Back to Journals » Journal of Pain Research » Volume 17
Distribution of Nerve Fibers in Abdominal Wall Endometriosis and Their Clinical Significance
Authors Zhang C, Dai Y, Zhang J, Li X, Jia S, Shi J, Leng J
Received 4 December 2023
Accepted for publication 20 March 2024
Published 24 April 2024 Volume 2024:17 Pages 1563—1570
DOI https://doi.org/10.2147/JPR.S453148
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Wendy Imlach
Chenyu Zhang,1 Yi Dai,1 Junji Zhang,1 Xiaoyan Li,1 Shuangzheng Jia,2 Jinghua Shi,1,* Jinhua Leng1,*
1Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, National Clinical Research Center for Obstetric & Gynecologic Diseases, Beijing, People’s Republic of China; 2Department of Gynecologic Oncology, National Cancer Center / National Clinical Research Center for Cancer / Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jinghua Shi; Jinhua Leng, Tel +86 151-0101-6294 ; +86 137-0129-8616, Email [email protected]; [email protected]
Objective: This study aimed to explore the distribution of nerve fibers in abdominal wall endometriosis (AWE) and discern their association with pain.
Methods: A retrospective case–control study was conducted. The cases comprised 30 patients diagnosed with AWE, while the control group consisted of 17 patients who had undergone laparotomy without any history of endometriosis. We analyzed clinical characteristics and examined the innervation patterns in samples using stains for S-100, neuron-specific enolase (NSE), protein gene product 9.5 (PGP9.5), neurofilament (NF), and substance P (SP) antibodies.
Results: There was a notable increase in the density of S-100, NSE and PGP9.5 immunoreactive nerve fibers and a higher proportion of SP positivity in AWE lesions compared to standard abdominal wall scars (p < 0.05). However, there were no significant differences in the density or proportion of NF-immunoreactive nerve fibers between the cases and the controls. Moreover, no statistically significant correlation was observed between the density of S-100, NSE, PGP9.5, NF, or SP-positive nerve fibers and pain scores.
Conclusion: This study demonstrated an increased immunoreactive nerve fiber density located in AWE lesions compared to normal abdominal wall scars. Further high-quality studies are needed to investigate the mechanisms responsible for pain in women with endometriosis.
Keywords: abdominal wall endometriosis, pain, nerve fibers, immunohistochemistry
Introduction
Endometriosis (EMs) is characterized by the ectopic presence of endometrial tissue outside the uterine cavity, which can respond to ovarian hormonal stimuli. Predominantly observed in women of reproductive age, it leads to various pain manifestations such as dysmenorrhea, rectalgia, dyschezia, dyspareunia, and chronic pelvic pain.1 EMs can manifest outside the genital tract and has been reported in nearly all organs, including the skin, brain, and lungs.2 The abdominal wall endometriosis (AWE) remains the most prevalent form of extragenital EMs.3
Patients diagnosed with AWE often report palpable pain around scars from prior gynecological procedures.4 This pain, which tends to intensify in severity during menstruation, significantly impacts the patient’s quality of life. Nevertheless, the precise relationship between pain and AWE is yet to be elucidated. Notably, deep infiltrating pelvic endometriosis frequently results in severe pain and discomfort.5 Prior clinical studies have indicated a potential correlation between pain severity and nerve fiber distribution in endometriotic lesions,6,7 prompting our research team to investigate the pain mechanisms in AWE. To date, few researchers have delved into the innervation of these nodules.
Painful scars could result from nerve injury or entrapment following surgical intervention or traumatic injury, leading to disruption of the structural integrity of the dermis and underlying soft tissues.8 Our objective is to perform a comparative analysis of the collagen-rich and fibrous characteristics exhibited by both scar subcutaneous tissue and AWE lesion while also investigating the underlying factors contributing to pain associated with AWE. In this investigation, we will utilize the painless subcutaneous tissue of scars as a control group. The presence of various nerve fibers was confirmed through immunohistochemistry using specific markers, including S-100, neuron-specific enolase (NSE), protein gene product 9.5 (PGP9.5), neurofilament (NF), and substance P (SP), to differentiate between myelinated, unmyelinated, and other types of nerve fibers. Additionally, we collected clinical data with a focus on pain symptoms to explore potential associations with AWE lesion innervation.
Methods
Study Protocol
A retrospective case–control study was conducted. The specimens were gathered during routine surgical procedures at the Department of Gynecology and Obstetrics, Peking Union Medical College Hospital, from March to December 2009. The participants consisted of women within the reproductive age range, specifically between 18 and 45 years old. All participants were required to abstain from any endometriosis-related treatment for a period of three months prior to surgery. The AWE group comprised individuals with severely agonizing nodules that exhibited resistance to conservative treatment. The control group consisted of participants who had undergone a previous laparotomy for benign gynecological conditions (eg, uterine leiomyoma) but did not have EMs. They subsequently underwent a secondary laparotomy and did not report any pain at the surgical scar. All the patients underwent standardized laparotomy by the same senior surgical team. Based on histopathological findings, participants were categorized into an AWE (case) group and non-AWE (control) group. The presence of various nerve fibers, including S-100, NSE, PGP9.5, NF, and SP, was confirmed by immunohistochemistry analysis of the paraffin section. Clinical data on patients’ ages, initial symptoms, examines, surgery details, and follow-up were analyzed. The study received approval from the Human Ethics Committees of Peking Union Medical College Hospital, and informed consent was obtained from all participants. Our research adheres to the principles outlined in the Declaration of Helsinki.
Clinical Outcomes
The dataset included preoperative data, including demographic details, such as age and parity, and clinical characteristics, such as asymptomatic duration (from previous surgery to symptom occur), symptomatic duration, number of abdominal masses, diameter of mass, blood flow surrounded the lesion, location of the lesion, pain of the lesion, dysmenorrhea, type of incision, and depth of infiltration. The assessment of pain using the visual analogue scale (VAS) involves the utilization of a ruler equipped with precise markings, enabling patients to accurately indicate their pain intensity by marking the corresponding position on the ruler. Subsequently, physicians assign a score based on the marked position, facilitating a relatively objective evaluation of pain levels before and after treatment while accounting for potential individual variations.9 The postoperative follow-up data were extracted from the medical records of outpatients. The documentation of pain relief was also collected.
Immunohistochemistry
Surgical tissue samples, from either the AWE lesions or control subcutaneous tissue of scars, were immediately preserved in 10% neutral buffered formalin and forwarded to the Pathomorphology Department. Two experienced pathomorphologists independently assessed the hematoxylin and eosin (H.E.) stained slides obtained from paraffin-embedded tissues, meticulously selecting appropriate samples for immunohistochemistry. Subsequently, sections measuring 4 µm in thickness were carefully prepared for further analysis.
Sections from each sample were immunostained overnight at 4°C using specific antibodies, including polyclonal rabbit anti-S-100 protein (dilution 1: 100; Abcam Inc. USA), monoclonal mouse anti-human NSE (dilution 1: 100; Abcam Inc. USA), monoclonal mouse anti-human PGP9.5 protein (dilution 1: 100; Abcam Inc. USA), monoclonal mouse anti-human NF protein (dilution 1: 100; Zeta Corporation USA), and polyclonal rabbit anti-human SP protein (dilution 1: 100; Abcam Inc. USA). After washing with Phosphate Buffered Saline (PBS), sections were incubated with PV9001/9002 agent (GBI, USA) for 30 minutes at 37°C. The antigen–antibody reactions were visualized using 3,3’-diaminobenzidine (DAB). Sections were counterstained with hematoxylin, dehydrated, and mounted. PBS was employed as a negative control to the antibodies.
Specimens were visualized using a Nikon OPTIPHOT-2 optical microscope and images captured with the ACT-2U Digital Image Acquisition System. Nerve fibers in EMs lesions or control group subcutaneous tissues were enumerated per low-power field (×100). The total number of nerve fibers was divided by actual squares of low-power field (each square of 0.425 mm2) to obtain an average of nerve fibers per mm2. Results were presented as mean (±SD) nerve fibers per mm2 for all EMs and control sections. Two histopathologists, blinded to sample origins, independently assessed the immunohistochemical reactions, focusing on areas with high nerve density.
Statistical Analysis
Continuous variables were presented as the mean ± standard deviation and compared using Student’s t-test. Categorical variables were presented as counts (percentages) and were compared using the chi-squared test. The Spearman correlation analysis was employed to ascertain the association between nerve fibers count in EMs lesions and pain severity. Statistical analyses were finished with Statistical Package for the Social Sciences (SPSS, Version 22.0. Armonk, NY: IBM Corp). A p-value <0.05 were deemed statistically significant.
Results
Clinical Characteristics of Participants
A total of 47 eligible patients were included in the study. Among the participants, thirty individuals (average age: 32.7 years; range: 27–45 years) in the AWE group presented with a painful nodule that enlarged during menstruation, located either on a previous cesarean section scar (27 cases) or another laparotomy scar (3 cases) following excision of an endometriotic lesion. In the control group, seventeen patients (average age: 34.2 years; range: 30–42 years) without any pain on their surgical scars were recruited. Their demographic and clinical data are detailed in Table 1.
Seventy percent (21/30) of AWE patients experienced dysmenorrhea, with a visual analogue scale (VAS) score of 4.8±2.4. In contrast, only 23.5% (4/17) of control group patients reported dysmenorrhea with a VAS score of 1.5±1.2. Among the AWE patients, the average time from previous surgery to symptom onset was 25.0±16.8 months, with an average symptom duration of 26.8±17.6 months. Thirty percent (9/30) of patients exhibited multiple endometriomas, 17.5% (7/30) had masses distanced from the scar, 86.7% (26/30) experienced cyclic pain on the lesions, and the remainder reported noncyclic pain. The average mass diameter was 1.8±1.1 cm. Eleven patients had masses that infiltrated the fascia, while four required synthetic mesh for abdominal wall reconstruction.
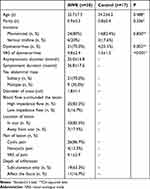 |
Table 1 Clinical Features of the Patients in AWE and Control Groups |
Immunohistochemistry Findings
S-100 antibody displayed positive immunoexpression in all 47 patients. A significant difference in nerve fiber density stained with S-100 existed between the AWE group (7.00±4.68/mm2) and the control group (3.05±1.92/mm2, p = 0.002; Table 2; Figure 1a). Immunohistochemical analysis using NSE antibody was positive in 38 (80.9%) patients. Among these, 86.7% (26/30) were from the AWE group and 70.6% (12/17) from the control. The nerve fiber density in AWE lesions (7.99±3.97/mm2) significantly surpassed that in control (2.89±2.06/mm2, p < 0.001). NE cells intensely stained with NSE were evident in the glands of ectopic endometrium of all 30 AWE patients (Table 2; Figure 1b). The density of PGP9.5 in the AWE group was 7.60±5.15/mm2, whereas it was 4.02±2.83/mm2 in the control group, with a significant p value of 0.011 observed between the two groups. However, there was no significant difference in the positive ratios between the two groups (p = 0.606; Table 2; Figure 1c).
SP positivity was observed in 56.7% (17/30) of AWE samples, substantially higher than the 17.6% (3/17) in the control group (p = 0.009). However, there was no significant difference in SP-immunoreactive nerve fiber density between the groups (0.57±0.26/mm2 in AWE vs 0.42±0.30 /mm2 in control; p = 0.079; Table 2; Figure 1d). Similarly, we found no statistical difference in NF-immunoreactive nerve fiber densities between the two groups (p >0.05; Table 2; Figure 1e). The discrepancy in nerve fiber density between AWE and control groups was illustrated in Figure 1f. While no significant correlation was identified between nerve fiber densities and pain VAS scores (p > 0.05; Table 2).
 |
Table 2 Nerve Fiber Density and Composition in AWE Lesions and Control Scars |
Follow-Up Details
The average follow-up post-procedure was 19.2 months. Over 96% (29/30) of AWE patients remained asymptomatic at their last examination. One patient still reported mild pain around the scar, without a palpable mass. Combined oral contraceptives alleviated the discomfort.
Discussion
Our study identified increased densities of S-100, NSE and PGP9.5 immunoreactive nerve fibers and a greater proportion of SP immunoreactive nerve fibers in AWE lesions compared to normal abdominal wall scars. However, there were no significant differences in NF-immunoreactive nerve fiber densities between groups. Furthermore, no significant correlation emerged between nerve fiber densities and pain severity as quantified by the visual analogue score.
Previous reports have identified nerve fiber proliferation in various endometriotic lesions.7,10–13 Multiple factors can contribute to nerve fiber growth in ectopic endometrium, including hormonal stimulations,14–16 inflammatory mediators,17–19 and potential interactions between nerve fibers, blood vessels, and ectopic endometrium.20,21 In this study, several markers of nerve fibers, such as S-100, NSE, PGP9.5, NF and SP, were chosen, and the clinical meanings of these markers were tried to explain the potential mechanism of the pain of AWE lesions.
S-100 is a dimer intracellular calcium-binding protein, expressed in melanocytes, glial cells and Schwann’s cells.22 The S-100 family has been reported involved in the regulation of a cellular processes,23 such as the participation in mediating fibroproliferative remodeling through the RAGE-NA-kB axis,24 the vascular remodeling in endometriotic angiogenesis,25 and the activating NF-κB signaling pathway in endometrial stromal cells which promotes the development of EMs.26 NSE is a dimer intracellular enzyme of glucose metabolism27 and exists in neurons and peripheral nervous system tissue. NSE is a sensitive marker of neuronal damage. These two biochemical markers above have been widely used in several central nervous system diseases, including hypoxic brain injury, acute ischemic stroke, head injury and epilepsy, as potent predictors of neurological outcome.28 In our study, the elevated expression of S-100 and NSE may be related to the presence of possible neuroendocrine pathways and the injury and regeneration of nerve fibers in AWE lesions.
NSE is also one of the most commonly used immunohistochemical markers of neuroendocrine (NE) cells. The NE system is defined as cells present either in endocrine organs or dispersed throughout the human body. NE cells can produce neurotransmitters, neuromodulators, neuropeptide hormones, paracrine regulators and other bioactive substances.29 Vittoria et al30 presented that a few NE cells might be contained in the normal human endometrium and increased in proliferative conditions, such as endometrial carcinomas. In this study, large number of NSE-stained cells in ectopic endometrium were observed, which was consistent with Wang’s observation,31 and he also reported no NSE- positive NE cells were detected in proliferative phase endometrium in women without EMs and very low density in secretory and menstrual period. We postulated that the intense expression of NSE in NE cells in AWE lesions may stimulate nerve fibers and nociceptors to induce pain signals.
PGP9.5, a cytoplasmic thiol-esterase restricted to nervous and neuroendocrine cells, has been proved to be increased in the proliferative phase of endometriotic women with pain.32 NF is a specific marker for myelinated nerve fibers,33 whereas SP34 is sensory nerve fiber marker that represents both Aδ and C fibers. Published articles proved a higher nerve fiber density with the positive presentation of the above markers in EMs patients compared with controls.35–37 Our data revealed a significant difference in PGP9.5 expression between AWE and control groups, along with a relatively high proportion of SP expression but no difference in NF expression. These findings suggest that the involvement of type Aδ and C fibers in pain generation associated with abdominal wall endometriosis.
The pain caused by EMs lesions can be variable. Some lesions are not painful, while others can cause neuroinflammation at a distance up to 28 mm.38 In this study, no significant connection was proved between nerve fiber density and the severity of patients’ pain. This result might also be restricted by the small number of patients, and the correlation might change with increased patients.
This is the first time to observe the distribution of nerve fiber in abdominal wall endometriotic lesions. The increased innervation of AWE gives us another explanation of the typical experience a palpable mass with cyclic or noncyclic pain. Besides the mechanical and thermal irritation caused by cyclic congestion, they may also combine with hyperalgesia, which is an exacerbation of pain or initiation of painful sensation to a non-painful stimulus. In fact, EMs have been proposed to be a consequence of neurological dysfunction, and possibly involved in a process of denervation and reinnervation.39 The infiltrating of endometrium in abdominal wall subcutaneous tissue might impair the local nerve fiber and lead to a series of denervation and reinnervation process.
Our study has limitations. The sample size was modest, pain severity assessments were subjective, and we only considered certain immunoreactive nerve fibers. It is essential to understand that pain in EMs may result from a combination of factors, including hormonal interactions, angiogenesis, neurotransmitters, inflammation, and others. Further comprehensive studies could provide a more nuanced understanding of these mechanisms.
Conclusion
In summary, this study demonstrated an increased immunoreactive nerve fiber density in AWE lesions compared to normal abdominal wall scars. However, no correlation was observed with clinical pain. The presence of augmented nerve fibers in AWE lesions may play a substantial role in the pathogenesis of pain and tenderness. Further comprehensive, high-quality studies are essential to elucidate the mechanisms underlying pain in women with EMs.
Abbreviations
EMs, endometriosis; AWE, abdominal wall endometriosis; NSE, neuron-specific enolase; PGP9.5, protein gene product 9.5; NF, neurofilament; SP, substance P; VAS, visual analogue scale.
Data Sharing Statement
The article does not involve sequencing data. Demographic information and scientific results are already shown in the main text. All the data used in the study are available from the corresponding author upon reasonable request.
Details of Ethics Approval
Approved from The Institutional Review Board (IRB) of Peking Union Medical College Hospital.
Informed Consent Addressed
All participants gave their informed consent for participation.
Acknowledgments
We thank all the patients who are willing to participate in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Key R&D Program of China (2022YFC2704000) and National Natural Science Foundation of China (Grant No. 82071628).
Disclosure
The authors declared no potential conflicts of interest.
References
1. Francica G, Giardiello C, Angelone G, Cristiano S, Finelli R, Tramontano G. Abdominal wall endometriomas near cesarean delivery scars: sonographic and color Doppler findings in a series of 12 patients. J Ultrasound Med. 2003;22:1041–1047. doi:10.7863/jum.2003.22.10.1041
2. Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021;397(10276):839–852. doi:10.1016/s0140-6736(21)00389-5
3. Douglas C, Rotimi O. Extragenital endometriosis--a clinicopathological review of a Glasgow hospital experience with case illustrations. J Obstet Gynaecol. 2004;24:804–808. doi:10.1080/01443610400009568
4. Yıldırım D, Tatar C, Doğan O, et al. Post-cesarean scar endometriosis. Turk J Obstet Gynecol. 2018;15:33–38. doi:10.4274/tjod.90922
5. Li T, Xu XX, Dai Y, Zhang JJ, Lang JH, Leng JH. 直肠阴道隔子宫内膜异位症部分切除联合药物治疗的效果及对生命质量的影响 [Efficacy and impact on quality of life of different drug treatments after partial resection of rectovaginal endometriosis]. Zhonghua Fu Chan Ke Za Zhi. 2017;52:307–313. Chinese. doi:10.3760/cma.j.issn.0529-567X.2017.05.004
6. Williams C, Hoang L, Yosef A, et al. Nerve bundles and deep dyspareunia in endometriosis. Reprod Sci. 2016;23:892–901. doi:10.1177/1933719115623644
7. Wang YY, Leng JH, Shi JH, Li XY, Lang JH. 子宫内膜异位症患者不同部位病灶中神经纤维分布及其与疼痛症状的关系 [Relationship between pain and nerve fibers distribution in multiple endometriosis lesions]. Zhonghua Fu Chan Ke Za Zhi. 2010;45:260–263. Chinese.
8. Bijlard E, Uiterwaal L, Kouwenberg CA, Mureau MA, Hovius SE, Huygen FJ. A systematic review on the prevalence, etiology, and pathophysiology of intrinsic pain in dermal scar tissue. Pain Physician. 2017;20:1–13. doi:10.36076/ppj.2017.2.13
9. Delgado DA, Lambert BS, Boutris N, et al. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev. 2018;2:e088. doi:10.5435/JAAOSGlobal-D-17-00088
10. García-Solares J, Dolmans MM, Squifflet JL, Donnez J, Donnez O. Invasion of human deep nodular endometriotic lesions is associated with collective cell migration and nerve development. Fertil Steril. 2018;110:1318–1327. doi:10.1016/j.fertnstert.2018.08.016
11. Arnold J, Barcena de Arellano ML, Rüster C, et al. Imbalance between sympathetic and sensory innervation in peritoneal endometriosis. Brain Behav Immun. 2012;26:132–141. doi:10.1016/j.bbi.2011.08.004
12. Zhang X, Lu B, Huang X, Xu H, Zhou C, Lin J. Innervation of endometrium and myometrium in women with painful adenomyosis and uterine fibroids. Fertil Steril. 2010;94:730–737. doi:10.1016/j.fertnstert.2009.03.026
13. Tokushige N, Markham R, Russell P, Fraser IS. Different types of small nerve fibers in eutopic endometrium and myometrium in women with endometriosis. Fertil Steril. 2007;88:795–803. doi:10.1016/j.fertnstert.2006.12.078
14. Dunselman GA, Willebrand D, Evers JL. Immunohistochemical analysis of oestrogen and progesterone receptors of eutopic and ectopic endometrium in the rabbit model of endometriosis: the effect of pregnancy. Hum Reprod. 1992;7:73–75. doi:10.1093/oxfordjournals.humrep.a137563
15. Kim YA, Kim JY, Chang SH, Chang DY, Chun KC, Koh JW. Progesterone reduces neurofilament (NF)--positive nerve fibers in eutopic endometrium of patients with endometriosis and myomata. J Reprod Med. 2014;59:481–487.
16. Krizsan-Agbas D, Pedchenko T, Hasan W, Smith PG. Oestrogen regulates sympathetic neurite outgrowth by modulating brain derived neurotrophic factor synthesis and release by the rodent uterus. Eur J Neurosci. 2003;18:2760–2768. doi:10.1111/j.1460-9568.2003.03029.x
17. Wu J, Xie H, Yao S, Liang Y. Macrophage and nerve interaction in endometriosis. J Neuroinflammation. 2017;14:53. doi:10.1186/s12974-017-0828-3
18. Anaf V, Chapron C, El Nakadi I, De Moor V, Simonart T, Noël JC. Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertil Steril. 2006;86:1336–1343. doi:10.1016/j.fertnstert.2006.03.057
19. Kim YA, Kim JY, Kim MR, Hwang KJ, Chang DY, Jeon MK. Tumor necrosis factor-alpha-induced cyclooxygenase-2 overexpression in eutopic endometrium of women with endometriosis by stromal cell culture through nuclear factor-kappaB activation. J Reprod Med. 2009;54:625–630.
20. Chopin V, Lagadec C, Toillon RA, Le Bourhis X. Neurotrophin signaling in cancer stem cells. Cell Mol Life Sci. 2016;73:1859–1870. doi:10.1007/s00018-016-2156-7
21. Berkley KJ, Dmitrieva N, Curtis KS, Papka RE. Innervation of ectopic endometrium in a rat model of endometriosis. Proc Natl Acad Sci U S A. 2004;101:11094–11098. doi:10.1073/pnas.0403663101
22. Schäfer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996;21:134–140. doi:10.1016/s0968-0004(96)80167-8
23. Yang F, Ma J, Zhu D, et al. The role of S100A6 in human diseases: molecular mechanisms and therapeutic potential. Biomolecules. 2023;13:1139. doi:10.3390/biom13071139
24. Carlsson H, Yhr M, Petersson S, Collins N, Polyak K, Enerbäck C. Psoriasin (S100A7) and calgranulin-B (S100A9) induction is dependent on reactive oxygen species and is downregulated by Bcl-2 and antioxidants. Cancer Biol Ther. 2005;4:998–1005. doi:10.4161/cbt.4.9.1969
25. Hayrabedyan S, Kyurkchiev S, Kehayov I. FGF-1 and S100A13 possibly contribute to angiogenesis in endometriosis. J Reprod Immunol. 2005;67:87–101. doi:10.1016/j.jri.2005.07.001
26. Sun Q, Cao Y, Lan Y, Lei L, Zhang B, Wang S. S100A7 promotes the development of human endometriosis by activating NF-κB signaling pathway in endometrial stromal cells. Cell Biol Int. 2021;45:1327–1335. doi:10.1002/cbin.11578
27. Påhlman S, Esscher T, Bergvall P, Odelstad L. Purification and characterization of human neuron-specific enolase: radioimmunoassay development. Tumour Biol. 1984;5:127–139.
28. Ekmektzoglou KA, Xanthos T, Papadimitriou L. Biochemical markers (NSE, S-100, IL-8) as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation. Resuscitation. 2007;75:219–228. doi:10.1016/j.resuscitation.2007.03.016
29. Lai M, Lü B, Xing X, Xu E, Ren G, Huang Q. Secretagogin, a novel neuroendocrine marker, has a distinct expression pattern from chromogranin A. Virchows Arch. 2006;449:402–409. doi:10.1007/s00428-006-0263-9
30. Vittoria A, Paino G, La Mura E, Budetta G, Cecio A. Chromogranin- and somatostatin-containing neuroendocrine cells in the porcine uterus. An immunocytochemical study. Anat Histol Embryol. 1989;18:136–142. doi:10.1111/j.1439-0264.1989.tb00589.x
31. Wang G, Tokushige N, Russell P, Dubinovsky S, Markham R, Fraser IS. Neuroendocrine cells in eutopic endometrium of women with endometriosis. Hum Reprod. 2010;25:387–391. doi:10.1093/humrep/dep379
32. Zevallos HB, McKinnon B, Tokushige N, Mueller MD, Fraser IS, Bersinger NA. Detection of the pan neuronal marker PGP9.5 by immuno-histochemistry and quantitative PCR in eutopic endometrium from women with and without endometriosis. Arch Gynecol Obstet. 2015;291:85–91. doi:10.1007/s00404-014-3379-1
33. Schlaepfer WW. Neurofilaments: structure, metabolism and implications in disease. J Neuropathol Exp Neurol. 1987;46:117–129. doi:10.1097/00005072-198703000-00001
34. Heinrich D, Reinecke M, Forssmann WG. Peptidergic innervation of the human and Guinea pig uterus. Arch Gynecol. 1986;237:213–219. doi:10.1007/bf02133783
35. Velho RV, Sehouli J, Mechsner S. Mechanisms of peripheral sensitization in endometriosis patients with peritoneal lesions and acyclical pain. Arch Gynecol Obstet. 2023;308:1327–1340. doi:10.1007/s00404-023-07110-9
36. Bokor A, Kyama CM, Vercruysse L, et al. Density of small diameter sensory nerve fibres in endometrium: a semi-invasive diagnostic test for minimal to mild endometriosis. Hum Reprod. 2009;24:3025–3032. doi:10.1093/humrep/dep283
37. Cagiran FT, Kali Z, Kirici P, Celik O. Comparison of immunohistochemical characteristics of endometriomas with non-endometriotic benign ovarian cysts. Eur Rev Med Pharmacol Sci. 2022;26:7594–7599. doi:10.26355/eurrev_202210_30034
38. Demco L. Review of pain associated with minimal endometriosis. JSLS. 2000;4:5–9.
39. Quinn MJ. Endometriosis: the consequence of uterine denervation-reinnervation. Arch Gynecol Obstet. 2011;284:1423–1429. doi:10.1007/s00404-011-2063-y
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

