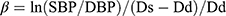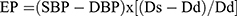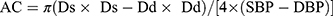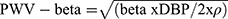Back to Journals » Clinical Interventions in Aging » Volume 19
Early Cardiac Rehabilitation Improves Carotid Arterial Stiffness in Patients with Myocardial Infarction
Authors Ołpińska B , Wyderka R, Łoboz-Rudnicka M, Brzezińska B, Łoboz-Grudzień K , Jaroch J
Received 18 December 2023
Accepted for publication 24 February 2024
Published 14 March 2024 Volume 2024:19 Pages 471—480
DOI https://doi.org/10.2147/CIA.S452362
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Bogusława Ołpińska,1 Rafał Wyderka,1,2 Maria Łoboz-Rudnicka,1 Barbara Brzezińska,1 Krystyna Łoboz-Grudzień,1 Joanna Jaroch1,2
1Department of Cardiology, T Marciniak Lower Silesian Specialist Hospital, Emergency Medicine Center, Wrocław, Poland; 2Faculty of Medicine, University of Science and Technology, Wrocław, Poland
Correspondence: Bogusława Ołpińska, Department of Cardiology, T Marciniak Lower Silesian Specialist Hospital, Emergency Medicine Center, Ul. Generała Augusta Fieldorfa 2, Wrocław, 54-049, Poland, Email [email protected]
Background: Little is known about the effect of cardiac rehabilitation (CR) on carotid arterial stiffness (CAS) in patients with myocardial infarction (MI).
Patients and Methods: Rehabilitation group (B) included 90 patients with MI subjected to CR, control group (K) consisted of 30 patients with MI not participating in CR, and healthy group comprised 38 persons without cardiovascular risk factors. CAS was determined using echo-tracking before and after CR.
Results: At baseline, patients with MI (B+K) presented with significantly higher mean values of CAS parameters: beta-stiffness index (7.1 vs 6.4, p = 0.004), Peterson’s elastic modulus (96 kPa vs 77 kPa, p < 0.001) and PWV-beta (6.1 m/s vs 5.2 m/s, p < 0.001) than healthy persons. Age (beta: r = 0.242, p = 0.008; EP: r = 0.250, p = 0.006; PWV-beta: r = 0.224, p = 0.014) and blood pressure: SBP (EP: r = 0.388, PWV-beta: r = 0.360), DBP (AC: r = 0.225) and PP (PWV-beta: r = 0.221) correlated positively with the initial parameters of CAS. Beta-stiffness index (Rho=− 0.26, p = 0.04) and PWV-beta (Rho = 0.29, p = 0.03) correlated inversely with peak exercise capacity expressed in METs. After CR, mean values of beta-stiffness index (6.2 vs 7.1, p = 0.016), EP (78 kPa vs 101 kPa, p = 0.001) and PWV-beta (5.4 m/s vs 6.2 m/s, p = 0.001) in group B were significantly lower than in group K. In group B, CAS parameters decreased significantly after CR. Univariate analysis demonstrated that the likelihood of an improvement in CAS after CR was significantly higher in patients with baseline systolic blood pressure < 120 mm Hg (OR = 2.74, p = 0.009) and left ventricular ejection fraction < 43% (OR = 5.05, p = 0.005).
Conclusion: In patients with MI, CR exerted a beneficial effect on CAS parameters. The improvement in CAS was predicted by lower SBP and LVEF at baseline.
Keywords: carotid arterial stiffness, managed care after myocardial infarction, acute coronary syndrome, echo-tracking
Introduction
The global incidence of myocardial infarction (MI), the most severe clinical presentation of coronary artery disease, remains high, with more than 7 million people suffering annually and with especially high prevalence in the growing population of people >60 years old.1,2 While the increased frequency of invasive treatment in the acute phase of MI has contributed recently to a significant decrease in in-hospital mortality, annual mortality rate after MI remains high (up to 10%), mostly due to the underestimation of the role of secondary prevention: inadequate control of cardiovascular risk factors, inadequate pharmacotherapy and lifestyle modifications.3
Cardiac rehabilitation (CR) is a comprehensive multidisciplinary intervention, consisting of exercise training, risk factor management and psychosocial counselling. Early post-discharge CR (the first 4–6 weeks after discharge) is based mostly on supervised, physician prescribed exercise training. As CR reduces rates of hospitalization and mortality and improves quality of life in post-MI patients, it is recommended in all patients after MI, both by the ESC4 and the American Cardiology Societies (AHA, ACC).5
Managed Care after Myocardial Infarction program, implemented in Poland in 2017 by the Polish Cardiac Society, National Health Fund and Ministry of Health and dedicated to patients with MI, contributed to a significant improvement in short- term6 and 1-year prognosis.7 This beneficial effect results, among others, from better access to early post-infarction cardiac rehabilitation.8
Arterial stiffness contributes to the progression of atherosclerosis and plays an essential role in the pathophysiology of cardiovascular disorders.9 Increased arterial stiffness has been shown to be an independent predictor of both cardiovascular and all-cause mortality.10 Locally measured arterial stiffness has also a proven prognostic value: in Hoorn Population Study, carotid arterial stiffness (CAS) proved to be an independent predictor of death from all cause.11 However, data on the factors influencing arterial stiffness in patients with myocardial infarction are scarce, little is also known on the impact of early post-MI cardiac rehabilitation on arterial stiffness. Moreover, in previous studies, arterial stiffness has been determined with methods based on regional parameters using applanation tonometry (cfPWV, crPWV) and to our best knowledge no study on the influence of early post-MI cardiac rehabilitation on CAS, measured with the use of echo-tracking, has been performed to date.12 Recently, echo-tracking is the subject of growing interest among researchers, as an easy to perform, bedside method of CAS measurement, that can be performed with the same device as transthoracic echocardiography. Age- and sex-specific reference values for carotid arterial stiffness measured with echo-tracking were provided as a result of the study performed by the E-tracking International Collaboration Group (ETIC), with the contribution of our Department of Cardiology.13
Thus, the aim of the study was to measure CAS in patients with MI using the ultrasound echo-tracking method and to analyze the effect of early CR on CAS in patients with a recent MI, as well as determine the predictors of the post-rehabilitation improvement of CAS in this group.
Materials and Methods
The study group consisted of 120 patients hospitalized for MI (non-ST-elevation MI or ST-elevation MI) in the Department of Cardiology of T Marciniak Lower Silesian Specialist Hospital – Emergency Medicine Centre in Wroclaw, Poland. The rehabilitation group (group B) consisted of 90 patients (mean age 64.2 years) who after having been discharged from the hospital, have completed the 2nd stage of cardiac rehabilitation within the framework of the Managed Care after Myocardial Infarction program (Table 1). The control group (group K) included 30 patients (mean age, 63.5 years) with myocardial infarction who have not participated in the cardiac rehabilitation program due to personal or logistical reasons, or because of contraindications to kinesiotherapy, such as the presence of a free-floating left ventricular thrombus, cognitive dysfunction or impaired mobility. Healthy group which comprised 38 persons without cardiovascular risk factors examined to establish norms for CAS parameters. Exclusion criteria from the study included severe valvular heart disease, arrhythmia, lack of compliance, and age below 18 years.
The indices of CAS (beta-stiffness index [beta], Peterson’s elastic modulus [Ep], local one-point pulse wave velocity [PWV-beta] and arterial compliance [AC]) were measured with the use of echo-tracking twice, during initial hospitalization due to myocardial infarction and six weeks after the discharge from hospital. The echo-tracking measurements were conducted with an Aloka ProSound Hitachi α-10 device (the method described elsewhere).14
The following indices of arterial stiffness were calculated automatically:
- Beta-stiffness index (beta): the ratio of the natural logarithm of systolic/diastolic blood pressure to the relative change in diameter:
- Peterson’s elastic modulus (EP) (kPa), the pressure change required for (theoretical) 100% increase in arterial diameter, relative to the resting diameter:
- Arterial compliance (AC) (mm2/kPa) defined as a change in the absolute dimension of the vessel in response to a given change in blood pressure:
- Local one-point pulse wave velocity (PWV-beta) (m/s):
where: ln – natural logarithm, SBP – systolic blood pressure, DBP – diastolic blood pressure, Ds – arterial diameter in systole, Dd – arterial diameter in diastole, ρ – blood density (1.050 kg/m3).
Left ventricular ejection fraction was calculated twice (during the hospitalization and six weeks after the discharge) with the use of Simpson method on transthoracic echocardiography.
In group B, an exercise stress electrocardiogram was obtained twice, before and after the cardiac rehabilitation program; the analyzed parameters included peak exercise capacity expressed in metabolic equivalents of task (METs) and duration of the exercise. The cardiac rehabilitation conducted within the framework of the Managed Care after Myocardial Infarction program was held in an inpatient (13 patients) or outpatient (77 patients) setting. The rehabilitation program lasted five weeks and consisted of 25 training sessions with aerobic endurance exercises and resistance exercises. Aside from the kinesiotherapy, the program included also the optimization of pharmacotherapy, management of cardiovascular risk factors, dietary counseling and education.15 All patients included in the study provided their written informed consent to participate. The protocol of the study complied with the Declaration of Helsinki and was approved by the Local Bioethics Committee at the Wroclaw Medical University.
Statistical Analysis
Statistical analysis of the results was carried out with STATISTICA v. 13 package (TIBCO Software Inc., Palo Alto, Ca, USA).
The normal distribution of quantitative variables was verified with the Shapiro–Wilk test, with the critical value for the test set at p < 0.05. Homogeneity of variances was tested with the Brown–Forsyth test and Levene’s test. Quantitative parameters (variables) were summarized as medians (Me), lower (Q1) and upper quartiles (Q3). The significance of differences between the median values of non-normally distributed parameters for two groups was verified with the Mann–Whitney U-test.
The significance and direction of relationships between the pairs of variables were estimated based on Spearman’s rank correlation coefficients (Rho) values.
The distributions of qualitative (nominal and ordinal) variables were summarized in contingency tables as numbers (n) and percentages (%). The significance of relationships between the pairs of qualitative variables was verified with Pearson’s chi-squared test or Fisher’s exact test. Whenever the relationship turned out to be significant, the odds ratio (OR) was calculated, along with its 95% confidence interval.
Cut-off values for continuous variables (SBP, PP) determining the effect of rehabilitation on CAS indices were obtained from the Receiver Operating Characteristic (ROC) curves.
The results of all tests were considered significant whenever the p-value for the test was <0.05.
Results
Patients with myocardial infarction (group B and group K analyzed together) presented with significantly higher baseline values of the parameters of arterial stiffness: beta-stiffness index (7.1 vs 6.4, p = 0.004), EP (96 kPa vs 77 kPa, p < 0.001) and PWV-beta (6.1 m/s vs 5.2 m/s, p < 0.001) than healthy persons.
At baseline, the factors correlating positively with the initial parameters of CAS were age (beta: r = 0.242, p = 0.008; EP: r = 0.250, p = 0.006; PWV-beta: r = 0.224, p = 0.014) and blood pressure parameters: SBP (EP: r = 0.388, PWV-beta: r = 0.360), DBP (AC: r = 0.225) and PP (PWV-beta: r = 0.221). Significantly lower mean values of CAS parameters: EP (t=−2030; p = 0.045), PWV-beta (t=−2142; p = 0.035) and higher AC values (t = 2.558; p = 0.012) were found in smoking patients than in non-smoking patients. There was also a positive correlation between BMI and AC (r = 0.203; p = 0.029). The baseline values of two parameters of CAS, beta-stiffness index (Rho=−0.26, p = 0.04) and PWV-beta (Rho-0.29, p = 0.03) correlated inversely with peak exercise capacity (expressed in METs), during stress electrocardiography (Table 2).
 |
Table 2 Correlations Between Baseline Parameters of Arterial Stiffness and Peak Exercise Capacity and Exercise Duration in the Rehabilitation Group Along with the Significance Levels |
At baseline, no significant differences in the parameters of CAS: beta-stiffness index (p = 0.322), EP (p = 0.347), AC (p = 0.104) and PWV-beta (p = 0.364), were found between the two groups of patients with myocardial infarction: group B and group K. However, six weeks later, some parameters of CAS, namely beta-stiffness index (6.2 vs 7.1, p = 0.016), EP (78 kPa vs 101 kPa, p = 0.001) and PWV-beta (5.4 m/s vs 6.2 m/s, p = 0.001) turned out to be significantly lower in the group B than in the group K (Table 3). In group B, CAS parameters decreased significantly after CR: beta (6.2 vs 7.4; p = 0.005), EP (85 vs 95; p = 0.001), PWV-beta (5.6 vs 6.0; p = 0.016); AC (0.89 vs 0.81; p = 0.011).
Moreover, after six weeks, group B presented with significantly lower arterial pressure values, SBP (124 mm Hg vs 130 mm Hg, p = 0.006), DBP (80 mm Hg vs 83 mm Hg, p = 0.033) and MAP (95 mm Hg vs 99 mm Hg, p = 0.010) than group K.
After six weeks, group B was divided into two subgroups, a subgroup consisting of patients who showed an improvement in at least two parameters of arterial stiffness (a decrease in EP, beta-stiffness index and/or PWV-beta and/or an increase in AC) and a subgroup including patients who showed an improvement in only one parameter or showed no improvement at all. The analysis of ROC curves for clinical, biochemical and echocardiographic factors identified the cut-off values for those factors determining a benefit from CR. Then, a univariate analysis was conducted to identify the significant predictors of the post-rehabilitation improvement in CAS parameters. Patients with pre-rehabilitation SBP no higher than 120 mm Hg turned out to have nearly three times greater odds for the post-rehabilitation improvement in the CAS parameters (OR = 2.74, p = 0.009) than those with higher systolic blood pressure (SBP >120 mm Hg). Moreover, approximately five times greater odds for the improvement in the CAS parameters (OR = 5.05, p = 0.005) were found in patients from group B with lower LVEF values at the baseline (up to 43%) (Table 4).
Discussion
To the best of our knowledge, this is the first study documenting a beneficial effect of early post-infarction cardiac rehabilitation, conducted within the framework of the Managed Care after Myocardial Infarction program, on carotid arterial stiffness parameters.
In this study, patients with myocardial infarction presented with significantly higher values of CAS parameters than healthy controls, like in the literature.16 This observation seems particularly interesting given a recent change in the acute coronary syndrome pathophysiology paradigm. In view of current evidence, the development of acute coronary syndrome seems to follow a “perfect storm” scenario; aside from the instability of atherosclerotic plaques, a coexistence of another two components is required: hemodynamic factors interfering with the plaques and a proatherogenic environment with inflammation and local or systemic suppression of the fibrinolytic system.17 Some evidence suggests a link between increased arterial stiffness and the coexistence of atherosclerotic plaque instability and proatherogenic hemodynamic conditions within the arterial lumen. A turbulent blood flow within the arteries associated with increased arterial pressure, as well as resultant oscillations in endothelial shear stress, causes weakening of the fibrous cap and hence increases the likelihood of plaque rupture.18
Increased local aortic stiffness (aPWV), determined by means of MRI immediately after myocardial infarction, was shown to exert an unfavorable effect on early myocardial healing.19 Patients after myocardial infarction whose parameters of aortic stiffness at the baseline were increased presented with elevated levels of cardiac troponins at one-year follow-up, suggesting chronic subclinical myocardial ischemia,20 and had higher concentrations of natriuretic peptides (markers of left ventricular wall stress); the latter finding implicates the role of aortic stiffness in post-infarction remodeling.21 An unfavorable prognostic value of arterial stiffness was also demonstrated in a group of 160 patients with STEMI: baseline aPWV >7.3m/s turned out to be a predictor of adverse cardiovascular events, namely death, non-fatal reinfarction, congestive heart failure and stroke, at one year.22 Thus, identifying interventions that could reduce arterial stiffness in patients with myocardial infarction is of utmost importance.
In the present study, early CR contributed to an improvement in CAS parameters within six weeks of myocardial infarction. Most previous studies demonstrating a beneficial effect of physical exercise on arterial stiffness included healthy people with cardiovascular risk factors or without; the studies of the problem in question in patients with symptomatic CVD are sparse. Laskey et al observed a significant decrease in arterial stiffness (cfPWV) in patients with the chronic coronary syndrome who completed a program of complex cardiac rehabilitation.23 In the Polish population, Trzos et al examined stiffness parameters (cfPWV) in 119 patients with MI and noted their significant reduction in the group of 64 patients undergoing CR lasting 4–9 weeks.24 In the only randomized controlled study analyzing the effect of a complex rehabilitation program on the parameters of arterial stiffness (cfPWV, Alx) in 86 patients after myocardial infarction, no significant changes in cfPWV were found in the intention-to-treat analysis, and only per-protocol analysis (including solely the patients who completed >80% of the sessions) demonstrated an improvement in this parameter in the rehabilitation group.12
The interpretation of the results of interventional studies is hindered by the fact that most parameters of arterial stiffness derive from arterial blood pressure at the time of measurement; therefore, a decrease in EP, AC and cfPWV might reflect solely a shift in the pressure/volume curve. Meanwhile, the beta-stiffness index, measured by echo-tracking and representing the slope of the curve, is a stable, pressure-independent parameter that reflects the adaptability of the arterial wall to changing hemodynamic conditions. In other words, the beta-stiffness index shows how the stiffness of a given vascular segment increases in response to a given change in arterial pressure.25 This illustrates how the choice of an inappropriate measurement method may influence the outcome of an intervention study.
It is worth noting that in our study a significantly higher percentage of the rehabilitated group in comparison with the control group was treated with MRA. Kalizki et al showed a reduction in cfPWV in the group of patients with refractory hypertension after a treatment with low-dose eplerenone.26 The beneficial effect of RAAS inhibitors on AS parameters results not only from passive reduction resulting from their antihypertensive result but also from their additional actions, such as inhibition of proliferation and migration of endothelial cells and smooth muscle cells, reducing oxidative stress, improving endothelial function, and reducing fibrosis of the vascular wall.27
In the present study, the post-rehabilitation improvement of CAS parameters was predicted by lower left ventricular ejection fraction (up to 43%) and lower systolic blood pressure (<120 mm Hg) before the rehabilitation. A total of 8 (9%) patients from the rehabilitation group had baseline LVEF <40%, with mean LVEF for the entire group equal to 49.3%. Notably, a substantially reduced left ventricular ejection fraction constituted an exclusion criterion in the previously mentioned studies analyzing the effects of cardiac rehabilitation on arterial stiffness in coronary artery disease.12,24 Patients with higher systolic blood pressure benefited less in the reduction of CAS presumably due to long-term, difficult-to-reverse changes in the vascular wall structure and function, altered autonomic activity and balance between vasodilative and vasoconstrictive factors.28,29
Our study demonstrated inverse correlations between pre-rehabilitation parameters of local arterial stiffness (beta, PWV-beta) and peak exercise capacity expressed in METs. Previous studies demonstrating lower physical capacity in people with higher arterial stiffness included primarily healthy subjects with cardiovascular risk factors or without. To the best of our knowledge, a link between physical capacity and arterial stiffness (cfPWV) determined using tonometry in a group of patients with myocardial infarction was analyzed in only one previous study, conducted by Alves et al.30 Meanwhile, arterial stiffness, a determinant of ventricular-arterial coupling, plays a vital role in the adaptation of the cardiovascular system to physical exercise.31
Conclusion
- Patients with myocardial infarction presented with increased carotid arterial stiffness compared to healthy subjects.
- Age and blood pressure correlated positively with initial CAS parameters, while baseline inverse correlations were found between arterial stiffness parameters and peak exercise capacity (MET).
- Early cardiac rehabilitation contributed to an improvement in arterial stiffness parameters within six weeks of myocardial infarction.
- Lower baseline systolic blood pressure (SBP) and lower baseline left ventricular ejection fraction (LVEF) were predictors of the post-rehabilitation improvement in arterial stiffness parameters.
Strength of the Study
There are few studies in the literature on arterial stiffness in patients with myocardial infarction, in particular those undergoing cardiac rehabilitation. To my best knowledge, presented study is the first to report an influence of cardiac rehabilitation on the parameters of CAS in patients with myocardial infarction. A novel element in presented study is the method of measuring local arterial stiffness in patients with myocardial infarction – the ultrasound echo-tracking method. The results of the presented study indicate the benefits of cardiac rehabilitation in a diverse population of patients with myocardial infarction, unselected in terms of left ventricular ejection fraction or cardiac rehabilitation mode (inpatient vs outpatient).
Limitations of the Study
This study included a relatively small sample of patients with myocardial infarction, exclusively Caucasians. Blood pressure used to calculate CAS parameters was measured on the brachial artery; however, in studied population of older patients (mean age 64.2 years), the effect of pulse pressure amplification should not have a relevant effect on CAS parameters.
Acknowledgments
This paper is part of a PhD thesis publication under the title “Effect of early post-myocardial infarction rehabilitation on arterial stiffness in patients with myocardial infarction”.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Salari N, Morddarvanjoghi F, Abdolmaleki A, et al. The global prevalence of myocardial infarction: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2023;23(1):206. doi:10.1186/s12872-023-03231-w
2. White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372(9638):570–584. doi:10.1016/S0140-6736(08)61237-4
3. Gierlotka M, Zdrojewski T, Wojtyniak B, et al. Incidence, treatment, in-hospital mortality and one-year outcomes of acute myocardial infarction in Poland in 2009–2012--nationwide AMI-PL database. Kardiol Pol. 2015;73(3):142–158. doi:10.5603/KP.a2014.0213
4. Ibanez B, James S, Agewall S, et al. Wytyczne ESC dotyczące postępowania w ostrym zawale serca z uniesieniem odcinka ST w 2017 roku [2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation.]. Kardiol Pol. 2018;76(2):229–313. doi:10.5603/KP.2018.0041
5. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction) [published correction appears in J Am Coll Cardiol. 2005 45(8):1376]. J Am Coll Cardiol. 2004;44(3):671–719. doi:10.1016/j.jacc.2004.07.002
6. Wita K, Kułach A, Wita M, et al. Managed Care after Acute Myocardial Infarction (KOS-zawał) reduces major adverse cardiovascular events by 45% in 3-month follow-up - single-center results of Poland’s National Health Fund program of comprehensive post-myocardial infarction care. Arch Med Sci. 2020;16(3):551–558. doi:10.5114/aoms.2019.85649
7. Wita K, Wilkosz K, Wita M, et al. Managed Care after Acute Myocardial Infarction (MC-AMI) - a Poland’s nationwide program of comprehensive post-MI care - improves prognosis in 12-month follow-up. Preliminary experience from a single high-volume center. Int J Cardiol. 2019;296:8–14. doi:10.1016/j.ijcard.2019.06.040
8. Kubielas G, Diakowska D, Uchmanowicz I. Survival analysis of patients with acute coronary syndrome receiving comprehensive coordinated care after myocardial infarction (KOS-Zawał). Kardiol Pol. 2022;80(3):415. doi:10.33963/KP.a2022.0035
9. Safar ME, Blacher J, Jankowski P. Arterial stiffness, pulse pressure, and cardiovascular disease-is it possible to break the vicious circle? Atherosclerosis. 2011;218(2):263–271. doi:10.1016/j.atherosclerosis.2011.04.039
10. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. doi:10.1016/j.jacc.2009.10.061
11. van Sloten TT, Schram MT, van den Hurk K, et al. Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all-cause mortality: the Hoorn study. J Am Coll Cardiol. 2014;63(17):1739–1747. doi:10.1016/j.jacc.2013.12.041
12. Oliveira NL, Ribeiro F, Silva G, et al. Effect of exercise-based cardiac rehabilitation on arterial stiffness and inflammatory and endothelial dysfunction biomarkers: a randomized controlled trial of myocardial infarction patients. Atherosclerosis. 2015;239(1):150–157. doi:10.1016/j.atherosclerosis.2014.12.057
13. Uejima T, Dunstan FD, Arbustini E, et al. Age-specific reference values for carotid arterial stiffness estimated by ultrasonic wall tracking [published correction appears in J Hum Hypertens. 2020 Jan 30]. J Hum Hypertens. 2020;34(3):214–222. doi:10.1038/s41371-019-0228-5
14. Jaroch J, Łoboz Grudzień K, Bociąga Z, et al. The relationship of carotid arterial stiffness to left ventricular diastolic dysfunction in untreated hypertension. Kardiol Pol. 2012;70(3):223–231.
15. Jankowski P, Gąsior M, Gierlotka M, et al. Coordinated care after myocardial infarction. The statement of the Polish Cardiac Society and the Agency for Health Technology Assessment and Tariff System [article in Polish]. Kardiol Pol. 2016;74(8):800–811. indexed in Pubmed: 27553352. doi:10.5603/KP.2016.0118
16. Hirai T, Sasayama S, Kawasaki T, Yagi S. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation. 1989;80(1):78–86. doi:10.1161/01.CIR.80.1.78
17. Libby P, Pasterkamp G, Crea F, Jang IK. Reassessing the Mechanisms of Acute Coronary Syndromes. Circ Res. 2019;124(1):150–160. doi:10.1161/CIRCRESAHA.118.311098
18. Van der Donckt C, Herck JL, Schrijvers DM, et al. Elastin fragmentation in atherosclerotic mice leads to intraplaque neovascularization, plaque rupture, myocardial infarction, stroke, and sudden death. Eur Heart J. 2015;36(17):1049–1058. doi:10.1093/eurheartj/ehu041
19. Reindl M, Tiller C, Holzknecht M, et al. Aortic stiffness and infarct healing in survivors of acute ST-segment-elevation myocardial infarction. J Am Heart Assoc. 2020;9(3):e014740. doi:10.1161/JAHA.119.014740
20. Feistritzer H-J, Klug G, Reinstadler SJ, et al. Aortic stiffness is associated with elevated high-sensitivity cardiac troponin T concentrations at a chronic stage after ST-segment elevation myocardial infarction. J Hypertens. 2015;33(9):1970–1976. doi:10.1097/HJH.0000000000000644
21. Klug G, Feistritzer HJ, Reinstadler SJ, et al. Association of aortic stiffness with biomarkers of myocardial wall stress after myocardial infarction. Int J Cardiol. 2014;173(2):253–258. doi:10.1016/j.ijcard.2014.02.038
22. Feistritzer H-J, Klug G, Reinstadler SJ, et al. Prognostic value of aortic stiffness in patients after ST-elevation myocardial infarction. J Am Heart Assoc. 2017;6(9):e005590. doi:10.1161/JAHA.117.005590
23. Laskey W, Siddiqi S, Wells C, Lueker R. Improvement in arterial stiffness following cardiac rehabilitation. Int J Cardiol. 2013;167(6):2734–2738. doi:10.1016/j.ijcard.2012.06.104
24. Trzos E, Kurpesa M, Rechciński T, Wierzbowska-Drabik K, Krzemińska-Pakuła M. The influence of physical rehabilitation on arterial compliance in patients after myocardial infarction. Cardiol J. 2007;14(4):366–371.
25. Gavish B, Izzo JL Jr. Arterial stiffness: going a step beyond. Am J Hypertens. 2016;29(11):1223–1233. doi:10.1093/ajh/hpw061
26. Kalizki T, Schmidt BMW, Raff U, et al. Low dose-eplerenone treatment decreases aortic stiffness in patients with resistant hypertension. J Clin Hypertens. 2017;19(7):669–676. doi:10.1111/jch.12986
27. Laurent S, Boutouyrie P. Vascular mechanism collaboration. Dose-dependent arterial destiffening and inward remodeling after olmesartan in hypertensives with metabolic syndrome. Hypertension. 2014;64(4):709–716. doi:10.1161/HYPERTENSIONAHA.114.03282
28. Ferrier KE, Waddell TK, Gatzka CD, Cameron JD, Dart AM, Kingwell BA. Aerobic exercise training does not modify large-artery compliance in isolated systolic hypertension. Hypertension. 2001;38(2):222–226. doi:10.1161/01.hyp.38.2.222
29. Miura H, Takahashi Y, Maki Y, Sugino M. Effects of exercise training on arterial stiffness in older hypertensive females. Eur J Appl Physiol. 2015;115(9):1847–1854. doi:10.1007/s00421-015-3168-y
30. Alves AJ, Oliveira NL, Lopes S, et al. Arterial stiffness is related to impaired exercise capacity in patients with coronary artery disease and history of myocardial infarction. Heart Lung Circ. 2019;28(11):1614–1621. doi:10.1016/j.hlc.2018.08.023
31. Pucci G, Battista F, Schillaci G. Aerobic physical exercise and arterial de-stiffening: a recipe for vascular rejuvenation? Hypertens Res. 2012;35(10):964–966. doi:10.1038/hr.2012.107
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.