Back to Journals » Clinical Interventions in Aging » Volume 19
Does Comprehensive Geriatric Assessment Reduce the Incidence of Postoperative Delirium? A Quasi-experimental Study in Older Adults Undergoing Transcatheter Aortic Valve Implantation
Authors Schwesinger A, Tsai LT, Lang W, Mantegazza N, Bauernschmitt R, Wilhelm MJ, Bischoff-Ferrari HA, Gagesch M
Received 4 November 2023
Accepted for publication 6 February 2024
Published 28 February 2024 Volume 2024:19 Pages 347—355
DOI https://doi.org/10.2147/CIA.S448167
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Nandu Goswami
Anna Schwesinger,1,* Li-Tang Tsai,1,* Wei Lang,1 Noemi Mantegazza,1 Robert Bauernschmitt,2 Markus Johannes Wilhelm,2 Heike Annette Bischoff-Ferrari,1,3,4 Michael Gagesch1,5,6
1Center on Aging and Mobility, University of Zurich, Zurich, Switzerland; 2Clinic for Cardiac Surgery, University Hospital Zurich, Zurich, Switzerland; 3Department of Geriatrics and Aging Research, University of Zurich, Zurich, Switzerland; 4IHU HealthAge, University Hospital Toulouse and University Toulouse III Paul Sabatier, Toulouse, France; 5Department of Aging Medicine, University Hospital Zurich, Zurich, Switzerland; 6University Clinic of Aging Medicine, Zurich City Hospital, Zurich, Switzerland
*These authors contributed equally to this work
Correspondence: Michael Gagesch, University Clinic for Aging Medicine, Zurich City Hospital, Tièchestrasse 99, 8037 Zürich, Switzerland, Tel +41 44 417 31 98, Email [email protected]
Purpose: Postoperative delirium (POD) after transcatheter aortic valve implantation (TAVI) is frequent in older adults and associated with multiple negative outcomes including a higher mortality. We aimed to investigate whether a comprehensive geriatric assessment (CGA) prior to TAVI reduces the odds of POD and results in a positive change in self-care ability, intended to lay a foundation for future geriatric comanagement.
Patients and methods: We used a retrospective, single-center study with a quasi-experimental design enrolling patients aged 70 years and older undergoing CGA before elective TAVI, and a nonrandomized comparison group without preoperative CGA. Data on POD occurrence during the first 5 days after TAVI (primary outcome) and change in self-care ability index (SPI) between admission and discharge (secondary outcome) were collected from electronic health records and CGA data (exposure) by clinical assessment. To explore associations between (1) CGA and POD, and (2) CGA and SPI, multivariate logistic regression and linear regression models were applied adjusting for age, sex, BMI, and number of medications.
Results: Among 435 patients (mean age 81.0 ± 5.6 years, 43.6% women, median [IQR] SPI at baseline 40 [39, 40] points), POD incidence was 14.3% in the CGA group vs 18.8% in the non-CGA group (P 0.219). Undergoing CGA before TAVI was not associated with the odds for POD (OR: 1.15; 95%CI: 0.65– 2.04) or improved SPI (P 0.073).
Conclusion: We observed no association of CGA prior to TAVI with POD incidence or postoperative self-care, highlighting the need for additional studies investigating the effect of POD preventive measures in older TAVI patients integrated into a comprehensive geriatric comanagement program.
Keywords: POPS, frail older adults, perioperative care, aortic stenosis, TAVI, delirium
Introduction
Aortic stenosis (AS) is among the top three cardiovascular diseases in older adults from highly developed countries with a prevalence of 12.4%.1,2 Transcatheter aortic valve implantation (TAVI) is the current standard of care in older adult patients not eligible for surgical valve replacement (SAVR) as it is considered superior to medical treatment with regard to survival and quality of life.3–6
Although being a minimally invasive procedure, TAVI comprises a non-negligible risk for important adverse events including postoperative delirium (POD).7 As a multifactorial syndrome of brain dysfunction, delirium is characterized by an acute onset and often fluctuating course of symptoms such as inattention, impaired reasoning and altered level of consciousness.8 A systematic review and meta-analysis found a pooled estimated mean POD rate of 8.1% after TAVI in 32,389 individuals from 31 studies, with incidence ranging from 0% to 45%.7 Variation in reported incidence rates can be attributed to a large heterogeneity in diagnostic tools, the presence of procedural risk factors as general anesthesia or nontransfemoral access site and patient-related risk factors of the investigated population (eg age and frailty parameters including impaired mobility and low self-care abilities).7,9–12
Given that POD is strongly associated with several downstream complications and high mortality after TAVI,9,13 it appears important to reduce the occurrence of POD in older patients in acute care. However, traditional risk predictors in cardiac surgery like the STS-Score or EuroSCORE 2 do not take into account geriatric risk determinants assessing functional impairments.14 In addition, older age, cognitive impairment, previous episodes of delirium and physical frailty have been identified as common risk factors for POD after TAVI.9,10,12 Consequently, the 2017 European Society of Cardiology guideline for valvular heart disease included a recommendation for routine frailty screening in all older adults evaluated for aortic repair15 which is also included in the current 2021 guideline.16
Addressing the specific needs of older adults undergoing surgery, the American Geriatrics Society and the Association of Anaesthetists have published evidence based consensus guidelines for optimal perioperative care of older surgical patients.17 The innovative method of “Proactive care of Older Patients undergoing Surgery” (POPS) offers an approach to identify functional limitations and individual care needs of older adults by a preoperatively performed comprehensive geriatric assessment (CGA) in order to address otherwise under-diagnosed high-risk conditions including functional impairment and delirium by providing information for individualized and multidisciplinary care and potentially preventive measures.18–21 Subsequently, a POPS program for TAVI patients was developed and implemented at our center in 2017.
Despite this rising awareness for the need of a holistic management of geriatric patients in acute care, to our knowledge, there are no studies to date reporting on the potential effect of CGA on POD occurrence in older patients with AS referred for TAVI. Therefore, the primary aim of this quasi-experimental study was to compare the odds of incident POD in TAVI patients who did or did not undergo a CGA on the day prior to the procedure. In addition, we aimed to explore whether preoperative CGA leads to a positive change in self-care ability during hospitalization. Further, we aimed to identify potential risk factors for POD in this particular patient group, including age, sex and polypharmacy.
Materials and Methods
We report on a single-center retrospective quasi-experimental cohort study conducted at the University Hospital Zurich (USZ), Switzerland between 2017 and 2021. We included patients 70 years and older electively referred for TAVI at the Clinic for Cardiac Surgery with available data from routinely collected clinical information. Our study has a quasi-experimental design including an intervention group with patients undergoing CGA before TAVI (CGA group), and a comparison group consisting of patients without preoperative CGA (non-CGA group). We hypothesized that POD incidence was lower in patients undergoing CGA before TAVI compared to patients who do not. As no well-established global outcome parameter to measure the effect of CGA on clinical outcomes exist, we investigated the change in activities of daily living (ADL) by a self-care ability index (German: Selbstpflege-Index: SPI) between admission and discharge in both groups as a secondary aim, hypothesizing, that undergoing CGA may result in improved ADL after TAVI compared to the non-CGA group.
Patient Selection and Data Collection
Eligible patients were 70 years and older, diagnosed with aortic stenosis and referred for TAVI after discussion in the local interdisciplinary heart team. Conscious sedation was the anesthesiological method of choice in our investigated sample. Patients in the CGA group were enrolled into the Zurich Peri-operative care Project for Senior patients in cardiac surgery (POPS-Heart) TAVI 70+ program (ie undergoing a CGA on the day before TAVI), which reflects a CGA and is considered standard of care for this group at our institution. Planning CGA on the day prior to the procedure appeared most practical as a first step of implementation, as TAVI patients were referred from a wide geographical region without access to local geriatric centers. Thus, we expected low feasibility for any additional ambulatory visits at a greater time interval prior to the planned procedure. For logistical reasons (ie absence of the assessor, lost patient registration, lack of time resources and others), not all patients could be enrolled onto the program. Given that the selection of patients who received CGA was independent of medical reasons, the TAVI patients who did not receive CGA serve as a comparison group. Except for the absence of a CGA, medical team members, location of the patient or perioperative procedures did not differ in between both groups. At the same time, patients receiving TAVI as an emergency procedure or by nontransfemoral access or who died during hospital stay were excluded. In addition, patients who underwent CGA during a previous hospital stay were excluded. Individual patient demographics were collected at baseline from the hospitals’ electronic health records system.
Outcomes
Our primary outcome was the incidence of POD within the first five days following TAVI, when POD mostly occurs.13 At our hospital, delirium is routinely assessed in all in-patients by trained nursing staff at 8-h intervals (once per shift) by a standardized screening tool based on Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) criteria. At intensive care units (ICU), the Intensive Care Delirium Screening Checklist (ICDSC) is used, and at regular wards the Delirium Observation Screening Scale (DOS) is used. The ICDSC had a pooled sensitivity of 83% (95%CI: 74–90) and specificity of 87% (95%CI: 78–93) in prior studies22 and was used for TAVI patients who were initially treated in the ICU or intermediate care (step-down) unit. Since the assessment of older patients in the ICU is particularly complex and the reliability of the ICDSC is considered to be lower than for other delirium assessment tools, a positive screening result in the ICDSC was followed by a second diagnostic tool, mostly the DOS. The DOS is a validated tool for the diagnosis of delirium with expanding use in various clinical settings showing a pooled sensitivity and specificity of 90% and 92% (95%CI: 76–97 and 88–94), respectively, and has the advantage of being easily integrated in routine nursing care.23 For our study, presence of POD was defined as a score of ≥3 points in the Delirium Observation Screening Scale (DOS) or ≥4 points in the Intensive Care Delirium Screening Checklist (ICDSC).
Our secondary outcome was to investigate a potential change in independence on ADL, recorded daily from admission to discharge by nursing staff using a standardized self-care index (SPI).24 The SPI has been previously used to describe functional status in medical inpatients upon admission and at discharge in order to investigate hospital-acquired functional decline and short-term mortality.24 The SPI constitutes the standard-of-care instrument for evaluation of ADL in all USZ inpatients and is routinely obtained once daily. The SPI score is composed of 10 categories (activity/mobility, personal hygiene upper body and lower body, dressing/undressing upper body and lower body, eating, drinking, urinary excretion, stool excretion, and ability to acquire knowledge), each of which is evaluated with 1 to 4 points, resulting in a minimal score of 10 points (full dependence) and a maximal score of 40 points indicating complete self-care independence. SPI information was collected by data extraction request from electronic health records for all included patients.
Exposure
Receiving CGA upon admission by a board-certified staff geriatrician from the USZ Department of Aging Medicine was studied as the exposure. Results and corresponding recommendations were documented on a standardized POPS-CGA paper form report that was later scanned and included in the patients’ electronic health records for review and consideration by the competent heart surgeons and the respective nursing care team. Provided recommendations included eg advice about the support of early mobilization and physical therapy, nonpharmacological delirium prevention strategies and nutrition optimisation. However, recommendations were not discussed verbally between teams or discussed in an interdisciplinary team meeting.
The POPS-CGA covers physical function and mobility, basic ADL and instrumental ADL, cognitive screening, risk assessment of post-interventional delirium, eye and ear function, nutritional screening, frailty screening, depression screening, polypharmacy screening, assessment of comorbidity burden and assessment of quality of life by validated screening tools. Assessments indicating noticeable findings, limitations or impairments, were addressed by specific recommendations for the treating heart surgeons. For example, early physical therapy was recommended for limited mobility, optimizing nutrition for risk of or prevalent malnutrition, potentially inadequate medications are identified, and specific follow-up is mentioned for evidence of depressive or cognitive impairment.
Statistical Analysis
Baseline demographics were compared between the intervention and comparison group to evaluate the baseline equivalence, using the chi-squared test for categorical data and the independent samples t-test or Wilcoxon rank sum test for continuous data depending on the normality of distribution. In addition, we performed exploratory analyses investigating the association between baseline characteristics of the study population with the incidence of POD after TAVI. To explore the association between CGA and POD, we used multivariable logistic regression adjusted for age, sex, BMI and number of medications. To investigate the association between change in SPI and CGA, a linear regression model was fitted with change in SPI score as outcome, and adjusted for potential confounders (age, sex, BMI and number of medications). All statistical analyses were performed using SAS v 9.4 (SAS Institute, Inc., Cary, NC, United States). The analyses were two-sided and the type I error rate was set at 5%. The results were presented as an odds ratio and 95% confidence interval.
Ethics Approval and Consent to Participate
This study was approved by ethics committee of the Canton of Zurich, Switzerland that is part of the Swiss Association of Research Ethics Committees (Swissethics). It is registered under the Business Administration System for Ethics Committees (BASEC) number 2021–00903. The study was conducted in accordance with the 1964 Declaration of Helsinki. All participants signed the General Informed Consent of the University Hospital Zurich that allows the further use of health-related personal data and biological material for research purposes.
Results
A total of 637 patients met the baseline requirements and were screened for inclusion. The study flow is depicted in Figure 1. After applying exclusion criteria, the final study sample consisted of 435 patients, with 254 (58.4%) in the intervention group (CGA group) and 181 (41.6%) in the comparison group (non-CGA group). Overall, mean age was 81 (±5.6) years with 221 (53.6%) patients aged 80 and older, 179 (43.6%) were women and mean BMI was 27.4 (±4.8) kg/m2. Median length of stay in the hospital was 7 days (IQR 5, 8) in the CGA group and 7 days (IQR 6, 9) in the non-CGA group. Both investigated groups indicated a comparably high rate of polypharmacy (n=145 [65.0%] vs n=119 [65.8%]) with a median count of six medications (IQR 4, 7 vs 4, 9). In regard to cognitive function and frailty status in the CGA-group, mean Mini-mental State Examination (MMSE) total score was 26.4 (SD: 3.0) points while 21 (9.3%) were frail, 104 (46.0%) pre-frail, and 101 (44.7%) robust according to the Fried frailty phenotype. Baseline patient characteristics are summarized in Table 1.
 |
Table 1 Patients’ Characteristics |
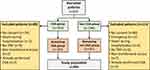 |
Figure 1 Study Flow Diagram. Abbreviations: CGA, comprehensive geriatric assessment; TAVI, transcatheter aortic valve implantation. |
Patients with and without POD (Table 2) did not differ statistically significant in terms of age (82.2 ± 5.3 vs 80.8 ± 5.6, P 0.078), proportion of women (35.8% vs 45.1%, P 0.163) and BMI (27.4 ± 5.6 vs 27.4 ± 4.8, P 0.730). POD occurred in 67 (16.3%) individuals, with a nonsignificant 4.5% lower incidence of POD in the CGA group (14.3% vs 18.8%, P 0.219). Performing CGA before TAVI was not associated with lower odds for POD in comparison with the non-CGA group (OR: 1.15; 95%CI: 0.65–2.04). Patients with POD took a higher number of medications compared to patients without POD (median 7.0 [IQR 5.0, 10.0]) vs 6.0 [4.0, 8.0], P 0.003). Compared to male patients, women had 51% lower odds of developing POD (OR: 0.49; 95%CI: 0.26–0.89), and taking one additional medication was associated with 13% increased odds of POD (OR: 1.13; 95%CI: 1.04–1.23). Further, older age was associated with a borderline significant 5% increased odds for POD (OR: 1.05; 95%CI: 1.00–1.11).
 |
Table 2 Factors Associated with Postoperative Delirium (POD) |
In regard to self-care abilities, median SPI at admission as well as at discharge extended to the maximal score of 40 points (IQR 39, 40) for both groups upon admission (P 0.536) vs 40 points (IQR 37, 40) for both groups at discharge (P 0.483). Median of change in SPI between admission and discharge was 0 points (IQR −2, 0) for both CGA vs non-CGA group (P 0.073). Of note, results from the linear regression model (R2 = 0.54) showed that higher SPI at admission (β coefficient 0.21, P <0.001), older age (β −0.11, P 0.004) and a higher number of medications (β −0.28, P <0.001) were associated with decrease in SPI at discharge (Table 3).
 |
Table 3 Factors Associated with Change in Self-care Abilities (SPI) |
Discussion
In this quasi-experimental study among 435 patients age 70 years and older undergoing elective TAVI for aortic stenosis, performing CGA on the day prior to TAVI was not associated with lower odds for developing POD during the first 5 days after the procedure, while patients in the CGA-group demonstrated a nonsignificant 4.5% lower incidence in POD compared to the non-CGA group. In regard to self-care abilities assessed by SPI, no difference was observed between admission and discharge in both investigated groups.
Overall, our observed POD incidence of 16.3% falls within the range to prior reports on POD after TAVI (0–45%) with a pooled estimate rate of 8.1% and a marginally higher incidence when using CAM-defined delirium (13.5%).7 At the same time, our results appear somewhat lower compared to earlier results for older Swiss TAVI recipients (20% POD).25 As all included patients were electively referred to TAVI in a multidisciplinary decision-making process, this may have led to the exclusion of patients with advanced geriatric syndromes, including risk factors for POD. However, the incidences of POD in the selected patient group would be rather underestimated as the patients at an even higher risk for adverse outcomes did not meet the indication for TAVI.
In TAVI patients, procedural and periprocedural complications such as the occurrence of new cerebral ischemic lesions26 as well as patient-related risk factors including impaired mobility, cognitive impairment, present frailty and malnutrition10,12,18 have been identified as important predictors for POD. Therefore, our primary hypothesis was based on the intention to detect those patient-related POD risk factors by CGA and provide derived recommendations to the treating heart surgeons.
While multidomain interventions such as physical exercise, cognitive training and optimizing nutrition have been previously described as effective with regard to the reduction of adverse postoperative outcomes including delirium in older adults referred for surgery,27,28 our study did not include interdisciplinary management after TAVI beyond the implementation of a CGA. At the same time, only a few interventions (eg systematic neurocognitive assessment, encouraging mobility and self-care and pain management) have shown a positive effect on delirium incidence after TAVI in individual studies.7 As CGA in our study was performed directly on the day of admission before TAVI, the time frame to implement clinical interventions might have been too short. This may be especially true for physical training and cognitive support, even though early mobilization within 4–6 h after the intervention has been associated with a decrease in delirium incidence in recent studies.29 Besides, approximately half of the predisposing and precipitating factors of delirium are regarded as being nonmodifiable,10,13 impeding the establishment of effective perioperative interventions even if those risk factors were identified by the CGA. Despite those limitations and to the best of our knowledge, this is the first study comparing the occurrence of POD and investigating a potential change in self-care abilities during hospitalization in older TAVI patients with or without prior CGA and written recommendations.
When investigating the effect of CGA-based recommendations on ADL abilities (measured by SPI), we assumed that perioperative prevention measures such as intensified mobilization as recommended by CGA would improve self-care abilities and, therefore, our intervention group might demonstrate less ADL deterioration. However, baseline ADL independence in our sample was unexpectedly high for a geriatric population with a majority of patients scoring the maximum SPI score of 40 points (ie reflecting full independence in ADL), and no improvement could be detected during hospitalization. This might be attributable to the elective setting with cautious indication in this patient group.
Further, we investigated the association of patient characteristics with the incidence of POD as they may be identified as possible risk factors for delirium during the preoperative assessment. Among those, a higher number of medications was significantly associated with POD. This finding is supported by other studies that have established polypharmacy as an independent risk factor for postoperative delirium, eg in hip fracture patients30 and general surgery.31 Subsequently, a systematic preoperative medication review as part of a CGA evaluation may be effective in the prevention of delirium by reducing the number of medications, drug interactions and the use of delirium promoting medications.32 In addition, patients developing delirium after TAVI were predominantly men and of older age. However, both male sex and age have not been proven as independent risk factors for POD. These results are in line with findings from a systematic review about risk factors for delirium after TAVI.33
Our study has several strengths. To our knowledge, this is the first study investigating the association of a preoperatively performed CGA and POD incidence in a real-world sample of Swiss older adults undergoing TAVI, also incorporating an instrument to measure ADL independence as an outcome parameter (ie the SPI index) for the potential effect of the intervention. Screening for POD was routinely performed in all TAVI patients by means of reliable diagnostic tools. Therefore, incidence of POD should be less biased compared to selective screening in patients with poor condition or retrospectively diagnosed delirium using documented behavioural alterations.
Our study also has its limitations, including a lack of randomization and sample size calculation while using only data from a single center with a limited sample size and implementation of the CGA on the day prior to the procedure. Also, there may be differences in the delirium risk profile between the groups that we did not consider and that were not included in the statistical analyses. For instance, we excluded all patients with nontransfemoral access site (ie carotid, subclavian or transapical access), but did not screen our cohort for coronary and carotid artery diseases or a high blood transfusion rate, all known periprocedural risk factors for POD.7,13,34,35 Neither did we analyze the proportion of cerebral embolic protection device (CEPD) usage to limit the occurrence of brain lesions and neurological complications after TAVI.26,36 The current analysis also lacks data on the perioperative administration of potentially prodelirogenic drugs during surgery, in the recovery room, and postoperatively as well as data on perioperative hemodynamic and cardiologic status during surgery and data on postoperative events. Moreover, the recommendations of the CGA were only provided in writing reports and not verbally discussed with the surgical team and we did not investigate the implementation rate of the advised recommendations. In addition, our measure of choice to assess ADL (SPI) has limited validity to predict postoperative functional changes. Further, there may exist differences in patient characteristics that were assessed by the CGA for the intervention group only and therefore could not be included in the analysis of baseline equivalence between the groups. However, we consider these differences to be minimal because there was, as a fundamental property of the quasi-experimental study design, no intentional selection of patients for inclusion in the intervention group.
Conclusion
Even though we were not able to demonstrate a statistically significant reduction of POD by the performance of a pre-interventional CGA in older TAVI recipients, we identified a higher number of medications as an important independent risk factor for POD in our patient group. Our study may contribute to the optimization of perioperative care in older adults undergoing TAVI. Further research in larger populations is warranted in order to confirm and extend our findings. This should include structured management plans based on the results of a CGA, as the proactive and timely implementation of CGA-driven interventions can optimize the effect of geriatric comanagement on reducing delirium incidence.37
Abbreviations
ADL, activities of daily living; AS, aortic stenosis; BASEC, Business Administration System for Ethics Committees; CAM, confusion assessment method; CEPD, cerebral embolic protection device; CGA, comprehensive geriatric assessment; DOS, Delirium Observation Screening Scale; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders IV; ICDSC, Intensive Care Delirium Screening Checklist; ICU, intensive care unit; MMSE, Mini-mental State Examination; POD, postoperative delirium; POPS, proactive care of older patients undergoing surgery; SAVR, surgical aortic valve replacement; SPI, self-care ability index (German “Selbstpflegeindex”); SPPB, short physical performance battery; TAVI, transcatheter aortic valve implantation; USZ, University Hospital Zurich.
Disclosure
LTT is currently an employee of Nestlé SA Research, Lausanne, Switzerland. All the other authors have no competing interest in this work.
References
1. Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc. 2010;85(5):483–500. doi:10.4065/mcp.2009.0706
2. Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62(11):1002–1012. doi:10.1016/j.jacc.2013.05.015
3. Khanji MY, Ricci F, Galusko V, et al. Management of aortic stenosis: a systematic review of clinical practice guidelines and recommendations. Eur Heart J Qual Care Clin Outcomes. 2021;7(4):340–353. doi:10.1093/ehjqcco/qcab016
4. Ak A, Porokhovnikov I, Kuethe F, Schulze PC, Noutsias M, Schlattmann P. Transcatheter vs. surgical aortic valve replacement and medical treatment: systematic review and meta-analysis of randomized and non-randomized trials. Herz. 2018;43(4):325–337. doi:10.1007/s00059-017-4562-5
5. Elmalem S, Dumonteil N, Marcheix B, et al. Health-related quality of life after transcatheter aortic valve implantation in elderly patients with severe aortic stenosis. J Am Med Dir Assoc. 2014;15(3):201–206. doi:10.1016/j.jamda.2013.11.010
6. Fu J, Popal MS, Li Y, et al. Transcatheter versus surgical aortic valve replacement in low and intermediate risk patients with severe aortic stenosis: systematic review and meta-analysis of randomized controlled trials and propensity score matching observational studies. J Thorac Dis. 2019;11(5):1945–1962. doi:10.21037/jtd.2019.04.97
7. Abawi M, Pagnesi M, Agostoni P, et al. Postoperative delirium in individuals undergoing transcatheter aortic valve replacement: a systematic review and meta-analysis. J Am Geriatr Soc. 2018;66(12):2417–2424. doi:10.1111/jgs.15600
8. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi:10.1016/S0140-6736(13)60688-1
9. van der Wulp K, van Wely M, van Heijningen L, et al. Delirium after transcatheter aortic valve implantation under general anesthesia: incidence, predictors, and relation to long-term survival. J Am Geriatr Soc. 2019;67(11):2325–2330. doi:10.1111/jgs.16087
10. van der Wulp K, van Wely MH, Schoon Y, et al. Geriatric assessment in the prediction of delirium and long-term survival after transcatheter aortic valve implantation. J Thorac Cardiovasc Surg. 2020;161(6):2095–2102.e3. doi:10.1016/j.jtcvs.2020.02.076
11. Husser O, Fujita B, Hengstenberg C, et al. Conscious sedation versus general anesthesia in transcatheter aortic valve replacement: the German aortic valve registry. JACC. 2018;11(6):567–578. doi:10.1016/j.jcin.2017.12.019
12. Assmann P, Kievit P, van der Wulp K, et al. Frailty is associated with delirium and mortality after transcatheter aortic valve implantation. Open Heart. 2016;3(2):e000478. doi:10.1136/openhrt-2016-000478
13. Abawi M, Nijhoff F, Agostoni P, et al. Incidence, predictive factors, and effect of delirium after transcatheter aortic valve replacement. JACC. 2016;9(2):160–168. doi:10.1016/j.jcin.2015.09.037
14. Schoenenberger AW, Stortecky S, Neumann S, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J. 2013;34(9):684–692. doi:10.1093/eurheartj/ehs304
15. Pulignano G, Gulizia MM, Baldasseroni S, et al. ANMCO/SIC/SICI-GISE/SICCH executive summary of consensus document on risk stratification in elderly patients with aortic stenosis before surgery or transcatheter aortic valve replacement. Eur Heart J Suppl. 2017;19(Suppl D):D354–d369. doi:10.1093/eurheartj/sux012
16. Vahanian A, Beyersdorf F, Praz F, et al. ESC/EACTS guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European society of cardiology (ESC) and the European association for cardio-thoracic surgery (EACTS). Eur Heart J. 2021;43(7):561–632. doi:10.1093/eurheartj/ehab395
17. Mohanty S, Rosenthal RA, Russell MM, Neuman MD, Ko CY, Esnaola NF. Optimal Perioperative Management of the Geriatric Patient: a best practices guideline from the American College of Surgeons NSQIP and the American Geriatrics Society. J Am Coll Surg. 2016;222(5):930–947. doi:10.1016/j.jamcollsurg.2015.12.026
18. Khan MM, Lanctôt KL, Fremes SE, et al. The value of screening for cognition, depression, and frailty in patients referred for TAVI. Clin Interv Aging. 2019;14:841–848. doi:10.2147/cia.S201615
19. Damier E, Chidlovskii E, Bertrand B, Dang VM, Vanzetto G, Couturier P. Multidimensional geriatric assessment before transcatheter aortic valve implantation in frail elderly patients with one-year follow-up. Cardio-geriatrician collaboration benefits? Ann Cardiol Angeiol. 2016;65(4):250–254. doi:10.1016/j.ancard.2016.05.001
20. Partridge J, Sbai M, Dhesi J. Proactive care of older people undergoing surgery. Aging Clin Exp Res. 2018;30(3):253–257. doi:10.1007/s40520-017-0879-4
21. Eamer G, Taheri A, Chen SS, et al. Comprehensive geriatric assessment for older people admitted to a surgical service. Cochrane Database Syst Rev. 2018;1(1). doi:10.1002/14651858.CD012485.pub2
22. Chen TJ, Chung YW, Chang HR, et al. Diagnostic accuracy of the CAM-ICU and ICDSC in detecting intensive care unit delirium: a bivariate meta-analysis. Int J Nurs Stud. 2021;113:103782. doi:10.1016/j.ijnurstu.2020.103782
23. Park J, Jeong E, Lee J. The Delirium observation screening scale: a systematic review and meta-analysis of diagnostic test accuracy. Clin Nurs Res. 2021;30(4):464–473. doi:10.1177/1054773820961234
24. Koch D, Kutz A, Haubitz S, et al. Association of functional status and hospital-acquired functional decline with 30-day outcomes in medical inpatients: a prospective cohort study. Appl Nurs Res. 2020;54:151274. doi:10.1016/j.apnr.2020.151274
25. Humbert M, Büla CJ, Muller O, Krief H, Monney P. Delirium in older patients undergoing aortic valve replacement: incidence, predictors, and cognitive prognosis. BMC Geriatr. 2021;21(1):153. doi:10.1186/s12877-021-02100-5
26. Pagnesi M, Martino EA, Chiarito M, et al. Silent cerebral injury after transcatheter aortic valve implantation and the preventive role of embolic protection devices: a systematic review and meta-analysis. Int J Cardiol. 2016;221:97–106. doi:10.1016/j.ijcard.2016.06.143
27. Harari D, Hopper A, Dhesi J, Babic-Illman G, Lockwood L, Martin F. Proactive care of older people undergoing surgery (‘POPS’): designing, embedding, evaluating and funding a comprehensive geriatric assessment service for older elective surgical patients. Age Ageing. 2007;36(2):190–196. doi:10.1093/ageing/afl163
28. Thillainadesan J, Yumol MF, Hilmer S, Aitken SJ, Naganathan V. Interventions to improve clinical outcomes in older adults admitted to a surgical service: a systematic review and meta-analysis. J Am Med Dir Assoc. 2020;21:1833–1843.e20. doi:10.1016/j.jamda.2020.03.023
29. Vendrik J, Vlastra W, van Mourik MS, et al. Early mobilisation after transfemoral transcatheter aortic valve implantation: results of the MobiTAVI trial. Neth Heart J. 2020;28(5):240–248. doi:10.1007/s12471-020-01374-5
30. Oh ES, Li M, Fafowora TM, et al. Preoperative risk factors for postoperative delirium following Hip fracture repair: a systematic review. Int J Geriatr Psychiatry. 2015;30(9):900–910. doi:10.1002/gps.4233
31. Saljuqi AT, Hanna K, Asmar S, et al. Prospective Evaluation of Delirium in Geriatric Patients undergoing emergency general surgery. J Am Coll Surg. 2020;230(5):758–765. doi:10.1016/j.jamcollsurg.2020.01.029
32. Jeong YM, Lee E, Kim KI, et al. Association of pre-operative medication use with post-operative delirium in surgical oncology patients receiving comprehensive geriatric assessment. BMC Geriatr. 2016;16:134. doi:10.1186/s12877-016-0311-5
33. Tilley E, Psaltis PJ, Loetscher T, et al. Meta-analysis of prevalence and risk factors for delirium after transcatheter aortic valve implantation. Am J Cardiol. 2018;122(11):1917–1923. doi:10.1016/j.amjcard.2018.08.037
34. Tse L, Bowering JB, Schwarz SK, Moore RL, Burns KD, Barr AM. Postoperative delirium following transcatheter aortic valve implantation: a historical cohort study. Can J Anaesth. 2015;62(1):22–30. doi:10.1007/s12630-014-0254-2
35. Bagienski M, Kleczynski P, Dziewierz A, et al. Incidence of postoperative delirium and its impact on outcomes after transcatheter aortic valve implantation. Am J Cardiol. 2017;120(7):1187–1192. doi:10.1016/j.amjcard.2017.06.068
36. Lansky AJ, Brown D, Pena C, et al. Neurologic complications of unprotected transcatheter aortic valve implantation (from the Neuro-TAVI Trial). Am J Cardiol. 2016;118(10):1519–1526. doi:10.1016/j.amjcard.2016.08.013
37. Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital elder life program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry. 2018;26(10):1015–1033. doi:10.1016/j.jagp.2018.06.007
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
