Back to Journals » Cancer Management and Research » Volume 14
Distribution of CD8 T Cells and NK Cells in the Stroma in Relation to Recurrence or Metastasis of Nasopharyngeal Carcinoma
Authors Li Y, Dong H, Dong Y, Wu Q, Jiang N, Luo Q, Chen F
Received 6 March 2022
Accepted for publication 28 July 2022
Published 27 September 2022 Volume 2022:14 Pages 2913—2926
DOI https://doi.org/10.2147/CMAR.S365230
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kattesh Katti
Yi Li, Hui Dong, Yudi Dong, Qiaoyuan Wu, Ni Jiang, Qing Luo, Fang Chen
Department of Cancer Research Laboratory, Department of Pathology, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, People’s Republic of China
Correspondence: Qing Luo; Fang Chen, Department of Cancer Research Laboratory, Department of Pathology, Affiliated Hospital of Zunyi Medical University, 149 Dalian Road, Huichuan District, Zunyi, Guizhou, 563003, People’s Republic of China, Tel +85128608074, Email [email protected]; [email protected]
Objective: The purpose of this study was to explore the expression and distribution of tumor-infiltrating immune cells (TIICs) and their relationship with recurrence and metastasis of nasopharyngeal carcinoma (NPC).
Methods: The gene expression profiles of NPC were downloaded from GEO database (GSE53819 and GSE64634). The abundance of TIICs in NPC samples was calculated by the CIBERSORT algorithm, and TIICs with higher expression were screened in NPC. Then, we performed immunohistochemistry experiments to evaluate the expression of selected TIICs in 94 NPC samples from the Affiliated Hospital of Zunyi Medical University. We further explored the relationship between TIICs and recurrence and metastasis of NPC.
Results: The results based on the GEO database showed that the expression of CD8 T cells, NK cells, macrophages and plasma cells was higher than that in normal tissues. Immunohistochemistry results showed that CD8 T cells, NK cells, macrophages and plasma cells were mainly expressed in the stroma, and the expression of CD8 T cells and NK cells in the stroma of patients without recurrence or metastasis was significantly higher than that in patients with recurrence or metastasis of NPC. Kaplan–Meier analysis showed that patients with high CD8 T cells and high NK cells expression in the stroma had favorable recurrence or metastasis-free survival and overall survival (P< 0.05). Univariate and multivariate Cox analyses indicated that CD8 T cells and NK cells in the stroma were independent factors for the recurrence or metastasis of NPC.
Conclusion: The expression of CD8 T cells, NK cells, macrophages and plasma cells is significantly higher than that in normal tissues. Among them, the expression of CD8 T cells and NK cells is closely related to the recurrence and metastasis of NPC. They are independent factors affecting the recurrence and metastasis of NPC.
Keywords: TIICs, nasopharyngeal carcinoma, recurrence and metastasis, CD8 T cells, NK cells
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant epithelial cancer that usually occurs in the epithelial lining of the nasopharynx with the highest rate of recurrence or metastasis in head and neck cancers.1,2 NPC has unique ethnic and geographical distribution characteristics, and is mainly prevalent in southern China and Southeast Asia.1 Radiotherapy is the main treatment for NPC, and with the development of intensity-modulated radiotherapy (IMRT) and the optimization of chemotherapy regimens, the overall survival (OS) of NPC patients has significantly improved.3 However, 5% to 15% of patients will develop local failure, and 15% to 30% will experience failure at distant metastasis after chemoradiotherapy.4 Recurrent and metastatic NPC is ineffective with a variety of treatments, which is the main cause of treatment failure and mortality, and the mechanisms involved are still unclear.5 Therefore, accurate biomarkers that can predict the recurrence and metastasis of NPC are urgently needed.
The development and progression of malignant tumors require interaction with other cells in the tumor microenvironment (TME), including tumor-infiltrating immune cells (TIICs).6,7 TIICs are the main components of the TME, and are mainly composed of T cells, B cells, macrophages, dendritic cells, natural killer (NK) cells, and polymorphonuclear leukocytes.8 Increasing evidence indicates that the composition and abundance of TIICs are potential predictors of patient survival, such as breast cancer,9 renal cell carcinoma,10 lung cancer,11 esophageal cancer12 and muscle-invasive bladder cancer.13 The above studies show that TIICs have the potential to be used as clinical biomarkers. In NPC, a number of studies assessing the prognostic and treatment value of TIICs density and distribution patterns in the TME have been performed. Zhao et al used single-cell RNA-seq to reveal the landscape of tumor and TIICs in NPC, and found that immune cells varied in composition and functional status.14 The latest research also suggests that the characterization of immune status in the TME has becoming a breakthrough for the treatment of recurrent and metastatic NPC.15 The composition of T cells changes significantly during the recurrence of NPC.16
Although many studies have confirmed the prognostic significance of TIICs in NPC, the findings remain controversial. Moreover, the complex relationship between TIICs and recurrence or metastasis of NPC is still not clear. Therefore, we believe that it is of great significance to further explore the correlation between the distribution and density of TIICs and the recurrence and metastasis of NPC. To investigate the differential infiltration of TIICs in NPC patients, we measured the expression of TIICs in NPC by CIBERSORT based on GEO data. Then, we performed immunohistochemistry to evaluate the expression of TIICs in NPC, and further explored the relationship between TIICs and recurrence or metastasis. This study is expected to provide early diagnosis and personalized treatment for people at high risk of recurrence and metastasis of NPC.
Materials and Methods
Evaluation of TIICs in NPC
The gene expression profiles of patients with NPC were downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). To obtain more reliable experimental results, this research combined multiple sample sets for analysis including GSE53819 and GSE64634. The Perl (The Perl Programming Language, version 5.28.1, http://www.perl.org) was used to merge the two sets of data, and the R (The R Project for Statistical Computing, version 3.6.0, https://www.r-project.org) sva package was used for experimental batch correction and the limma package was used for normalization. CIBERSORT was applied to calculate the P value for the deconvolution for each sample by using Monte Carlo sampling, which provided a measure of confidence in the result.17 The bar chart and heatmap were drawn to show the composition of TIICs of each sample, and correlations between TIICs’ abundance were discussed. A heat map was drawn to display the correlation between cells using the pheatmap package. A violin plot was drawn to visualize the expression differences in 22 types of infiltrating immune cells by using the vioplot package of R.
Patients
Informed consent was obtained from all enrolled patients, and this study was approved by the Ethics Committee of the Affiliated Hospital of Zunyi Medical University (KLLY-2020-116). We retrospectively evaluated 96 nonmetastatic NPC patients who were first diagnosed at the Affiliated Hospital of Zunyi Medical University (Guizhou, China) from 2013 to 2017.The following criteria were met in all cases: a) histologically diagnosed NPC, b) complete clinical data, including age, sex, and TNM stage (eighth edition of AJCC) were available, and c) no other malignant tumor were present and the patients did not accept tumor-related treatment in the past, such as radiotherapy, chemotherapy, immunotherapy and surgery. All patients received comprehensive treatment with radiotherapy as the mainstay. Patients in stage I were mainly treated with radiotherapy alone, patients in stage II were treated with chemoradiotherapy, and patients in stage III–IVA were treated with induction chemotherapy + chemoradiotherapy. The exclusion criteria were as follows: a) tissue paraffin specimens were not available, b) incomplete following data, and c) nonstandard treatment. Recurrence or metastasis-free survival (RMFS) was computed as the time from the day of diagnosis to the first recurrence and metastasis (identified by physical examination, CT or MRI) or the most recent follow-up. OS was calculated from the date of diagnosis to the date of death or the last follow-up. In the first 2 years of follow-up, patients were examined by routine imaging methods every 3 months, every 6 months from the third year to the fifth year, and annually thereafter. We ended the follow-up period in December 2020.
Immunohistochemistry
Formalin-fixed paraffin-embedded (FFPE) tissue blocks from tissue biopsy obtained at the time of first diagnosis of puncture sample for NPC were used for this study. Hematoxylin and eosin (H&E) sections were available from each tissue block as routine hospital care for NPC patients. Immunostaining was performed on sections (2 μm) of the tissue blocks, which were mounted on glass slides and dried in an oven at 60 °C for 1 h. Immunostaining was performed automatically using the Bond Max fully automated IHC stainer (Leica). Immune infiltration pattern analysis identified 4 TIICs highly expressed in NPC: CD8 T cells (CD8), NK cells (CD56), macrophages (CD68) and plasma (CD138). These TIICs were stained with different antibodies as follows: an anti-human CD8 mouse monoclonal antibody (TA802079S, dilution 1:150, Origene), an anti-human CD56 mouse monoclonal antibody (TA805376S, dilution 1:150, Origene), an anti-human CD68 mouse monoclonal antibody (TA802949S, dilution 1:500, Origene), and an anti-human CD138 mouse monoclonal antibody (TA813799S, dilution 1:1000, Origene).
Immunohistochemical Evaluation
All slides were observed with a Leica DM3000 (Leica Microsystems, Germany). To evaluate the expression of stained immune cells, 3 respective areas of stroma and tumor core were evaluated at ×200 magnification, and the mean value was adopted. All images were acquired using the same microscope and camera set. The captured images were transferred to a computer for image analysis. The intensities of the positive staining in the stroma and tumor core were determined using the mean optical density (MOD) as follows: MOD=IOD/area of the tumor section by image pro plus 6.0. All immunohistochemistry data were measured by two experienced pathologists who were unaware of the clinical data.
Statistical Analysis
The datasets in our study were mainly analyzed by the R package software (version 3.6.0) and integrated by Perl (version 5.30.0.1). For continuous variables, significant differences were detected by Student’s t-test and ANOVA. For categorical variables, significant differences were detected by Pearson’s χ2 test. The survival analysis was constructed with the Kaplan–Meier curve, and the significance of differences was examined by the Log rank test. The Cox proportional hazards regression model was used for univariate and multivariate analyses. The hazard ratio (HR) and 95% confidence interval (CI) were calculated to estimate the hazard risk of variables. P<0.05 was considered statistically significant.
Results
CD8 T Cells, NK Cells, Macrophages, and Plasma Cells are Highly Expressed in NPC
The occurrence of NPC is more common in southern China, while only 5% of the cases are reported in European countries.18 Therefore, this study was based on Chinese NPC tissue samples. The available dataset (GSE53819 and GSE64634) contained 52 samples, including 30 NPC samples and 22 normal nasopharyngeal samples. The CIBERSORT algorithm was used to calculate the proportion of 22 types of TIICs in 30 NPC samples and 22 normal nasopharyngeal samples and is displayed in a bar chart (Figure 1A). The proportions of TIICs in the sample varied significantly between both intragroup and intergroup. The abundance of TIICs in each sample was different, and T cells, and macrophages accounted for most of the TIICs in the NPC microenvironment (Figure 1B). Additionally, the TIICs infiltration levels showed a strong correlation with each other in the NPC microenvironment (Figure 1C). For example, activated dendritic cells is positively correlated with eosinophils (correlation coefficient = 0.88), and activated mast cells were positively correlated with activated dendritic cells (correlation coefficient = 0.93). Compared with normal samples, plasma cells (p<0.001), T cells CD8 (p=0.007), NK cells activated (p<0.001), macrophages M0 (p<0.001) and macrophages M1 (p<0.001) were highly expressed in NPC tissues, while B cells naive (p<0.001), B cells memory (p<0.001), T cells CD4 memory resting (p=0.017), T cells follicular helper (p<0.001), NK cells resting (p=0.032), macrophages M2 (p=0.029) and mast cells resting (p=0.01) were lowly expressed in NPC tissues (Figure 1D). Based on the above results, we found that the distribution and expression of TIICs in NPC tissues were significantly different. Among them, the expression of CD8 T cells, macrophages, NK cells and plasma cells was significantly higher than that in normal tissues and may play an important clinical prognostic role in NPC. Therefore, this study mainly focuses on CD8 T cells, NK cells, macrophages and plasma cells and further explores their correlation with the recurrence and metastasis of NPC.
Clinicopathological Features of the Patients
We recruited 246 patients with NPC who were diagnosed at the Affiliated Hospital of Zunyi Medical University. After applying the inclusion and exclusion criteria, a total of 96 patients were included in this study. The median follow-up time was 45 months. The clinical characteristics of the 96 NPC patients in this study are summarized in Table 1. The median age was 42 years (range=16–73 years). The majority of NPC patients were male (67%). Patients in stages I–II accounted for 14 (14%) cases, while those in stages III–IV included 82 (86%) patients. During the follow-up, 24 patients relapsed or metastasized, and 22 patients died.
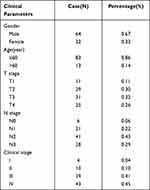 |
Table 1 Clinical Parameters of Patients with Nasopharyngeal Carcinoma |
Distribution of CD8 T Cells, NK Cells, Macrophages and Plasma Cells in NPC
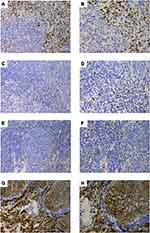
Tumor core and stromal CD8, CD56, CD68 and CD138 were stained in a distinguishable and clear manner, as shown in Figure 2. CD8, CD56, and CD68 were significantly more abundant in the stroma, but CD138 was more abundant in the tumor core (all P<0.05) (Figure 3). The MOD of CD8, CD56, CD68 and CD138 in the tumor core were 0.045±0.064, 0.001±0.004, 0.002±0.004 and 0.036±0.037, respectively. Correspondingly, the MOD of these TIICs in the stroma were 0.079±0.064, 0.047±0.009, 0.008±0.012 and 0.016±0.017, respectively. Immunohistochemical results showed that CD8 T cells, NK cells and macrophages were mainly expressed in stromal cells, while CD138 was expressed not only on stromal plasma cells but also on NPC tumor cells. This experiment mainly focused on the expression of TIICs. CD8 T cells, NK cells, macrophages and plasma cells infiltrated mainly within the stromal region. In addition, a large of studies have confirmed that the expression of TIICs in the stroma is more closely related to prognosis. Therefore, we used the expression of CD8 T cells, NK cells, macrophages, and plasma cells in the stroma for subsequent experiments.
CD8 T Cells and NK Cell Were Associated with Recurrence or Metastasis of NPC
We next examined the expression of CD8 T cells, NK cells, macrophages and plasma cells in different states of recurrence or metastasis (Figure 4). The expression of CD8 T cells and NK cells in the no recurrence or metastasis group was significantly lower than that in the recurrence or metastasis group (0.016±0.022 vs 0.022±0.023, 0.001±0.002 vs 0.003±0.003)(P<0.05). However, macrophages and plasma cells were not significantly different between the two groups (P>0.05). Kaplan–Meier analysis was used to evaluate the associations between CD8 T cell, NK cell, macrophages and plasma cell expression and RMFS and OS, as shown in Figures 5 and 6. Patients with high CD8 T cells and high NK-cell expression showed favorable RMFS and OS (P<0.05). Macrophages and plasma cells had no significant correlation with RMFS and OS. We also performed univariate and multivariate analyses of the Cox proportional hazard model to analyze the risk of recurrence or metastasis of CD8 T cells, NK cells, macrophages and plasma cells and other clinicopathological variables (Figure 7). In the univariate analysis, T, age, CD8 T cells and NK cells showed a significant correlation with the RMFS of NPC patients (P<0.05). The independent value was examined by multivariate analysis. The results found that CD8 T cell and NK cell expression were independent factors for RMFS in NPC patients (P<0.05).
Discussion
Recurrence and metastasis are the main reasons for the poor prognosis of NPC. It is of great significance to predict the recurrence and metastasis risk of patients and carry out appropriate monitoring and intervention for patients with high recurrence and metastasis risk. In recent years, the role of the TME, which plays an important role in the occurrence and development of cancer, has gained attention from an increasing number of researchers. The TME is composed of various immune cells, cancer-related fibroblasts, and endothelial cells, and the interaction between these cells also involves chemokines, cytokines, angiogenic mediators, growth factors, and so on.7 The components and complex interactions of TIICs exert significant effects on the aggressiveness of malignant cells. Recent evidence suggests that the types and densities of TIICs not only have predictive value in patient survival but also affect tumor responses to therapy and therefore hold great promise as clinical biomarkers for malignancies.19,20 However, the relationship between TIICs and prognosis is still controversial, and the complex relationship between TIICs and recurrence or metastasis of NPC is still not clear. In this study, we analyzed the role of TIICs in NPC using bioinformatics tools, and we further explored the correlation between TIICs and the recurrence or metastasis of NPC by performing IHC. The analysis of the infiltration pattern of NPC based on the GEO database showed that the distribution and expression of TIICs were significantly different in NPC. The expression of CD8 T cells, NK cells, macrophages and plasma cells was higher than that in normal tissues. Immunohistochemistry results showed that the expression of CD8 T cells and NK cells in the recurrence or metastasis group was significantly higher than that in the nonrecurrence or metastasis group. Kaplan–Meier analysis showed that patients with high CD8 T cell and NK cells expression had favorable RMFS and OS. Univariate and multivariate Cox analyses suggested that CD8 T cells and NK cells were independent influencing factors for the recurrence or metastasis of NPC.
Currently, CIBERSORT can calculate the relative proportions of TIICs by integrating genomic profiles and the deconvolution algorithm. Gentles et al reported that the CIBERSORT results were in accordance with the results of flow cytometry and immunohistochemistry experiments.21 The incidence and mortality of NPC in China are higher than the world average, and NPC tissue microarray gene data are limited. Therefore, in this study, we combined multiple Chinese NPC datasets to conduct CIBERSORE analysis to evaluate the expression of 22 immune cells in NPC for the first time. The results showed that the expression of CD8 T cells, NK cells, macrophages, and plasma cells was significantly higher than that in normal tissues. Luo et al used single gene expression data based on the GEO database to perform CIBERSORT calculations and found that NPC samples contained a higher proportion of M1 macrophages, whereas memory B cells and CD4 memory resting T cells were relatively lower.22 Compared with our results, there are certain differences. The reason may be that only a single gene expression profile file was included, and the sample size was small. Additional and larger NPC data should be obtained to better assess and explain immune infiltration.
Then, we used FFPE for immunohistochemical analysis to evaluate the correlation between CD8 T cells, NK cells, macrophages, and plasma cells and the recurrence or metastasis of NPC. In this study, an automatic immunohistochemical instrument was used for staining experiments and semi-quantitative analysis of immunohistochemical results used image pro plus. Image pro plus can delineate the core area and stroma area of the tumor, and accurately measure the optical density parameters. It can digitally process the pathological image to avoid the influence of personal error, and is widely used in immunohistochemical results analysis. Previous studies have used image pro plus to calculate the infiltration and expression of CD4+ T cells, CD8+ T cells and CD19+ B cells in the gastric cancer microenvironment.23 Deng et al also used the software to count the IOD of CD163+ and CD68+ to reflect the infiltration degree of macrophages in NPC24. Our immunohistochemical semi-quantitative results showed that the expression of CD8 T cells and NK cells was higher in the nonrecurrence or metastasis group, which was related to better prognostic survival and lower recurrence or metastasis. Univariate and multivariate Cox regression further found that they were independent factors affecting the recurrence or metastasis of NPC.
CD8 T cells play a key role in regulating tumor growth by recognizing tumor‐specific or ‐associated antigens and thus represent a positive prognostic marker in multiple solid tumors.25. High levels of infiltrating CD8 + T cells are associated with better prognosis in many cancer patients.26,27 Higher CD8 T cells density is also an independent, significant, and favorable predictive factor for local recurrence-free survival (LRFS) in patients receiving chemoradiotherapy for NPC.28 Furthermore, our report also showed an association between increased CD8+ T cell expression and reduced recurrence or metastasis and increased survival. These studies were all affected by various treatment factors, such as surgery, radiotherapy and chemotherapy. Gerber et al reported that elimination of CD8 T cells decreased the antitumor effects of radiotherapy,29 and chemotherapy changed the TME by releasing tumor antigens and promoting the accumulation of dendritic cells, which stimulate CD8 T cells and activate the IFN pathway.30 CD8 T cells may improve the curative effect of chemoradiotherapy to reduce the rate of recurrence and metastasis, thereby improving patient survival. Moreover, NPC is closely related to Epstein–Barr virus infection, and research has indicated that virus-related tumors induce a more active antitumor immune response than nonvirus-related tumors. Kawaguchi et al, through subgroup analysis, found that EBV+ patients with higher CD8+ TIL density showed significantly favorable LRFS or OS.28 However, due to the limited EB virus data collected, EB virus correlation analysis was not possible in this study. Follow-up research will continue to collect EB virus related data and further discuss the relationship between TIICs and EB virus.
NK cells were discovered for their ability to rapidly recognize and efficiently kill tumor cells.31 The infiltration of tumors by NK cells has been linked to favorable outcomes in non-small-cell lung cancer,32 colorectal cancer,33 endometrial cancer34 and so on. A recent study reported that the density of NK cells in NPC correlated positively with prognostic outcomes.35 We also obtained the same result indicating that high NK-cell expression was associated with favorable RMFS and OS. Intuitively, the result is plausible since a higher number of NK cells diminishes the likelihood of disease recurrence and metastasis because of the antitumor properties of NK cells.36 Similar to CD8 T cells, NK cells target virus-infected cells, but through germline-encoded receptors, unlike CD8 T cells.37
Macrophages are an abundant population of TIICs in TMEs.38 Under different stimulations, macrophages M0 can differentiate into two further phenotypes: antitumor M1 and pro-tumor M2. TAMs play a controversial role in tumor progression depending on different tumor types. TAM infiltration has been shown to correlate with worse outcome in cancers, including triple-negative breast cancer39 and oesophageal cancer.40 However, some studies indicated that TAMs were correlated with better prognosis in colorectal carcinoma.41 In NPC, Deng et al reported that positive PD-L1 expression on tumor cells in combination with lower CD163+ TAM density was significantly associated with favorable prognosis.24 However in our analysis, we did not find any association between macrophages and clinical outcomes. This result may be explained by the immunohistochemical labelling of pan-macrophages (CD68) that we chose, which did not distinguish between MI and M2.
CD138 is a plasma cell marker and a proteoglycan, syndecan 1, which is expressed by solid tumor cells and plasma cells. It has been shown that plasma cells present the cell type with the strongest association with long term survival in many cancer types including lung cancer,42 and brain cancer.43 Plasma cells are often present at sites of inflammation and are generated from activated B cells by T cell-dependent and independent mechanisms.32 Previous studies have reported that CD138 is expressed in tumor cells and stromal plasma cells in NPC and is associated with advanced disease and poor prognosis.44 However, our study did not find a correlation between plasma cells and NPC, and its possible mechanism deserves further exploration.
In this study, we found that CD8 T cells and NK cells are important influencing factors for the prognosis, recurrence and metastasis of NPC. There is a special lymphocyte subset-NKT cells, which are a type of lymphocyte subsets that express both NK cell receptors and T cell receptors and have both immune characteristics. It produces a wide range of immune responses and plays an important role in anti-tumor immune regulation. Previous studies have found that the expression of NKT cells positively correlated with survival in breast cancer,45 High expression of NKT cells in pancreatic cancer patients also significantly improves OS.46 However, there are few studies on NKT cells in NPC. Our study firstly discovered the effect of CD8 T cells and NK cells on the recurrence and metastasis of NPC, but whether they are directly related to NKT cells, and the mechanisms need further explore.
Several limitations should be addressed in this study. First, none of the patients received immunotherapy, and the correlation analysis between TIICs and immunotherapy could not be further analyzed. Then, this was a retrospective study, and the cohort sizes were relatively small. Therefore, larger independent and prospective validation is needed in the future. Additionally, we selected several immune cells from CIBERSORT instead of evaluating them all. Other important immune phenotypes might have been missed. However, simply evaluating all immune cells might be costly and wasteful in clinical practice. A better screening method could be used to identify those important immune markers and fulfil the immunotype classification.
Conclusion
The expression of CD8 T cells, NK cells, macrophages and plasma cells in NPC tissues is significantly higher than that in normal tissues. Further studies have found that the high expression of CD8 T cells and NK cells reduced the recurrence and metastasis rate and improved the prognosis of NPC, which were independent factors affecting the recurrence or metastasis of NPC.
Ethics Statement
Written informed consent was obtained from all patients for the use of their tissues in this study. All experiments using human tissue were approved by the Ethics Committee of Zunyi Medical University (No. KLLY-2020-116) and was conducted in accordance with the Declaration of Helsinki.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81860469) and the Science and Technology Fund of the Science and Technology Department of Guizhou Province (No. qiankehejichu [2020] 1Z063).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen Y-P, Chan ATC, Le Q-T, et al. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80.
2. Lin L, Lu Y, Wang XJ, et al. Delineation of neck clinical target volume specific to nasopharyngeal carcinoma based on lymph node distribution and the international consensus guidelines. Int J Radiat Oncol Biol Phys. 2018;100(4):891–902.
3. Au KH, Ngan RKC, Ng AWY, et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: a report of 3328 patients (HKNPCSG 1301 study). Oral Oncol. 2018;77:16–21.
4. Lee AW, Ma BB, Ng WT, et al. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33(29):3356–3364.
5. Perri F, Della Vittoria Scarpati G, Caponigro F, et al. Management of recurrent nasopharyngeal carcinoma: current perspectives. Onco Targets Ther. 2019;12:1583–1591.
6. Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50.
7. Arneth B. Tumor Microenvironment. Medicina. 2019;56(1):15.
8. Domingues P, Gonzalez-Tablas M, Otero A, et al. Tumor infiltrating immune cells in gliomas and meningiomas. Brain Behav Immun. 2016;53:1–15.
9. Sui S, An X, Xu C, et al. An immune cell infiltration-based immune score model predicts prognosis and chemotherapy effects in breast cancer. Theranostics. 2020;10(26):11938–11949.
10. Zhang S, Zhang E, Long J, et al. Immune infiltration in renal cell carcinoma. Cancer Sci. 2019;110(5):1564–1572.
11. Zhao J, Bao W, Cai W. Immune infiltration landscape in lung squamous cell carcinoma implications. Biomed Res Int. 2020;2020:5981870.
12. Hatogai K, Fujii S, Kitano S, et al. Relationship between the immune microenvironment of different locations in a primary tumour and clinical outcomes of oesophageal squamous cell carcinoma. Br J Cancer. 2020;122(3):413–420.
13. Peng YL, Wu ZS, Lu HM, et al. Prognostic significance of tumor-infiltrating immune cells in muscle-invasive bladder cancer. Am J Transl Res. 2020;12(10):6524–6536.
14. Zhao J, Guo C, Xiong F, et al. Single cell RNA-seq reveals the landscape of tumor and infiltrating immune cells in nasopharyngeal carcinoma. Cancer Lett. 2020;477:131–143.
15. Berele BA, Cai Y, Yang G. Prognostic value of tumor infiltrating lymphocytes in nasopharyngeal carcinoma patients: meta-analysis. Technol Cancer Res Treat. 2021;20:15330338211034265.
16. Wang Y, Peng Z, Wang Y, et al. Immune microenvironment change and involvement of circular RNAs in TIL cells of recurrent nasopharyngeal carcinoma. Front Cell Dev Biol. 2021;9:722224.
17. Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457.
18. Chang ET, Ye W, Zeng YX, et al. The evolving epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2021;30(6):1035–1047.
19. Palucka AK, Coussens LM. The Basis of Oncoimmunology. Cell. 2016;164(6):1233–1247.
20. Baudino TA. Targeted cancer therapy: the next generation of cancer treatment. Curr Drug Discov Technol. 2015;12(1):3–20.
21. Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–945.
22. Luo MS, Huang GJ, Liu BX. Immune infiltration in nasopharyngeal carcinoma based on gene expression. Medicine. 2019;98(39):e17311.
23. You Q, Fang T, Yin X, et al. Serum CD4 is associated with the infiltration of CD4(+)T cells in the tumor microenvironment of gastric cancer. J Immunol Res. 2021;2021:6539702.
24. Deng R, Lu J, Liu X, et al. PD-L1 expression is highly associated with tumor-associated macrophage infiltration in nasopharyngeal carcinoma. Cancer Manag Res. 2020;12:11585–11596.
25. Thommen DS, Schumacher TN, Cell T. Dysfunction in cancer. Cancer Cell. 2018;33(4):547–562.
26. Kim J, Kwon J, Kim M, et al. Low-dielectric-constant polyimide aerogel composite films with low water uptake. Polymer J. 2016;48(7):829–834.
27. Wang Y, Yin C, Geng L, et al. Immune infiltration landscape in clear cell renal cell carcinoma implications. Front Oncol. 2020;10:491621.
28. Kawaguchi T, Ono T, Sato F, et al. CD8+ T cell infiltration predicts chemoradiosensitivity in nasopharyngeal or oropharyngeal cancer. Laryngoscope. 2021;131(4):E1179–E1189.
29. Gerber SA, Sedlacek AL, Cron KR, et al. IFN-gamma mediates the antitumor effects of radiation therapy in a murine colon tumor. Am J Pathol. 2013;182(6):2345–2354.
30. Kang TH, Mao CP, Lee SY, et al. Chemotherapy acts as an adjuvant to convert the tumor microenvironment into a highly permissive state for vaccination-induced antitumor immunity. Cancer Res. 2013;73(8):2493–2504.
31. Shin MH, Kim J, Lim SA, et al. NK cell-based immunotherapies in cancer. Immune Netw. 2020;20(2):e14.
32. Backman M, La Fleur L, Kurppa P, et al. Infiltration of NK and plasma cells is associated with a distinct immune subset in non-small cell lung cancer. J Pathol. 2021;255(3):243–256.
33. Halama N, Braun M, Kahlert C, et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res. 2011;17(4):678–689.
34. Versluis MAC, Marchal S, Plat A, et al. The prognostic benefit of tumour-infiltrating Natural Killer cells in endometrial cancer is dependent on concurrent overexpression of Human Leucocyte Antigen-E in the tumour microenvironment. Eur J Cancer. 2017;86:285–295.
35. Liou AK, Soon G, Tan L, et al. Elevated IL18 levels in Nasopharyngeal carcinoma induced PD-1 expression on NK cells in TILS leading to poor prognosis. Oral Oncol. 2020;104:104616.
36. Lopez-Soto A, Gonzalez S, Smyth MJ, et al. Control of Metastasis by NK Cells. Cancer Cell. 2017;32(2):135–154.
37. Png YT, Yang AZY, Lee MY, et al. The role of NK cells in EBV infection and EBV-associated NPC. Viruses. 2021;13(2):300.
38. Evrard D, Szturz P, Tijeras-Raballand A, et al. Macrophages in the microenvironment of head and neck cancer: potential targets for cancer therapy. Oral Oncol. 2019;88:29–38.
39. Zhang WJ, Wang XH, Gao ST, et al. Tumor-associated macrophages correlate with phenomenon of epithelial-mesenchymal transition and contribute to poor prognosis in triple-negative breast cancer patients. J Surg Res. 2018;222:93–101.
40. Yagi T, Baba Y, Okadome K, et al. Tumour-associated macrophages are associated with poor prognosis and programmed death ligand 1 expression in oesophageal cancer. Eur J Cancer. 2019;111:38–49.
41. Ohnishi K, Komohara Y, Saito Y, et al. CD169-positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carcinoma. Cancer Sci. 2013;104(9):1237–1244.
42. Lohr M, Edlund K, Botling J, et al. The prognostic relevance of tumour-infiltrating plasma cells and immunoglobulin kappa C indicates an important role of the humoral immune response in non-small cell lung cancer. Cancer Lett. 2013;333(2):222–228.
43. Soike MH, Logue J, Qasem S, et al. CD138 plasma cells may predict brain metastasis recurrence following resection and stereotactic radiosurgery. Sci Rep. 2019;9(1):14385.
44. Chen CL, Ou DL. Expression of syndecan-1 (CD138) in nasopharyngeal carcinoma is correlated with advanced stage and poor prognosis. Hum Pathol. 2006;37(10):1279–1285.
45. Zhong S, Jia Z, Zhang H, et al. Identification and validation of tumor microenvironment-related prognostic biomarkers in breast cancer. Transl Cancer Res. 2021;10(10):4355–4364.
46. Tang R, Liu X, Liang C, et al. Deciphering the prognostic implications of the components and signatures in the immune microenvironment of pancreatic ductal adenocarcinoma. Front Immunol. 2021;12:648917.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.