Back to Journals » Journal of Inflammation Research » Volume 17
Dietary Inflammatory Index and Cognitive Function: Findings from a Cross-Sectional Study in Obese Chinese Township Population from 45 to 75 Years
Authors Huang H, Li J, Shen J, Zhao T, Xiao R, Ma W
Received 30 October 2023
Accepted for publication 2 April 2024
Published 18 April 2024 Volume 2024:17 Pages 2365—2382
DOI https://doi.org/10.2147/JIR.S447300
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Hongying Huang, Jinchen Li, Jingyi Shen, Tong Zhao, Rong Xiao, Weiwei Ma
School of Public Health, Beijing Key Laboratory of Environmental Toxicology, Capital Medical University, Beijing, 100069, People’s Republic of China
Correspondence: Weiwei Ma, School of Public Health, Beijing Key Laboratory of Environmental Toxicology, Capital Medical University, Beijing, 100069, People’s Republic of China, Tel/Fax +86-10-83911651, Email [email protected]
Background and Objective: Cognitive dysfunction is highly prevalent in obese people, and food is a key factor in obesity, and dietary inflammatory index (DII) can reflect whether diet has anti-inflammatory or pro-inflammatory potential. In addition, dietary fatty acid consumption is linked to inflammation, obesity, and cognitive impairment. Erythrocyte membrane fatty acids can reflect dietary fatty acid intake. Our hypothesis was that erythrocyte membrane fatty acids might have a significant impact on the relationship between DII and cognition in obese individuals, and we designed experiments to test the hypothesis.
Methods: In three villages in Beijing, we collected 579 respondents from individuals 45 to 75 years old and categorized them by body mass index. The Montreal Cognitive Assessment (MoCA) score and DII score was calculated and gas chromatography was used to measure the proportion of erythrocyte membrane fatty acids. The relationship between the DII score and cognition was examined using multiple linear regression and binary logistic regression. Mediation analysis can help to understand the causal chain between variables, deeply explore the internal relationship and mechanism of action between variables. So a multiple chain mediation model was developed to investigate the mediating factors between the DII score and cognitive association.
Results: According to adjusted linear regression, higher DII scores were linked to lower MoCA scores in the obese group. The negative correlation between DII score and cognitive function score remains in binary linear regression. We discovered through mediation analysis that erythrocyte membrane fatty acids mediate the detrimental link between DII and cognitive function in obese individuals.
Conclusion: We propose that higher DII scores in obese people are associated with a decline in cognitive function. In addition, this effect might be mediated via the fatty acids in the erythrocyte membrane.
Keywords: mild cognitive impairment, dietary inflammatory index, obesity, fatty acids, chain mediation effect
Introduction
Dementia has emerged as one of the most ominous medical challenges of the 21st century, affecting over 1.5 million individuals aged 60 years in China.1,2 In addition, mild cognitive impairment (MCI) affected 38.77 million people over the age of 60, with a prevalence of 15.5%.2,3 With the acceleration of the aging process of Chinese society, it is expected that by 2030, the proportion of elderly people aged 60 years and older in China will expand from the current 15% to 25%,4 which revealed that the harm of cognitive dysfunction is increasing year by year. Evidence indicates that obesity is associated with the development of the cognitive impairment and has a higher potential for acquiring dementia in the mild and later life of individuals.5–8 As is reported by a system review, the prevalence of MCI in obese persons in China and Egypt was 18.5% and 42.9%, respectively.8
Dietary intake has a significant impact on obesity induced cognitive impairment.9,10 Ozawa et al, discovered that an inflammatory dietary pattern strong in red and processed meat, peas, legumes, and fried food and deficient in whole grains was linked to higher levels of interleukin-6 (IL-6) and hastened cognitive impairment in later age.11 Contrarily, the Mediterranean diet is mostly made up of fruits and vegetables, fish, grains, legumes, and olive oil, which has anti-inflammatory and antioxidant characteristics that can reduce the incidence of obesity and cognitive dysfunction.12,13 A systematic review examined the correlation between elevated levels of circulating IL-6 and overall cognitive decline, finding a positive relationship between the two variables.14 Additionally, a large neuroimaging study revealed a relationship between reduced hippocampal and increased ventricular volumes in late life and increased mid-life inflammatory marker expression in plasma.15 These results suggest that inflammation may be associated with obesity-related cognitive dysfunction. Dietary inflammatory index (DII) is a literature-derived and population-based indicator, designed and developed by Shivappa et al, is used to evaluate the potential for dietary inflammation.16,17 According to reports, DII may be utilized to investigate the links between dietary inflammation potential and illnesses including obesity and cognitive dysfunction.18,19 Diets with the most pro-inflammatory potential were linked to a higher incidence of MCI or dementia in older women, according to Hayden et al.20 According to Frith et al, there is a continuous negative correlation between DII and many types of memory, such as episodic memory, working memory, and semantic memory.21 However, less frequently are such investigations conducted in obese individuals, despite obesity plays important roles in cognitive decline.
Dietary fatty acids can be determined by serum, plasma, and erythrocyte membrane fatty acids, erythrocyte membrane fatty acids represent a comprehensive measure of the interaction between dietary fatty acid intake, other dietary factors, and fatty acid metabolism patterns, and reflect several months of dietary intake,22,23 with sensitive and accurate characteristics, can be used as biomarkers for a variety of metabolic diseases, including obesity, diabetes, metabolic syndrome, etc.24 In our previous study, it was found that there was an association between erythrocyte membrane fatty acids and cognitive function in obese patients.25,26
Given the current research progress, we hypothesized that DII may be associated with cognitive dysfunction and that erythrocyte membrane fatty acids may play a mediating role in it. Therefore, we performed a cross-sectional study to assess the association between dietary inflammatory potential and cognitive function in normal and obese population. In determining if dietary inflammation worsens the negative effects of obesity on cognitive function, we looked at the relationships between DII and Montreal cognitive assessment (MoCA) by regression analysis in normal and obese individuals. Finally, mediation analysis was carried out to explore the mediating factors of this association.
Methods
Study Design
Participants (aged from 45 to 75) was recruited from November to December in 2020 in three townships of Beijing (Caiyu town, Weishanzhuang town and Lixian town). Subsequently, the basic information, diet and cognitive function of the subjects were collected through questionnaires, physical examination was performed and blood was collected for testing.
Participants and Groups
Similar to previous studies,25 the inclusion and exclusion criteria for this study were: subjects who volunteered were excluded if they were thin, had severe thyroid and renal dysfunction, had a history of central nervous system disease such as cancer, encephalitis, head trauma, or had a clear history of mental illness, had reading, hearing, or visual impairment, or lost self-care, or had missing or mandatory suspicious data. We further excluded the people with 24 kg/m2 ≤ body mass index (BMI) <28 kg/m2. A total of 1282 participants volunteered to participate in this investigation. After screening, 579 participants were subsequently included in this study and split into normal weight group (NM; 18.5 kg/m2 ≤BMI<24 kg/m2) and obese group (OB; BMI ≥ 28 kg/m2) based on BMI, according to the Chinese Obesity Working Group27 (Figure 1).
 |
Figure 1 Study flowchart. |
Informed Consent
Before formal signing, we submitted the informed consent form template to the ethics committee for review and obtained approval from the Ethics Review Committee of Capital Medical University (Z2019SY011). We performed the trial in strict accordance with the approved version of the ethical review and ensured that the informed consent was clear to the subject by reading the informed consent form to the subject before the trial and by carefully reading the informed consent form and signing the agreement entirely voluntary. During the conduct of the trial, any subject may ask the investigator any questions, and may withdraw from the study at any time without being influenced by the outside world, and we shall properly handle the subsequent work.
Baseline Data Collection
A face-to-face questionnaire survey were used to gather data on dietary intake, cognitive performance, and sociodemographic factors. To guarantee the caliber of the questionnaires, the investigators received specialized training. Name, age, gender, smoking, drinking, exercising, employment status, past medical history, and family history of possible Alzheimer’s diseases (AD) or dementia were collected as sociodemographic factors for this study. As in our earlier study, we assessed cognitive function and overall cognitive status using the Chinese translations of the MoCA scale.25,28,29 The study’s definition of the MCI group included people with no more than six years of education and a MoCA score ≤ 19, those with seven to twelve years of education and a MoCA score ≤ 22, and people with more than twelve years of education and a MoCA score ≤ 24.30 Later, a second examination by neurologists was performed to validate the clinical diagnosis. The 34 items of the food frequency questionnaire (FFQ) were used to obtain information on dietary intake during interviews. The questionnaire employed for the Dietary Investigation of Chinese Residents served as the basis for the FFQ.31 The “Guidelines for the Prevention and Treatment of Type 2 Diabetes in China” released by the Diabetes Society of Chinese Medical Association in 201732 states that diabetes mellitus is diagnosed by fasting blood glucose (FBG) ≥ 7.0 mmol/L. Triglyceride levels in serum ≥ 1.7 mmol/L were used to define hypertriglyceridemia.33
Physical Examination, Blood Sample Collection and Detection
As was mentioned in our previous study,25 briefly, measurements of height (m), weight (kg), waist circumference (cm), hip circumference (cm) were taken. BMI and waist-hip ratios (WHR) were calculated according to the following formula: BMI = weight (kg)/ height (m)2; WHR = waist circumference (cm)/ hip circumference (cm).
The fasting peripheral blood samples were collected (10mL), of which 6 mL were used for biochemical analysis, and the test items included: total cholesterol (TC), total triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), FBG, apolipoprotein E (ApoE), serotonin (5-HT) and inflammatory cytokines, including toll-like receptor 4 (TLR4), lipopolysaccharide (LPS), TLR1, TLR2, IL-3, IL-12 and interferon gamma (IFN-γ). An additional 4 mL blood sample was used for measurement of erythrocyte membrane fatty acid content. As mentioned earlier by us,25 after detection, erythrocyte membrane fatty acid content was represented as a percentage.
Dietary Inflammatory Index (DII)
The DII scores were calculated according to the research of Shivappa et al.16 In a nutshell, the dietary data is transformed into the food ingredient content found in the DII scale, per the food ingredient database (the Chinese food ingredient table). The Z-score for each nutrient was derived based on the mean and standard deviation of each participant’s dietary intake data by comparing to the worldwide standard dietary intake database. Z-score = (actual intake of food components - average intake level) / standard deviation. Z-scores were converted to a percentile approach to minimize the impact of “right bias”. Each percentile was multiplied by two and then “1” was subtracted to produce a symmetrical distribution. The distribution that results has a center point of 0 and upper and lower limits of −1 and +1. The “Food Parameter Specific DII Score” is then calculated by multiplying the center percentile value (ie, median) of each nutrient or food parameter by the corresponding “Overall Inflammatory Response Score”. The final step is to calculate the person’s “Overall DII score” by adding up all their “food-specific DII scores”. We ranked DII scores in order from small to large and divided them equally into three groups: tertile 1 (most anti-inflammatory potential), tertile 2 (neutral diet) and tertile 3 (most pro-inflammatory potential).
In this analysis, 20 kinds of food parameters were available from the FFQ, including energy (kcal), protein (g), carbohydrate (g), total fat (g), fiber (g), cholesterol (mg), vitamin A (RE), β-carotene (μg), thiamin (mg), riboflavin (mg), niacin (mg), vitamin C (mg), vitamin E (mg), saturated fatty acids (SFA; g), monounsaturated fatty acids (MUFA; g), polyunsaturated fatty acids (PUFA; g), magnesium (Mg; mg), ferrum (Fe; mg), zinc (Zn; mg), selenium (Se; μg).
Tool Validity and Reliability
We gave participants a quick physical check and a health assessment in an effort to boost patient cooperation. Every day once the survey is finished, its correctness and completeness are verified. Information that was inaccurate or lacking was quickly updated. To guarantee accuracy and completeness, two investigators were chosen to enter the data independently throughout the data entry stage.
Covariates
The covariates that adjusted in multivariable models were summarized as follows: age, gender, WHR, culture, energy intake, lifestyles (smoking, drinking and exercise) and diseases (history of hypertension, diabetes mellitus and hypertriglyceridemia).
Statistical Analysis
The data entry was done using the Epidata 3.1 software (The Epi Data Association, Odense, Denmark). To verify accuracy, every piece of data was checked twice, and a consistency test was run. In our study, SPSS 26.0 was used for all statistical analysis. The categorical variables were reported as n (%), while the continuous variables were shown with their mean values and standard deviation (Mean and SD). Continuous data were tested for normality. The DII scores were divided into tertiles. Using the one-way ANOVA test, Mann–Whitney U-test, Kruskal–Wallis test, Chi-squared test and Fisher’s exact test to detect the significance among continuous and categorical variables between the DII tertile groups in different groups.
The association between DII scores and MoCA scores was estimated using multivariable linear regression adjusted for three models, including the Model1 (adjusted for age, gender, WHR, energy intake and culture), the Model 2 (adjusted for the covariates in Model 1 plus lifestyles), the Model 3 (adjusted for the covariates in Model 2 plus diseases). The association between DII tertiles and incidence of MCI was further analyzed using binary logistics regression, using the same adjusted model as multivariable linear regression. Finally, multiple mediation analysis model was used to investigate the relationship between DII score, erythrocyte membrane fatty acids and cognitive function (MoCA score) (Figure 2). P<0.05 representing statistically significant differences.
Results
Demographic Sociology
A final total of 579 participants were included in the current study, with 248 in the NM group and 331 in the OB group (Figure 1). Results of Table 1 provided an overview of the demographic and sociological features of the NM and OB participants based on DII tertiles. The participants, who was in tertile3 group were more prone to have lower energy intake, either in NM or in OB group (P<0.01). In NM group, teritile 3 had the largest percentage of people who never drank, followed by tertile 2 and then tertile 1 (P<0.05).
 |
Table 1 Demographic Sociology of Study Population |
The Compositions of Dietary Inflammatory Index in Different BMI Based Groups
In the OB group, tertile3 (most pro-inflammatory potential) was more prevalent and tertile1 (most anti-inflammatory potential) was less than it was in the NM group, but the difference was not statistically significant (P=0.623) (Figure 3). The components of food intake and the mean values of the total DII scores were compared between the NM and OB groups (Figure 4). The OB group has a lower intake in protein, vitamin A, riboflavin, Mg and Se than the NM group (P<0.05), despite the fact that the difference in overall DII scores between the two groups was not significant.
 |
Figure 3 The proportions of DII tertiles in NM and OB groups. Univariate analysis was carried out using the chi-square test. Abbreviations: NM, normal weight group; OB, obese group. |
Cognitive Scores for Comparison Among the DII Tertiles
The MoCA scales was employed in this investigation to evaluate cognitive function. No significant difference in any of the scores between the normal and obese groups could be found in our earlier investigation.25 Following DII stratification, MoCA scores in the NM group revealed disparities in DII tertiles. Tertile3 (most pro-inflammatory potential) has worse MoCA visuospatial function score (P<0.05) and better MoCA language skills score (P<0.01) (Table 2).
 |
Table 2 Cognitive Score of the Study Population in the NM and OB Groups |
Comparison of the Blood Biochemistry Parameters and Inflammatory Related Cytokines Among the DII Tertiles
As shown in Supplementary Material Tables S1 and Table 3, after stratification according to DII, there was no discernible difference in the blood biochemistry parameters whatever in NM or OB group. Participants adhering to the tertile 2 diet tended to have higher IFN-γ level and greater levels of IL-12 in NM and OB groups, respectively (P<0.05).
 |
Table 3 Inflammatory Related Cytokines of the Study Population in the NM and OB Groups |
Comparison of the Erythrocyte Membrane Fatty Acid Among the DII Tertiles
In the NM group, participants in the highest DII group were more likely to have larger proportions of 1-tricosanoic acid (C23:0) and lower proportions of ginkgolic acid (C15:1) (P<0.05). Additionally, tertile2 has a higher proportion of C15:1 than tertile1 (P<0.05). In the OB group, participants in tertile3 revealed relatively lower proportion of C15:1 compared with tertile1 (P<0.05) (Table 4).
 |
Table 4 The Fatty Acid Composition of the Erythrocyte Membranes of Study Population |
Association Between the DII Scores and MCI: Results of Regression Analysis
No matter the adjustments, there was no linear correlation between DII scores and MoCA scores in the NM group that was statistically significant. Participants in the OB group who scored higher on DII significantly scored lower on MoCA in all adjusted models (P<0.05) (Table 5).
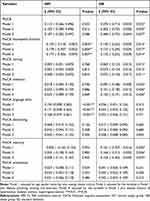 |
Table 5 β Values of DII in Linear Regression Models Between DII and Cognitive Scores After Adjusting for Different Covariates |
There was a negative relationship between DII score and MoCA visuospatial function (P<0.01) in NM group, meanwhile a positive relationship between DII score and MoCA language (P<0.01) was found in NM group. Among OB group, significant negative associations were also observed for DII scores and MoCA visuospatial function, MoCA naming, MoCA attention and MoCA memory (P<0.05) in each adjusted model.
DII score was divided into tertiles of categorical variables, the lowest DII score considered the anti-inflammatory diet (tertile1), tertile2 considered the neutral diet, and tertile with the highest DII score considered the pro-inflammatory diet (tertile3). We performed binary logistic regression with setting the tertile1 as reference (Figure 5). In NM group, there was no significance between DII tertiles and the occurrence of MCI, whatever the adjustments. In OB group, DII was positively associated with the risk of MCI in all models. The significantly increased Odds Ratio (95% Confidence Intervals) (ORs (95% CI)) of model 1 between the risk of MCI and DII score across tertile 3 compared with tertile1 was 2.343 (1.168, 4.699). In model 2, a 1-unit increase in DII was significantly associated with higher MCI occurrence by 2.008 and 2.512-fold in tertile2 and tertile3, respectively (P<0.05). Additionally, in Model 3, DII tertiles revealed a 2.142 or 2.739-fold (Tertile2: OR=2.142, 95% CI: 1.122, 4.089, P<0.05; Tertile3: OR=2.739, 95% CI: 1.330, 5.643, P<0.01) in the incidence of MCI for each tertile growth of the DII score.
Association between the DII scores and the fatty acid compositions of the erythrocyte membrane: Results of regression analysis
The β value of DII in linear regression models between DII and the fatty acid composition of the erythrocyte membranes after adjusting for different covariates was summarized on Supplementary Material Table S2. In NM group, there was a positive relationship between the proportions of lignoceric acid (C24:0) of the erythrocyte membranes and DII scores in Model 1, and this correlation persisted in Model 2 and Model 3 (P<0.01). A positive association between the proportions of C23:0 and pentadecanoic acid (C15:0) of the erythrocyte membrane and DII score was observed only in Model 3 and Model 1, respectively (P<0.05). In the OB group, there was a negative and positive relationship between the proportion of palmitic acid (C16:0) and arachidonic acid (C20:4n-6) in the erythrocyte membrane and DII score in Model 1, respectively, and this relationship persisted in Model 2 and Model 3 (P<0.05). The proportions of the ratio of omega-6 fatty acids (n-6) to omega-3 fatty acids (n-3) in the erythrocyte membranes was favorably correlated with DII score in three Models (P<0.05). A positive correlation between the proportion of MUFA, n-6 in the erythrocyte membrane and DII score was observed only in Model 2 (P<0.05).
The Erythrocyte Membrane Fatty Acids Mediate the Effect of DII on Cognition: Mediation Analysis
We investigated the association of DII score, erythrocyte membrane fatty acids, and MoCA score using a multiple mediation model in OB group. The adjustment variables of Model 3 that were previously discussed were utilized as covariates in this section. According to the findings, several erythrocyte membrane fatty acids function as mediators and form diverse chain mediation models. Regardless of the mediation paradigm, there were substantial total and direct effects between DII score and MoCA score (Supplementary Material Table S3) (P<0.05). While simple indirect effects mediated by single erythrocyte membrane fatty acids were not significant in these models (Supplementary Material Table S4), chain-mediated effects were substantial in each of these models (Table 6). The chain mediation effects were negative for models 1 (C16:0% for mediator 1, C11:0% for mediator 2), 5 (MUFA% for mediator 1, C11:0% for mediator 2), 8 (n-6% for mediator 1, C20:1% for mediator 2), 12 (n-3% for mediator 1, C11:0% for mediator 2), 14 (C18:1n-9% for mediator 1, C13:0% for mediator 2), and 15 (n-6/n-3% for mediator 1, C18:3n-3% for mediator 2) among them, whereas others showed positive chained mediation effects. These findings reveal a negative association between DII score and MoCA score that can be mediated by erythrocyte membrane fatty acids, while there is also a positive and negative chained indirect impact when various fatty acids function as mediating effects.
 |
Table 6 Chain Mediation Effect of Chain Mediation Effect Model |
Discussion
This study demonstrated that in the obese population, there was a negative correlation between DII scores and MoCA scores. Furthermore, even after the DII scores were separated into tertiles, this negative relationship remained consistent. Additionally, erythrocyte membrane fatty acids may be mediators of this negative connection.
Obesity was considered to be a major risk factor for cognitive dysfunction. Substantial evidence points to the link between obesity and cognitive decline, which is reflected in a number of cognitive function domains,34,35 including executive function,34–36 memory,34,37 attention34,35, and language.34,37 Evaluating cognitive function in middle-aged and elderly normal and obese participants using the MoCA test revealed that not only the total MoCA score differed, but also differed in eight cognitive domains. For instance, in our previous research, which reported the discrimination of the normal, overweight and obese groups for the scores of total MoCA,25,38 MoCA attention and MoCA memory.25 Therefore, in this study, we divided the study subjects into NM and OB groups according to BMI for subsequent studies. We also took into account the following variables as adjusted covariates: age,39 gender,39 WHR,40 energy intake,41 culture,42 lifestyle (ie, smoking,43 drinking43 and exercise43), and chronic illnesses that may lead to cognitive impairment (ie, history of hypertension,44 diabetes mellitus,45 hypertriglyceridemia).46
A growing body of research indicates that food (especially inflammatory diets) and nutrition are key factors in the emergence of obesity,47 giving rise to the concern about inflammatory food. The DII score, which is calculated by summing the special DII scores for each food item, can reflect whether a diet has an anti- or pro-inflammatory potential. Studies in different populations have shown that DII score affects the levels of inflammatory factors such as IL-6 and TNF-α,48,49 and the studies on these inflammatory factors have been relatively thorough, and we no longer repeat the study. In our study, DII score affects the levels of IFN-γ in normal patients and IL-12 in obese patients, but the significance of this effect is slight, and we believe that it may be related to the fact that the contents of these inflammatory factors themselves are not high enough. In addition, while high circulating cytokine levels are associated with poor cognitive performance, these are relatively nonspecific measures of disease, as circulating cytokines may arise from any vascularized tissue and may not be particularly related to diet- and obesity-related cognitive impairment.47 Therefore, more experiments are needed to demonstrate whether and how DII affects the level of inflammation in vivo. A large number of cross-sectional surveys have shown a positive association between DII and obesity,50–53 while few studies have shown no association or negative association between DII and obesity.54–56 In this study, although the difference in total DII scores between the two groups was not significant, the OB group consumed less protein, vitamin A, riboflavin, Mg and Se than the NM group (P<0.05) (Figure 4). This finding, like a large number of previous studies, implies that protein,57 vitamin A,58 riboflavin,59 Mg60 and Se57 are all food components that can play an anti-inflammatory role.
In the present study, the relationship between DII and cognitive function was explored in NM and OB populations. By grouped the DII scores into tertiles, the differences in MoCA scores among different tertile groups in NM and OB groups were compared respectively. As a result, only in the NM group, MoCA visual space capability scores decreased for Tertile 2 and Tertile 3 compared to Tertile 1 and MoCA language increased for Tertile 3 compared to Tertle1 and Tertile 2 (Table 2). We performed multiple linear regression found that higher DII scores were negatively correlated with MoCA visuospatial function scores and positively related to higher MoCA language skills scores in the NM group in all three adjusted models; There was a negative association between DII scores and total MoCA, MoCA visuospatial function, MoCA naming, MoCA attention and MoCA memory in OB group in all three adjusted model (Table 5). Using tertile1 as a control, we conducted binary logistics regression analysis, and the outcomes showed that DII was associated with the incidence of MCI in the OB group in any adjusted model. However, MCI incidence did not differ among NM group in any adjusted model (Figure 5). Wang et al18 indicated that, after adjustment, the findings of multiple linear regression among Chinese residents aged 65 to 85 years revealed that DII score was inversely connected with MoCA score. Subsequently, the higher risk of MCI was then linked to high DII scores, according to the findings of generalized linear regression analysis. Higher energy-adjusted dietary inflammatory index (E-DII) scores were specifically linked to a higher risk of MCI in the elderly Chinese population, according to Zhang et al.61 In a study by Liu et al62 it was discovered that MCI frequency in an older Chinese population was positively correlated with high DII scores. Song et al63 found that older persons with higher DII scores had lower digit symbol substitution test (DSST) and animal fluency (AF) scores, which implies a negative correlation between cognitive performance and DII score. According to Hayden et al20 higher DII scores were linked to faster cognitive impairment onset and more severe cognitive decline. Higher E-DII scores were linked to a higher incidence of cognitive impairment, according to a Korean study.64 Kesse-Guyot et al65 evaluated respondents’ cognitive abilities and found that higher DII was linked to worse performance and lower overall cognitive function. The results of these studies are consistent with our findings in obese individuals.
Fatty acids in erythrocyte membrane can reflect the intake level of dietary fatty acids. Meanwhile, inflammation is tightly correlated with the kind and quantity of dietary fatty acids. Study showed that high SFA levels in the diet can be considered a pro-inflammatory factor.66 Additionally, it has been proposed that C24:0 may increase fibroblast lipid peroxidation by promoting NADPH oxidase (NOX) activity,67 which was consistent with our findings that DII scores and the proportion of C24:0 in erythrocyte membrane were positively correlated in the three adjustment modes in this study, although this link was only observed in the normal population (P<0.01). Odd-chain saturated fatty acids (OCFAs), such as 15:0, have been linked to a lower prevalence of disorders, including type 2 diabetes, according to research.68–70 Contrarily, even-chain saturated fatty acids (ECFAs), including C16:0 and C24:0, potentially detrimental to health. Intriguingly, however, we discovered that the DII score in this study was negatively connected with the proportion of erythrocyte membranes C16:0 in the obese group and favorably correlated with the proportion of erythrocyte membranes C15:0 and C23:0 in the normal population. This may be due to the obese group consuming insufficient amounts of C16:0, while the normal population drank excessive amounts of C15:0 and C23:0.
There are few studies on the role of MUFA in inflammation, and some studies suggest that MUFAs have anti-inflammatory effects,71 but studies by Mika et al72 have found that elevated lipid MUFA/SFA ratios are associated with high levels of circulating C-reactive protein (CRP), suggesting that MUFAs may have pro-inflammatory effects.66 In our study, there was a positive correlation between the proportion of erythrocyte membrane MUFA and DII score in the OB group in Model 2 (P<0.05). Typically, n-6 is thought to have pro-inflammatory effects.73 In our study, a positive correlation was found between DII score and the proportion of erythrocyte membrane n-6 in OB group, after adjustment for model 2 (P<0.05). The pro-inflammatory effect of n-6 is mainly related to C20:4 n-6. C20:4 n-6 is metabolised by three enzymes (cyclooxygenase, lipoxygenase, and cytochrome P450) to elicit different inflammatory responses.74 In our study, in the OB group we found higher DII score was associated with the higher proportion of erythrocyte membrane C20:4n-6 whatever the adjusted models (P<0.05). Competitive metabolism exists between n-3 and n-6 polyunsaturated fatty acids, whereas n-3 is generally considered to have anti-inflammatory effects, and a higher n-6/n-3 ratio in the diet is related to inflammation and multiple chronic diseases.73,75 Our study found that DII scores were positively correlated with the proportion of the ratio of n-6 and n-3 of the erythrocyte membranes in OB group regardless of the adjusted models (P<0.05).
These fatty acids also have potential associations with cognitive function. In our previous study,25 although the proportion of erythrocyte membrane C20:4n-6 was not associated with total MoCA score in obese subjects, it was negatively correlated with the score of MoCA Orientation (B = − 0.016, P<0.05). Bigornia SJ et al76 conducted an observational study and found that erythrocyte C20:4n-6 concentration predicted cognitive impairment among participants aged 57 years (OR=1.26, P=0.01). In a cross-sectional study conducted by us in Chinese residents aged 35–64 years, discovered that the SFA, PUFA and MUFA were negatively correlated with cognitive functions in obese subjects.77 Therefore, we speculate that the effects of DII on cognitive function may be related to the changes in erythrocyte membrane fatty acids. Subsequently, we used mediation analysis to investigate the relationship among DII scores, erythrocyte membrane and MoCA scores. The findings revealed a substantial negative association between the DII and MoCA scores, which might be attributed to erythrocyte membrane fatty acids, although distinct fatty acid-mediated chain-mediating actions had both positive or negative impacts.
Our study, to the best of our knowledge, is the first to choose to look at the relationship between the DII score and cognitive abilities in obese and normal individuals, respectively. This can effectively reduce the interference effect of BMI in the effect of the DII on cognition and also close the gap that the relationship between the DII and cognitive capabilities has not been discussed in the obese population in the previously studies. Additionally, we used mediation analysis to find the chain-mediated relationship between DII scores, erythrocyte membrane fatty acids and cognition, which offers new suggestions for consuming anti-inflammatory diets to enhance cognition. The following issues with our study, however, should be noted: (1) the sample size of this study is small; (2) the use of the FFQ to assess participants’ dietary status may result in recall bias; (3) this study was only conducted in three villages in Beijing, which may only be representative of a portion of the city’s population. More investigations should be performed to further clarify the association between DII scores and cognitive function.
Conclusion
In this study, we investigated the cognitive differences between DII tertiles in obese adults aged from 45 to 75 years. Using regression analysis, we discovered a strong negative association between DII scores and cognitive scores in obese people, and the occurrence of MCI increased with increasing DII tertiles. Additionally, we discovered that in obese patients, DII had some influence over the erythrocyte membrane fatty acid ratio. By using mediation analysis, we discovered that the proportion of erythrocyte membrane fatty acid might mediate the negative connection between DII and cognitive function.
Abbreviations
DII, dietary inflammatory index; E-DII, energy-adjusted dietary inflammatory index; MCI, mild cognitive impairment; AD, Alzheimer’s diseases; MoCA, Montreal cognitive assessment; DSST, digit symbol substitution test; AF, animal fluency; OB, obese group; NM, normal weight group; BMI, body mass index; WHR, waist-hip ratios; C15:0, pentadecanoic acid; C16:0, palmitic acid; C23:0, 1-tricosanoic acid; C24:0, lignoceric acid; SFA, saturated fatty acids; OCFAs, odd-chain saturated fatty acids; ECFAs, even-chain saturated fatty acids; C15:1, ginkgolic acid; MUFA, monounsaturated fatty acids; n-6, omega-6 fatty acids; n-3, omega-3 fatty acids C20:4 n-6, arachidonic acid; PUFA, polyunsaturated fatty acids; TC, total cholesterol; TG, total triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FBG, fasting blood glucose; ApoE, apolipoprotein E; 5-HT, serotonin; TLR4, toll-like receptor 4; LPS, lipopolysaccharide; IL-6, interleukin-6; IFN-γ, interferon gamma; CRP, C-reactive protein; FFQ, food frequency questionnaire; NOX, NADPH oxidase; ORs, odds ratio; 95% CI, 95% confidence intervals; Mg, magnesium; Fe, ferrum; Zn, zinc; Se, selenium.
Data Sharing Statement
The research article data used to support the findings of this study are available from the corresponding author upon request.
Ethics Approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Review Board of Capital Medical University (Z2019SY011).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors have read and approved the final submitted manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number (81773406 and 82373556).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jia L, Du Y, Chu L, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. 2020;5:e661–e671.
2. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement; 2020.
3. Ren R, Qi J, Lin S, et al. The China Alzheimer report 2022. Gen Psychiatr. 2022;35:e100751.
4. Yee A, Tsui NB, Chang YN, et al. Alzheimer’s disease: insights for risk evaluation and prevention in the Chinese population and the need for a comprehensive programme in Hong Kong/China. Hong Kong Med J. 2018;24:492–500.
5. Hovens IB, Dalenberg JR, Small DM. A brief neuropsychological battery for measuring cognitive functions associated with obesity. Obesity. 2019;27::1988–1996. doi:10.1002/oby.22644
6. Kandimalla R, Thirumala V, Reddy PH. Is Alzheimer’s disease a type 3 diabetes? A critical appraisal. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1078–1089.
7. Dye L, Boyle NB, Champ C, Lawton C. The relationship between obesity and cognitive health and decline. Proc Nutr Soc. 2017;76:443–454. doi:10.1017/S0029665117002014
8. Karunathilaka N, Rathnayake S. Screening for mild cognitive impairment in people with obesity: a systematic review. BMC Endocr Disord. 2021;21:230.
9. Attuquayefio T, Stevenson RJ. A systematic review of longer-term dietary interventions on human cognitive function: emerging patterns and future directions. Appetite. 2015;95:554–570.
10. Edwards LM, Murray AJ, Holloway CJ, et al. Short-term consumption of a high-fat diet impairs whole-body efficiency and cognitive function in sedentary men. FASEB J. 2011;25:1088–1096. doi:10.1096/fj.10-171983
11. Ozawa M, Shipley M, Kivimaki M, Singh-Manoux A, Brunner EJ. Dietary pattern, inflammation and cognitive decline: the whitehall II prospective cohort study. Clin Nutr. 2017;36:506–512. doi:10.1016/j.clnu.2016.01.013
12. Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. 2016;2016(7432797):1–9. doi:10.1155/2016/7432797
13. Martinez-Lapiscina EH, Clavero P, Toledo E, et al. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. 2013;84:1318–1325. doi:10.1136/jnnp-2012-304792
14. Bradburn S, Sarginson J, Murgatroyd CA. Association of peripheral interleukin-6 with global cognitive decline in non-demented adults: a meta-analysis of prospective studies. Front Aging Neurosci. 2017;9:438.
15. Walker KA, Hoogeveen RC, Folsom AR, et al. Midlife systemic inflammatory markers are associated with late-life brain volume: the ARIC study. Neurology. 2017;89:2262–2270.
16. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–1696. doi:10.1017/S1368980013002115
17. Zhang J, Feng Y, Yang X, et al. Dose-response association of the dietary inflammatory potential with all-cause and cause-specific mortality. Adv Nutr. 2022;13:1834–1845. doi:10.1093/advances/nmac049
18. Wang X, Li T, Li H, et al. Association of dietary inflammatory potential with blood inflammation: the prospective markers on mild cognitive impairment. Nutrients. 2022;14.2417
19. Gholamalizadeh M, Ahmadzadeh M, BourBour F, et al. Associations between the dietary inflammatory index with obesity and body fat in male adolescents. BMC Endocr Disord. 2022;22(115). doi:10.1186/s12902-022-01001-x
20. Hayden KM, Beavers DP, Steck SE, et al. The association between an inflammatory diet and global cognitive function and incident dementia in older women. Women’s Health Initiative Memory Study Alzheimers Dement. 2017;13:1187–1196.
21. Frith E, Shivappa N, Mann JR, Hébert JR, Wirth MD, Loprinzi PD. Dietary inflammatory index and memory function: population-based national sample of elderly Americans. Br J Nutr. 2018;119:552–558.
22. Hirko KA, Chai B, Spiegelman D, et al. Erythrocyte membrane fatty acids and breast cancer risk: a prospective analysis in the nurses’ health study II. Int J Cancer. 2018;142:1116–1129.
23. Hashemi S, Amani R, cheraghian B, Neamatpour S. Stress and anxiety levels are associated with erythrocyte fatty acids content in young women. Iran J Psychiatry. 2020;15:47–54.
24. Svegliati-Baroni G, Pierantonelli I, Torquato P, et al. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Free Radic Biol Med. 2019;144:293–309. doi:10.1016/j.freeradbiomed.2019.05.029
25. Shen J, Li J, Hua Y, et al. Association between the erythrocyte membrane fatty acid profile and cognitive function in the overweight and obese population aged from 45 to 75 years old. Nutrients. 2022;14:914. doi:10.3390/nu14040914
26. Zhao T, Huang H, Li J, et al. Association between erythrocyte membrane fatty acids and gut bacteria in obesity-related cognitive dysfunction. AMB Express. 2023;13:148.
27. Chen C, Lu FC. Department of disease control ministry of health PRC: the guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17 Suppl:1–36.
28. Ding B, Xiao R, Ma W, Zhao L, Bi Y, Zhang Y. The association between macronutrient intake and cognition in individuals aged under 65 in China: a cross-sectional study. BMJ Open. 2018;8:e018573. doi:10.1136/bmjopen-2017-018573
29. Dong Y, Yean Lee W, Hilal S, et al. Comparison of the Montreal cognitive assessment and the mini-mental state examination in detecting multi-domain mild cognitive impairment in a Chinese sub-sample drawn from a population-based study. Int Psychogeriatr. 2013;25:1831–1838.
30. Huang H, Zhao T, Li J, Shen J, Xiao R, Ma W. Gut microbiota regulation of inflammatory cytokines and microRNAs in diabetes-associated cognitive dysfunction. Appl Microbiol Biotechnol. 2023;107:7251–7267. doi:10.1007/s00253-023-12754-3
31. Zhang W, Li Q, Shi L, et al. Investigation of dietary intake of cadmium in certain polluted area of south in China. Wei Sheng Yan Jiu. 2009;38(552–554):557.
32. Chinese Elderly Type 2 Diabetes P, Treatment of Clinical Guidelines Writing G, Geriatric E, Metabolism Branch of Chinese Geriatric S, Geriatric E, Metabolism Branch of Chinese Geriatric Health Care S, Geriatric Professional Committee of Beijing Medical Award F, National Clinical Medical Research Center for Geriatric D. [Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition)]. Zhonghua Nei Ke Za Zhi. 2022;61:12–50. Chinese.
33. Yuan G, Al-Shali KZ, Hegele RA. Hypertriglyceridemia: its etiology, effects and treatment. CMAJ. 2007;176:1113–1120.
34. Keawtep P, Wichayanrat W, Boripuntakul S, Chattipakorn SC, Sungkarat S. Cognitive benefits of physical exercise, physical-cognitive training, and technology-based intervention in obese individuals with and without postmenopausal condition: a narrative review. Int J Environ Res Public Health. 2022;19:13364. doi:10.3390/ijerph192013364
35. De Franciscis P, Barbieri M, Leo S, et al. Serum adiponectin levels are associated with worse cognitive function in postmenopausal women. PLoS One. 2017;12:e0186205. doi:10.1371/journal.pone.0186205
36. Kaur S, Gonzales MM, Tarumi T, et al. Serum brain-derived neurotrophic factor mediates the relationship between abdominal adiposity and executive function in middle age. J Int Neuropsychol Soc. 2016;22:493–500. doi:10.1017/S1355617716000230
37. Benito-Leon J, Mitchell AJ, Hernandez-Gallego J, Bermejo-Pareja F. Obesity and impaired cognitive functioning in the elderly: a population-based cross-sectional study (NEDICES). Eur J Neurol. 2013;20(899–906):e876–897. doi:10.1111/ene.12083
38. Fan R, Zhao L, Tong C, Qian XM, Xiao R, Ma WW. Inflammation and cognitive function in overweight and obese Chinese individuals. Cogn Behav Neurol. 2019;32:217–224. doi:10.1097/WNN.0000000000000206
39. Pavarini SCI, Brigola AG, Ottaviani AC, et al. Factors associated with cognitive performance in elderly caregivers. Arq Neuropsiquiatr. 2018;76:685–691.
40. Chen JM, Li QW, Jiang GX, et al. Association of neck circumference and cognitive impairment among Chinese elderly. Brain Behav. 2018;8:e00937. doi:10.1002/brb3.937
41. Shatenstein B, Kergoat MJ, Reid I. Poor nutrient intakes during 1-year follow-up with community-dwelling older adults with early-stage Alzheimer dementia compared to cognitively intact matched controls. J Am Diet Assoc. 2007;107:2091–2099.
42. Staekenborg SS, Kelly N, Schuur J, et al. Education as proxy for cognitive reserve in a large elderly memory clinic: ‘window of benefit’. J Alzheimers Dis. 2020;76:671–679. doi:10.3233/JAD-191332
43. Lee HJ, Jang J, Choi DW, Chae W, Park EC, Jang SI. Association between change in lifestyle and cognitive functions among elderly Koreans: findings from the Korean longitudinal study of aging (2006-2016). BMC Geriatr. 2020;20(317). doi:10.1186/s12877-020-01693-7
44. Wei J, Yin X, Liu Q, Tan L, Jia C. Association between hypertension and cognitive function: a cross-sectional study in people over 45 years old in China. J Clin Hypertens. 2018;20:1575–1583.
45. Damanik J, Yunir E. Type 2 diabetes mellitus and cognitive impairment. Acta Med Indones. 2021;53:213–220.
46. Gerber Y, VanWagner LB, Yaffe K, et al. Non-alcoholic fatty liver disease and cognitive function in middle-aged adults: the CARDIA study. BMC Gastroenterol. 2021;21(96). doi:10.1186/s12876-021-01681-0
47. Leigh SJ, Morris MJ. Diet, inflammation and the gut microbiome: mechanisms for obesity-associated cognitive impairment. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165767.
48. Kendel Jovanovic G, Mrakovcic-Sutic I, Pavicic Zezelj S, Susa B, Rahelic D, Klobucar Majanovic S. The efficacy of an energy-restricted anti-inflammatory diet for the management of obesity in younger adults. Nutrients. 2020;12:3583. doi:10.3390/nu12113583
49. Duggan C, Tapsoba JD, Shivappa N, et al. Changes in dietary inflammatory index patterns with weight loss in women: a randomized controlled trial. Cancer Prev Res. 2021;14:85–94. doi:10.1158/1940-6207.CAPR-20-0181
50. Mazidi M, Shivappa N, Wirth MD, et al. Dietary inflammatory index and cardiometabolic risk in US adults. Atherosclerosis. 2018;276:23–27. doi:10.1016/j.atherosclerosis.2018.02.020
51. Oliveira TMS, Bressan J, Pimenta AM, et al. Dietary inflammatory index and prevalence of overweight and obesity in Brazilian graduates from the Cohort of universities of Minas Gerais (CUME project). Nutrition. 2020;71(110635):110635. doi:10.1016/j.nut.2019.110635
52. Ramallal R, Toledo E, Martínez JA, et al. Inflammatory potential of diet, weight gain, and incidence of overweight/obesity: the SUN cohort. Obesity. 2017;25:997–1005. doi:10.1002/oby.21833
53. Shivappa N, Bonaccio M, Hebert JR, et al. Moli-sani study I: association of proinflammatory diet with low-grade inflammation: results from the Moli-sani study. Nutrition. 2018;54:182–188. doi:10.1016/j.nut.2018.04.004
54. Muhammad HFL, van Baak MA, Mariman EC, et al. Dietary inflammatory index score and its association with body weight, blood pressure, lipid profile, and leptin in Indonesian adults. Nutrients. 2019;11:148. doi:10.3390/nu11010148
55. Abdurahman AA, Azadbakhat L, Rasouli M, Chamari M, Qorbani M, Dorosty AR. Association of dietary inflammatory index with metabolic profile in metabolically healthy and unhealthy obese people. Nutr Diet. 2019;76:192–198.
56. Bazyar H, Zare Javid A, Bavi Behbahani H, et al. The association between dietary inflammatory index with sleep quality and obesity amongst Iranian female students: a cross-sectional study. Int J Clin Pract. 2021;75:e14061.
57. Mrityunjaya M, Pavithra V, Neelam R, Janhavi P, Halami PM, Ravindra PV. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front Immunol. 2020;11(570122). doi:10.3389/fimmu.2020.570122
58. Feng L, Sun F, Chen Y, Athari SS, Chen X. Studying the effects of vitamin a on the severity of allergic rhinitis and asthma. Iran J Allergy Asthma Immunol. 2021;20:648–692. doi:10.18502/ijaai.v20i6.8018
59. von Martels JZH, Bourgonje AR, Klaassen MAY, et al. Riboflavin supplementation in patients with crohn’s disease [the RISE-UP study]. J Crohns Colitis. 2020;14:595–607. doi:10.1093/ecco-jcc/jjz208
60. Shahi A, Aslani S, Ataollahi M, Mahmoudi M. The role of magnesium in different inflammatory diseases. Inflammopharmacology. 2019;27:649–661.
61. Zhang X, Wang Y, Liu W, et al. Diet quality, gut microbiota, and microRNAs associated with mild cognitive impairment in middle-aged and elderly Chinese population. Am J Clin Nutr. 2021;114:429–440.
62. Liu Q, Zhou D, Duan H, et al. Association of dietary inflammatory index and leukocyte telomere length with mild cognitive impairment in Chinese older adults. Nutr Neurosci. 2021;26:1–10.
63. Song W, Feng Y, Gong Z, Tian C. The association between dietary inflammatory index and cognitive performance in older adults aged 60 years and older. Front Nutr. 2022;9:748000. doi:10.3389/fnut.2022.748000
64. Shin D, Kwon SC, Kim MH, et al. Inflammatory potential of diet is associated with cognitive function in an older adult Korean population. Nutrition. 2018;55–56:56–62. doi:10.1016/j.nut.2018.02.026
65. Kesse-Guyot E, Assmann KE, Andreeva VA, et al. Long-term association between the dietary inflammatory index and cognitive functioning: findings from the SU.VI.MAX study. Eur J Nutr. 2017;56:1647–1655.
66. Ravaut G, Legiot A, Bergeron KF, Mounier C. Monounsaturated fatty acids in obesity-related inflammation. Int J Mol Sci. 2020;22:330. doi:10.3390/ijms22010330
67. Dhaunsi GS, Kaur J, Alsaeid K, Turner RB, Bitar MS. Very long chain fatty acids activate NADPH oxidase in human dermal fibroblasts. Cell Biochem Funct. 2005;23:65–68.
68. Venn-Watson S, Lumpkin R, Dennis EA. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: could it be essential? Sci Rep. 2020;10:8161.
69. Huang L, Lin JS, Aris IM, Yang G, Chen WQ, Li LJ. Circulating saturated fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Nutrients. 2019;11:998. doi:10.3390/nu11050998
70. Kurotani K, Sato M, Yasuda K, et al. Even- and odd-chain saturated fatty acids in serum phospholipids are differentially associated with adipokines. PLoS One. 2017;12:e0178192. doi:10.1371/journal.pone.0178192
71. Rocha DM, Bressan J, Hermsdorff HH. The role of dietary fatty acid intake in inflammatory gene expression: a critical review. Sao Paulo Med J. 2017;135:157–168.
72. Mika A, Sikorska-Wisniewska M, Malgorzewicz S, et al. Potential contribution of monounsaturated fatty acids to cardiovascular risk in chronic kidney disease. Pol Arch Intern Med. 2018;128:755–763. doi:10.20452/pamw.4376
73. Czepiel J, Gdula-Argasinska J, Garlicki A. n-3 and n-6 fatty acid changes in the erythrocyte membranes of patients with 658240251 clostridium difficile infection. Folia Biol. 2016;64:3–10. doi:10.3409/fb64_1.3
74. Wang T, Fu X, Chen Q, et al. Arachidonic Acid Metabolism and Kidney Inflammation. Int J Mol Sci. 2019;20:3683
75. Mariamenatu AH, Abdu EM. overconsumption of omega-6 polyunsaturated fatty acids (PUFAs) versus deficiency of omega-3 PUFAs in modern-day diets: the disturbing factor for their ”balanced antagonistic metabolic functions” in the human body. J Lipids. 2021:8848161.
76. Bigornia SJ, Scott TM, Harris WS, Tucker KL. Prospective associations of erythrocyte composition and dietary intake of n-3 and n-6 PUFA with measures of cognitive function. Nutrients. 2018;10:1253. doi:10.3390/nu10091253
77. Duan Q, Fan R, Lei R, Ma W, Ding B. Plasma fatty acid profile is related to cognitive function in obese Chinese populations (35–64 years): a cross-sectional study. Food Sci Nutr. 2020;8:4773–4781.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.