociceptive Pathways
Heat-evoked potentials represent an objective method to examine the integrity of the thermo-nociceptive neuroaxis14,15 and have been shown to be sensitive for small fiber loss in a variety of patients.16,41–43
Decreased amplitudes and prolonged latencies of heat-evoked potentials, as found by Caty et al, 2013,24 would therefore imply a loss of small fibers in the cohort of CRPS type I. Such a loss of peripheral fibers in CRPS has also been shown by studies quantifying the IENFD of the CRPS-affected limb. For instance, Oaklander et al, 2006,26 found a roughly 30% loss of IENFD in the painful, compared to the contralateral limb. In a more recent study,27 a reduction of IENFD was found in the painful, as well as in the contralateral limb of individuals with CRPS type I. However, our data provide no neurophysiological evidence of pathological loss within the nociceptive system in the cohort of CRPS type I, as there were no differences in latencies between individuals with CRPS and HC and also no decreased amplitudes compared to HC. This finding would be in line with literature, where the cohort of CRPS type I is typically described as a syndrome with no identified nerve lesion.10 Furthermore, a study by Kharkar et al 201244 did not find any differences in IENFD between the painful and unaffected areas in CRPS type I, nor compared to HC, which would also suggest structurally intact peripheral nociceptive pathways. Thus, the extent to which peripheral small fiber integrity is impaired, structurally and/or functionally, in individuals with CRPS type I remains unclear.
Increased CHEP Amplitudes and Pain Ratings as a Potential Indicator of Peripheral Sensitization
In this study, CHEPs were increased after stimulation of the painful area in individuals with CRPS compared to HC, but not after stimulation of the contralateral side or the remote, control area. While heat-evoked potentials have rarely been used in the cohort of CRPS, several LEP studies have shown such increases in vertex potentials in a centrally sensitized pain cohort of fibromyalgia,19–22 after stimulation of tender as well as nontender points. These studies suggested a generalized increase in perception of painful stimuli, as well as dysfunctional pain processing at central level and reduced brainstem or cortical inhibition in fibromyalgia.
Comparable alterations within the nociceptive system are also considered to be responsible for the maintenance and chronification of pain in CRPS. In particular, several studies suggested that central sensitization can lead to the spread of pain hypersensitivities in CRPS5,8,45 including one performed in the same CRPS cohort as in this study.9 While these studies relied on subjective readouts, increased pain-autonomic responses, a more objective readout, have also been used to indicate nociceptive sensitization in our CRPS cohort.28 In this study, we used CHEPs to verify their use as a possible additional objective surrogate for sensitization. If increased CHEP amplitudes would indicate widespread central sensitization, one would expect to also find them not only after stimulation of the painful, but also after stimulation of non-affected areas. As previous findings, using psychophysical measures such as temporal summation of pain, indicated that our CRPS cohort did indeed show signs of central sensitization,9 we can on one hand conclude that CHEP amplitudes alone cannot be used as a surrogate of central sensitization in CRPS. On the other hand, it is important to highlight that the other two studies9,28 used mechanical and not thermal stimuli to indicate central sensitization in CRPS. The importance of stimulation modality to reveal secondary and primary hyperalgesia has been shown in a seminal study by Treede et al, 1992.46 Therefore, multimodal approaches would be beneficial for an even more accurate assessment of pain processing on peripheral, as well as central level.
The increased CHEP amplitudes in combination with increased pain ratings and decreased sensory thresholds in the painful limb, but not the other areas, suggests a more pronounced peripheral sensitization, rather than a central phenomenon. Such thermal hyperalgesia in the cohort of CRPS has been previously reported26,47–49 and has also been shown to positively correlate with the evoked vertex potential amplitude.50 The present findings of increased pain ratings in the painful area of CRPS are in line with the notion that thermal hyperalgesia is related to primary hyperalgesia and inflammatory processes.46 Recently, a reduction in CHEP latencies has been used to detect capsaicin-induced peripheral sensitization in healthy participants.51 Such a shift in CHEP latency was, however, not observed in this study, potentially attributed to the use of a different stimulation protocol (ie increased (42°C) vs normal (35°C) baseline temperature of the thermode).
Taken together, by employing objective neurophysiological surrogates, we corroborate the concept of a peripheral involvement in the pathophysiology of pain in chronic CRPS type I, such as peripheral sensitization of A- and C-nociceptors.52
Affective-Motivational Components Can Increase CHEP Amplitudes
Psychological factors, such as pain catastrophizing or fear of pain, are additional factors that are known to shape the pain experience and processing.53 Fear of pain itself can significantly impact the perception of pain.54–57 For example, in CRPS type I it has been shown that pain-related fear and perceived harmfulness can lead to functional limitations in patients.58 In addition, pain catastrophizing was associated with increased activation of brain areas involved in attention towards pain and the emotional processing of pain, such as the anterior cingulate cortex, in fibromyalgia.54 In our patient cohort, even though not tested for fear of pain per se, pain catastrophizing was increased compared to HC and might also be a driving factor for increased CHEP amplitudes.
In addition to pain-related fear and catastrophizing, heightened attention towards painful heat stimuli was shown to increase heat-evoked potentials.50 Specifically, the N2-component of laser-evoked potentials was shown to be increased when attending a stimulus in healthy subjects.59 Interestingly, the N2-peak of heat-evoked potentials seems to be related to potential threats in the environment.60 Taken together, this could suggest that increased CHEPs observed in our CRPS cohort might be the result of fear of a potentially threatening painful stimulus (ie contact-heat stimulus) which shifted the attention of the individuals towards their painful limb. In contrast, neglect-like symptoms towards the painful limb have also been reported in more than 75% of individuals with CRPS,61 which would contradict the previous interpretation of heightened attention towards the painful area. However, neglect-like symptoms were present in only 11% of the current study sample. Therefore, the potential threat of an applied painful stimulus in the already painful area could be sufficient to shift the attention to exactly this area.
Limitations
This study combines well-established psychophysical measures (ie, QST) with less frequently explored heat-evoked potentials in well-characterized cohort of CRPS type I. There are a few limitations worth noting. First, the small sample size limits the power of our analyses. Secondly, not all individuals with CRPS had the same painful area and some did not tolerate the same intensity of heat stimuli, which rendered a direct comparison of raw values of the CHEP parameters impossible. That said, we managed to normalize the data with published reference values34,39 which allowed us to compare data sampled from different body areas and with different stimulation intensities. Thirdly, we could not exclude potential effects of medication on pain processing as most of the individuals with CRPS were on pain medication. Moreover, given the heterogeneity of the medication intake between subjects, a subgroup analysis was not possible in this cohort. This would however be an interesting topic to follow up on in future studies, as, for example, it is known that the use of opioids itself can lead to opioid-induced hyperalgesia in patients with chronic pain.62 On the other hand, the intake of analgesic medication could also lead to an underestimation of the hyperalgesia present in individuals with CRPS. In this regard, a recent review63 pointed out the different antihyperalgesic effects of medications, ranging from NSAIDs to opioids, on sensory testing in human evoked hyperalgesia pain models. However, as the medication of individuals with CRPS was very heterogenic and different medications interact with many distinct pain mechanisms,64 concrete conclusions could not be drawn from this study. Lastly, while all individuals with CRPS type I included in this study were considered to be in the chronic phase of the disease (ie, >6 months), it was not possible to clearly separate them, based on clinical signs and symptoms, into clear warm or cold CRPS subtypes. This distinction would be, however, interesting with regard to potential differences in thermo-nociceptive processing along the neuroaxis.
Conclusion
This is the first study providing neurophysiological evidence that in CRPS type I the thermo-nociceptive neuroaxis is intact and that hypersensitivities towards painful heat stimuli were restricted to the painful limb, while no remote changes in pain processing were observed. With the employed methods (ie, CHEPs), we depict predominantly peripheral changes of nociceptive processing, in addition to already shown central changes in our CRPS cohort.9,28 Whether this hypersensitivity is driven through peripheral sensitization alone or is also influenced by psychological factors remains to be elucidated. Future studies should also investigate the fear towards a given stimulus to estimate subjective attentional modulation of neurophysiological outcomes during the stimulation paradigm. Additionally, multimodal approaches, for example thermal and mechanical stimulations, would give additional insights into modality-specific pain processing in CRPS. Lastly, future studies including a larger sample size are needed to replicate these recent findings with higher statistical power.
Acknowledgments
The authors would like to thank L. Tauschek, A. Mollo, S. Carisch and R. Lütolf for their assistance with data acquisition.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Clinical Research Priority Program (CRPP) Pain of the University of Zurich and the Swiss National Science Foundation (SNSF) Grant (32003B_200482). P.S.S. is supported by a postdoctoral fellowship from the International Foundation for Research in Paraplegia (#PF198F). J.R. is supported by a postdoctoral fellowship from the International Foundation for Research in Paraplegia (#P191F) and the Lundbeck Foundation (R359-2020-2620).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Harden NR, Bruehl S, Perez RSGM., et al. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for Complex Regional Pain Syndrome. Pain. 2010;150(2):268–274. doi:10.1016/j.pain.2010.04.030
2. Bruehl S. Complex regional pain syndrome. BMJ. 2015;351. doi:10.1136/bmj.h2730
3. Birklein F, Ajit SK, Goebel A, Perez RSGM, Sommer C. Complex regional pain syndrome-phenotypic characteristics and potential biomarkers. Nat Rev Neurol. 2018;14(5):272–284. doi:10.1038/nrneurol.2018.20
4. Van Rijn MA, Marinus J, Putter H, Bosselaar SRJ, Moseley GL, Van Hilten JJ. Spreading of complex regional pain syndrome: not a random process. J Neural Transm. 2011;118(9):1301–1309. doi:10.1007/s00702-011-0601-1
5. Reimer M, Rempe T, Diedrichs C, Baron R, Gierthmühlen J. Sensitization of the nociceptive system in complex regional pain syndrome. PLoS One. 2016;11(5):1–25. doi:10.1371/journal.pone.0154553
6. Karpin H, Vatine JJ, Bachar Kirshenboim Y, Markezana A, Weissman-Fogel I. Central sensitization and psychological state distinguishing complex regional pain syndrome from other chronic limb pain conditions: a cluster analysis model. Biomedicines. 2023;11(1). doi:10.3390/biomedicines11010089
7. Maleki J, LeBel AA, Bennett GJ, Schwartzman RJ. Patterns of spread in complex regional pain syndrome, type I (reflex sympathetic dystrophy). Pain. 2000;88(3):259–266. doi:10.1016/S0304-3959(00)00332-8
8. Drummond PD, Finch PM, Birklein F, Stanton-Hicks M, Knudsen LF. Hemisensory disturbances in patients with complex regional pain syndrome. Pain. 2018;159(9):1824–1832. doi:10.1097/j.pain.0000000000001280
9. De Schoenmacker I, Mollo A, Scheuren PS, et al. Central sensitization in CRPS patients with widespread pain: a cross-sectional study. Pain Med. 2023;2:1–11. doi:10.1093/pm/pnad040
10. Marinus J, Moseley L, Birklein F, et al. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol. 2011;10(7):637–648. doi:10.1016/S1474-4422(11)70106-5
11. Eldufani J, Elahmer N, Blaise G. A medical mystery of complex regional pain syndrome. Heliyon. 2020;6(2):e03329. doi:10.1016/j.heliyon.2020.e03329
12. Verdugo RJ, Matamala JM, Inui K, et al. Review of techniques useful for the assessment of sensory small fiber neuropathies: report from an IFCN expert group. Clin Neurophysiol. 2022;136:13–38. doi:10.1016/j.clinph.2022.01.002
13. Jutzeler CR, Ulrich A, Huber B, Rosner J, Kramer JLK, Curt A. Improved diagnosis of cervical spondylotic myelopathy with contact heat evoked potentials. J Neurotrauma. 2017;34(12):2045–2053. doi:10.1089/neu.2016.4891
14. Treede RD, Lorenz J, Baumgärtner U. Clinical usefulness of laser-evoked potentials. Neurophysiol Clin. 2003;33(6):303–314. doi:10.1016/j.neucli.2003.10.009
15. Bromm B, Treede RD. Laser-evoked cerebral potentials in the assessment of cutaneous pain sensitivity in normal subjects and patients. Rev Neurol. 1991;147(10):625–643.
16. Casanova-Molla J, Grau-Junyent JM, Morales M, Valls-Solé J. On the relationship between nociceptive evoked potentials and intraepidermal nerve fiber density in painful sensory polyneuropathies. Pain. 2011;152(2):410–418. doi:10.1016/j.pain.2010.11.012
17. Garcia-Larrea L, Convers P, Magnin M, et al. Laser-evoked potential abnormalities in central pain patients: the influence of spontaneous and provoked pain. Brain. 2002;125(12):2766–2781. doi:10.1093/brain/awf275
18. Martini M, Valentini E, Aglioti SM. Emotional conflict in a model modulates nociceptive processing in an onlooker: a laser-evoked potentials study. Exp Brain Res. 2013;225(2):237–245. doi:10.1007/s00221-012-3365-4
19. Gibson SJ, Littlejohn GO, Gorman MM, Helme RD, Granges G. Altered heat pain thresholds and cerebral event-related potentials following painful CO2 laser stimulation in subjects with fibromyalgia syndrome. Pain. 1994;58(2):185–193. doi:10.1016/0304-3959(94)90198-8
20. Lorenz J, Grasedyck K, Bromm B. Middle and long latency somatosensory evoked potentials after painful laser stimulation in patients with fibromyalgia syndrome. Electroencephalogr Clin Neurophysiol Potentials Sect. 1996;100(2):165–168. doi:10.1016/0013-4694(95)00259-6
21. De Tommaso M, Federici A, Santostasi R, et al. Laser-evoked potentials habituation in fibromyalgia. J Pain. 2011;12(1):116–124. doi:10.1016/j.jpain.2010.06.004
22. De Tommaso M, Ricci K, Libro G, et al. Pain processing and vegetative dysfunction in fibromyalgia: a study by sympathetic skin response and laser evoked potentials. Pain Res Treat. 2017;2017. doi:10.1155/2017/9747148
23. Siracusa R, Di Paola R, Cuzzocrea S, Impellizzeri D. Fibromyalgia: pathogenesis, mechanisms, diagnosis and treatment options update. Int J Mol Sci. 2021;22(8):3891. doi:10.3390/ijms22083891
24. Caty G, Hu L, Legrain V, Plaghki L, Mouraux A. Psychophysical and electrophysiological evidence for nociceptive dysfunction in complex regional pain syndrome. Pain. 2013;154(11):2521–2528. doi:10.1016/j.pain.2013.07.038
25. Taylor SS, Noor N, Urits I, et al. Complex regional pain syndrome: a comprehensive review. Pain Ther. 2021;10(2):875–892. doi:10.1007/s40122-021-00279-4
26. Oaklander AL, Rissmiller JG, Gelman LB, Zheng L, Chang Y, Gott R. Evidence of focal small-fiber axonal degeneration in complex regional pain syndrome-I (reflex sympathetic dystrophy). Pain. 2006;120(3):235–243. doi:10.1016/j.pain.2005.09.036
27. Rasmussen VF, Karlsson P, Drummond PD, et al. Bilaterally reduced intraepidermal nerve fiber density in unilateral CRPS-I. Pain Med. 2018;19(10):2021–2030. doi:10.1093/pm/pnx240
28. Scheuren PS, De Schoenmacker I, Rosner J, Brunner F, Curt A, Hubli M. Pain-autonomic measures reveal nociceptive sensitization in complex regional pain syndrome. Eur J Pain. 2023;27(1):72–85. doi:10.1002/ejp.2040
29. Stern AF. The Hospital Anxiety and Depression Scale. Occup Med. 2014;64(5):393–394. doi:10.1093/occmed/kqu024
30. Sullivan M, Bishop S, Pivik J. The pain catastrophizing scale: user Manual. Psychol Assess. 1995;7(4):524–532.
31. Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–1920. doi:10.1185/030079906X132488
32. Kramer JLK, Haefeli J, Curt A, Steeves JD. Increased baseline temperature improves the acquisition of contact heat evoked potentials after spinal cord injury. Clin Neurophysiol. 2012;123(3):582–589. doi:10.1016/j.clinph.2011.08.013
33. Greffrath W, Baumgärtner U, Treede RD. Peripheral and central components of habituation of heat pain perception and evoked potentials in humans. Pain. 2007;132(3):301–311. doi:10.1016/j.pain.2007.04.026
34. Jutzeler CR, Rosner J, Rinert J, Kramer JLK, Curt A. Normative data for the segmental acquisition of contact heat evoked potentials in cervical dermatomes. Sci Rep. 2016;6:1–9. doi:10.1038/srep34660
35. Rosner J, Hubli M, Hostettler P, et al. Contact heat evoked potentials: reliable acquisition from lower extremities. Clin Neurophysiol. 2018;129(3):584–591. doi:10.1016/j.clinph.2017.12.034
36. De Schoenmacker I, Archibald J, Kramer JLK, Hubli M. Improved acquisition of contact heat evoked potentials with increased heating ramp. Sci Rep. 2022;12(1):1–11. doi:10.1038/s41598-022-04867-y
37. Rolke R, Magerl W, Campbell KA, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10(1):77. doi:10.1016/j.ejpain.2005.02.003
38. Magerl W, Krumova EK, Baron R, Tölle T, Treede RD, Maier C. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain. 2010;151(3):598–605. doi:10.1016/j.pain.2010.07.026
39. Rosner J, Hostettler P, Scheuren PS, et al. Normative data of contact heat evoked potentials from the lower extremities. Sci Rep. 2018;8(1):1–9. doi:10.1038/s41598-018-29145-8
40. Haefeli JS, Blum J, Steeves JD, Kramer JLK, Curt AEP. Differences in spinothalamic function of cervical and thoracic dermatomes: insights using contact heat evoked potentials. J Clin Neurophysiol. 2013;30(3):291–298. doi:10.1097/WNP.0b013e31827ed9ee
41. Atherton DD, Facer P, Roberts KM, et al. Use of the novel contact heat evoked potential stimulator (CHEPS) for the assessment of small fibre neuropathy: correlations with skin flare responses and intra-epidermal nerve fibre counts. BMC Neurol. 2007;7:1–10. doi:10.1186/1471-2377-7-21
42. Chao CC, Hsieh SC, Tseng MT, Chang YC, Hsieh ST. Patterns of contact heat evoked potentials (CHEP) in neuropathy with skin denervation: correlation of CHEP amplitude with intraepidermal nerve fiber density. Clin Neurophysiol. 2008;119(3):653–661. doi:10.1016/j.clinph.2007.11.043
43. Lagerburg V, Bakkers M, Bouwhuis A, et al. Contact heat evoked potentials: normal values and use in small-fiber neuropathy. Muscle and Nerve. 2015;51(5):743–749. doi:10.1002/mus.24465
44. Kharkar S, Venkatesh YS, Grothusen JR, Rojas L, Schwartzman RJ. Skin biopsy in complex regional pain syndrome: case series and literature review. Pain Physician. 2012;15(3):255–266. doi:10.36076/ppj.2012/15/255
45. Gierthmühlen J, Maier C, Baron R, et al. Sensory signs in complex regional pain syndrome and peripheral nerve injury. Pain. 2012;153(4):765–774. doi:10.1016/j.pain.2011.11.009
46. Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38(4):397–421. doi:10.1016/0301-0082(92)90027-C
47. Huge V, Lauchart M, Förderreuther S, et al. Interaction of hyperalgesia and sensory loss in complex regional pain syndrome Type I (CRPS I). PLoS One. 2008;3(7):1–10. doi:10.1371/journal.pone.0002742
48. Grothusen JR, Alexander G, Erwin K, Schwartzman R. Thermal pain in complex regional pain syndrome type I. Pain Physician. 2014;17(1):71–79. doi:10.36076/ppj.2014/17/71
49. Terkelsen AJ, Gierthmühlen J, Finnerup NB, Højlund AP, Jensen TS. Bilateral hypersensitivity to capsaicin, thermal, and mechanical stimuli in unilateral complex regional pain syndrome. Anesthesiology. 2014;120(5):1225–1236. doi:10.1097/ALN.0000000000000220
50. García-Larrea L, Peyron R, Laurent B, Mauguière F. Association and dissociation between laser-evoked potentials and pain perception. Neuroreport. 1997;8(17):3785–3789. doi:10.1097/00001756-199712010-00026
51. Linde LD, Haefeli J, Jutzeler CR, et al. Contact heat evoked potentials are responsive to peripheral sensitization: requisite stimulation parameters. Front Hum Neurosci. 2020;13:1. doi:10.3389/fnhum.2019.00459
52. Cuhadar U, Gentry C, Vastani N, et al. Autoantibodies produce pain in complex regional pain syndrome by sensitizing nociceptors. Pain. 2019;160(12):2855–2865. doi:10.1097/j.pain.0000000000001662
53. Sullivan MJL, Thorn B, Rodgers W, Ward LC. Path model of psychological antecedents to pain experience: experimental and clinical findings. Clin J Pain. 2004;20(3):164–173. doi:10.1097/00002508-200405000-00006
54. Gracely RH, Geisser ME, Giesecke T, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127(4):835–843. doi:10.1093/brain/awh098
55. Hirsh AT, George SZ, Bialosky JE, Robinson ME. Fear of pain, pain catastrophizing, and acute pain perception: relative prediction and timing of assessment. J Pain. 2008;9(9):806–812. doi:10.1016/j.jpain.2008.03.012
56. Zale EL, Lange KL, Ditre JW. The relation between pain-related fear and disability: a meta-analysis. J Pain. 2013;14(10):1019–1030. doi:10.1016/j.jpain.2013.05.005
57. Markfelder T, Pauli P. Fear of pain and pain intensity: meta-analysis and systematic review. Psychol Bull. 2020;146(5):411–450. doi:10.1037/bul0000228
58. De Jong JR, Vlaeyen JWS, De Gelder JM, Patijn J. Pain-related fear, perceived harmfulness of activities, and functional limitations in complex regional pain syndrome type i. J Pain. 2011;12(12):1209–1218. doi:10.1016/j.jpain.2011.06.010
59. Legrain V, Guérit JM, Bruyer R, Plaghki L. Attentional modulation of the nociceptive processing into the human brain: selective spatial attention, probability of stimulus occurrence, and target detection effects on laser evoked potentials. Pain. 2002;99(1–2):21–39. doi:10.1016/S0304-3959(02)00051-9
60. Moayedi M, Liang M, Sim AL, Hu L, Haggard P, Iannetti GD. Laser-evoked vertex potentials predict defensive motor actions. Cereb Cortex. 2015;25(12):4789–4798. doi:10.1093/cercor/bhv149
61. Ten Brink AF, Bultitude JH. Predictors of self-reported neglect-like symptoms and involuntary movements in complex regional pain syndrome compared to other chronic limb pain conditions. Pain Med. 2021;22(10):2337–2349. doi:10.1093/pm/pnab226
62. Guichard L, Hirve A, Demiri M, Martinez V. Opioid-induced hyperalgesia in patients with chronic pain. Clin J Pain. 2022;38(1):49–57. doi:10.1097/AJP.0000000000000994
63. van Amerongen G, de Boer MW, Groeneveld GJ, Hay JL. A literature review on the pharmacological sensitivity of human evoked hyperalgesia pain models. Br J Clin Pharmacol. 2016;903–922. doi:10.1111/bcp.13018
64. Patel R, Dickenson AH. Neuropharmacological basis for multimodal analgesia in chronic pain. Postgrad Med. 2022;134(3):245–259. doi:10.1080/00325481.2021.1985351
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.