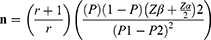Back to Journals » Nutrition and Dietary Supplements » Volume 13
Effect of Food Insecurity and Other Possible Factors Associated with Low Birth Weight Among Mothers Who Gave Birth to Live Newborns in West Ethiopia: A Facility-Based Unmatched Case–Control Study
Authors Desalegn M , Terefe B, Bikila H
Received 6 May 2021
Accepted for publication 24 August 2021
Published 7 September 2021 Volume 2021:13 Pages 133—143
DOI https://doi.org/10.2147/NDS.S317092
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Chandrika J Piyathilake
Markos Desalegn, Bekana Terefe, Haile Bikila
Department of Public Health, Institute of Health Science, Wollega University, Nekemte, Ethiopia
Correspondence: Markos Desalegn
Department of Public Health, Institute of Health Science, Wollega University, Nekemte, Ethiopia
Tel +251927619190
Email [email protected]
Background: More than 20 million births annually in developing countries are classed as low birth weight, with short- and long-term consequences. Food insecurity is the major determinant of low birth weight in developing countries.
Objective: This study aimed to identify the effects of food insecurity and other possible factors associated with low birth weight in West Wollega, West Ethiopia.
Methods: This facility-based unmatched case–control study was conducted among mothers who gave birth to live newborns in randomly selected hospitals in West Wollega. Data were collected using a structured interviewer-administered questionnaire. Collected data were coded and entered into Epi Info version 7.2.0.1 and analyzed by SPSS version 24. Adjusted odds ratios were used to identify predictors of low birth weight at a p-value less than 0.05.
Results: The study indicated that the mother living in a food-insecure household (AOR [95% CI] = 2.9 [1.05– 8]), uneducated mother (AOR [95% CI] = 5 [1.8– 14]), birth interval of < 24 months (AOR [95% CI] = 4.6 [1.2– 18]), age at first birth of < 18 years (AOR [95% CI] = 4 [1.1– 15]), late initiation of antenatal care (ANC) (AOR [95% CI] = 4.4 [1.3– 15.7]), pregnancy-induced hypertension (AOR [95% CI] = 3.6 [1.03– 12.9]), and maternal mid-upper arm circumference (MUAC) of < 23 cm (AOR [95% CI] = 11 [4– 35]) were predictors of low birth weight.
Conclusion: Household food insecurity, a birth interval of < 24 months, age at first birth < 18 years, late initiation of first ANC, pregnancy-induced hypertension, and maternal MUAC of < 23 cm were predictors of low birth weight in this study. Early screening for medical and obstetric conditions, as well as maternal nutritional status and household food insecurity, is a key action needed to reduce low birth weight in this study area.
Keywords: low birth weight, predictors, newborn, food insecurity
Background
Worldwide, low birth weight (LBW) continues to be a public health problem, with both short- and long-term consequences.1 About 15–20% of all births worldwide are LBW, representing more than 20 million births annually, and 95.6% of these are in developing countries.2 More than half of all LBW babies are born in south-central Asia, where more than one-quarter (27%) of all infants weigh less than 2500 g at birth.2,3
LBW is highly associated with fetal and neonatal mortality and morbidity, inhibited growth and cognitive development, and chronic disease later in life.4 LBW contributes to a high proportion of neonatal deaths. According to a report from the United Nations High Commissioner for Refugees (UNHCR), about 60–80% of neonatal deaths are due to direct or indirect causes of LBW.5 The burden of morbidity and mortality related to LBW is disproportionately shared among developed and developing countries.6 In Ethiopia, the level of mortality is not uniform across the different childhood age groups. About 44% of childhood deaths occur within the first 28 days of life, and a large proportions of these deaths is attributed to the poor nutritional status of the mother, which could result from food insecurity.7
Low family socioeconomic status, maternal age, parity, harmful parental behaviors such as smoking and excessive alcohol consumption, and poor nutrition during pregnancy, as well as a poor level of prenatal care, are risk factors for LBW.8
Food insecurity, the major predictor of LBW associated with poor-quality dietary intake and decreased nutritional status among women, and poor nutritional status in pregnancy and pre-pregnancy, is linked to poor birth outcomes.9,10 During pregnancy, women must have both an adequate quantity and quality of food; however, food insecurity prevents many women from meeting the guidelines for healthy nutrition during pregnancy and is associated with an increased risk of LBW.11 Undernutrition, which can result from food insecurity, is evidenced by decreased maternal height (stunting), and below-normal pre-pregnancy weight and pregnancy weight gain are among the strongest predictors of delivery of an LBW infant.12
In 2012, the World Health Organization endorsed a comprehensive implementation plan on maternal, infant, and young child nutrition to achieve a 30% reduction in the number of LBW infants by the year 2025. This would translate into a 3.9% relative reduction annually between 2012 and 2025, which is equivalent to the reduction from nearly 20 million to about 14 million newborns with LBW.13
Evidence has shown that LBW infants are at higher risk of prenatal mortality and morbidity than normal birth weight infants.14 Despite its importance in reducing LBW, research on the effects of food insecurity on LBW is scarce in this study area. Food insecurity has been associated with depression and anxiety among mothers. Pregnant women experiencing depressive symptoms are at risk for dysfunctional development and attachment of the fetus to the uterus during pregnancy, and intrauterine growth restriction (IUGR), all of which affect birth outcomes.11 Therefore, this study aimed to identify the effects of food insecurity and other predictors of LBW in the study area.
Methods
Study Area and Period
The study was conducted in selected public hospitals of West Wollega Zone, West Ethiopia from April 15 to July 15, 2019. The zone located at a distance of 441 km from Addis Ababa, to the west of the country. West Wollega is divided into 21 districts and three town administrations. Based on the 2019 census report, the total projected population size of the zone was 1,872,601, of whom 1,058,019 were males and 814,582 were females, among these 345,120 reproductive-age women. West Wollega has a total of seven hospitals (five public and two non-governmental). In addition, the zone has 67 health centers, 337 private clinics (21 medium and 316 small clinics), 36 rural drug vendors, and 51 drug stores.
Study Design and Population
A facility-based unmatched case–control study design was employed among mothers who gave birth to live newborns. Cases were term babies weighing <2500 g and controls were term babies weighing ≥2500 g. All live births at hospitals in West Wollega and all term live births in selected hospitals in West Wollega made up the source population and the study population, respectively. All term, live and singleton births were included for both cases and controls, whereas preterm newborns with congenital abnormalities and mothers who were seriously ill during the interview were excluded from the study.
Sample Size Determination
Sample size determination was carried out using the double population proportion formula by considering the 95% confidence level, 80% power, and control to case ratio of 3:1. The sample size determination was performed for four predictors of LBW separately (maternal food insecurity status, age of mothers (<20 years), maternal educational status, and place of residence) and the one yielded maximum was taken to calculate the final sample size of the study.11 Adding a 10% non-response rate, the final sample size was calculated to be 292 (73 cases and 219 controls) using the following formula:
where P=average proportion of P1 and P2, P1=proportion among cases (40.30%), P2= proportion among controls (21.70%), Zα/2=standard level of significance (1.96), Zβ=desired power (80%), n=sample size, and r=ratio of controls to cases (3:1).
Therefore, n=292 (73 cases and 219 controls).
Sampling Technique and Procedure
West Wollega has a total of seven hospitals, five governmental hospitals (Ghimbi, SayoNole, Nejo, Mandi, and Begi hospitals), and two non-governmental hospitals (Ayira Adventist and Ghimbi Adventist hospitals). Four hospitals were selected at random: three from the governmental and one from the non-governmental hospitals. The sample size was assigned to each selected hospital by considering average institutional delivery in the 2 months before actual data collection in each hospital. In the selection of cases and controls, the delivery registration logbook was used as the list of newborn babies in each facility. Based on this, samples of 112, 80, 64, and 36 ere assigned to Ghimbi, Nejo, Mandi, and Ghimbi Adventist hospitals, respectively.
In each selected health facility, all LBW who fulfilled the eligibility criteria during the study period were included, and three eligible newborns were selected using a simple random sampling technique next to each case, as the controls. Neonatal birth weight was measured immediately after birth using standard baby weighing scales; data collection and interviews were conducted after the mothers had become stable and been transferred to the postnatal room, using an interviewer-administered questionnaire.
Data Collection Tool and Procedure
Data were collected using a structured interviewer-administered questionnaire that was adopted from the reviewed literature. The questionnaire was first prepared in English and then translated into Afaan Oromo (the local language) for data collection. The tool has six sections: socio-demographic characteristics, obstetric characteristics, antenatal care (ANC) services and maternal behavior during pregnancy, maternal health condition during pregnancy, and nine generic questions to measure food insecurity status. The newborn’s weight was measured in grams using a standard baby weighing scale in the delivery room immediately after birth, by a trained health professional, and the trained data collectors used the measured newborn weight for this study. After the mother had stabilized, maternal mid-upper arm circumference (MUAC) was taken using a MUAC tape meter to estimate nutritional status.
Study Variables
- Dependent variable: Birth weight of a newborn baby.
- Independent variables: Household food insecurity, age, educational status, occupation, residence, family size, family income, history of low birth weight, parity, birth interval.
Operational Definitions and Measurements
- Birth weight: The first weight of a newborn, measured immediately after birth.
- Low birth weight: The first weight of a newborn less than 2500 g at birth.
- Household food security: Food insecurity status was assessed and labeled as secure and insecure. Food insecurity status was assessed based on the Household Food Insecurity Access Scale (HFIAS) of the Food and Nutrition Technical Assistance Project (FANTA), which was developed by USAID.
The tool used to assess food insecurity status consists of nine questions on occurrence and nine on frequency of occurrence, that were asked at each occurrence to determine how often the condition occurred. According to this scale, a given household is classified as food secure if they answer “no” to all the nine questions or “rarely” for only question 1. If “yes” and the frequency is “sometimes” or “often” for question 1; or “rarely”, “sometimes”, or “often” for question 2; or “rarely” for questions 3 and 4, that household has mild food insecurity.13 If they answer “yes” and the frequency is “sometimes” or “often” for questions 3 and 4; or “rarely” or “sometimes” for questions 5 and 6, the household has moderate food insecurity.13 Lastly, if answering “yes” and the frequency is “often” for questions 5 and 6; or “rarely”, “sometimes”, or “often” for questions 7–9, the household has severe food insecurity.13
- Household food insecurity: Limited or uncertain access to adequate food and healthy nutrition or uncertain ability to acquire food in a socially acceptable way.
- MUAC: A measurement of the mid-upper arm circumference of a pregnant woman to check her nutritional status, taken as normal for ≥23 cm and not normal for <23 cm.
Data Quality Assurance
The questionnaire was prepared in English and translated into Afaan Oromo (the local language). The pre-test was done before actual data collection, and amendments were made relating to clarity, wording, and logical sequence, and the skip pattern of the tool. Training was given to the data collectors on the objective of the study, technique, and procedure that should be followed during the interview and anthropometric measurements.
Data collectors were informed on the steps to be followed during MUAC measurement, standardization, and the importance of measuring the newborn baby with light clothes and remeasuring the clothes to determine the exact weight of the baby. The procedure was closely supervised by the principal investigator. Collected data were checked for completeness every day after data collection.
Data Processing and Analysis
After the data collection was completed, data were coded and entered into Epi Info version 7.2.0.1 and exported to SPSS version 24 for analysis. Descriptive statistics were computed for each study variable. All variables were changed to categorical and dichotomous types and summarized using frequencies and percentages. The outcome variable was labeled as a case for a birth <2500 g and a control for a birth weight ≥2500 g. Then, cases were coded with number ”1” and controls were coded with ”0”. Univariate analysis was performed for socio-demographic variables, food security-related variables, and other important variables in the study.
Bivariate analysis was carried out to compare the LBW with each independent variable. The multivariate logistic regression model was used to identify determinants of LBW. Independent variables with a p-value <0.25 were selected as candidate variables for the multiple logistic regression model to identify the association between dependent and independent variables. The adjusted odds ratio (AOR) was calculated at a 95% confidence interval (CI) with a p-value of <0.05 to identify the independent predictors of LBW.
Results
Socio-Demographic Characteristics of Mothers Who Gave Birth to Live Newborns in West Wollega Hospitals, 2019
In this study, a total of 292 mothers with newborn babies, comprising 73 cases and 219 controls, were interviewed, making a response rate of 100%. In total, 126 of the mothers (43%) were in the age group 25–29 years; 31 of these (42.5%) were cases and 95 (43.4%) were controls. The mean±SD age of the mothers was 24.7±3.9 years. Furthermore, 222 mothers (76%) were educated; of these, the majority, 192 (87.7%), were controls and 30 (41.1%) were cases. The family income for 122 mothers (41%), comprising 29 cases (39.7%) and 93 controls (42.5%), was below 2400.00 Ethiopian birrs monthly (Table 1).
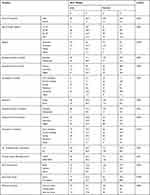 |
Table 1 Socio-Demographic Characteristics of Mothers and Their Respective Newborns’ Birth Weight in Selected Hospitals in West Wollega, Ethiopia, 2019 |
Obstetric Characteristics of Mothers Who Gave Birth to Live Newborns in West Wollega Hospitals, 2019
Of the total study participants, 50 mothers of cases (68.5%) and 156 mothers of controls (71.2%) were gravida two and above. Over half of the mothers, 111 (54%), had been told the birth weight of their newborn baby in the previous birth, 27 cases (54%) and 84 controls (53.8%). The majority of mothers, 174 (84%), who had given birth to two or more babies, 35 cases (70%) and 139 controls (89.1%), had a birth interval of greater than 24 months between the current and previous deliveries.
In total, 42 mothers (14%), comprising 24 cases (32.9%) and 18 controls (8.2%), reported that they had first given birth at the age of below 18 years. About one-quarter, 76 (26%), of maternal MUACs were less than 23 cm, 40 cases (54.8%) and 36 controls (16.4%). The study found that 277 mothers (95%) attended ANC during the current pregnancy, of whom 244 (88%), comprising 51 cases (74%) and 193 controls (92.8%), attended four times or more. The majority of mothers, 246 (84%), comprising 64 cases (87.7%) and 182 controls (83.1%), had received iron folate, and 53 cases (72.6%) and 163 controls (74.4%) had received nutritional counseling during the current pregnancy.
Food Insecurity, Other Maternal Health Conditions, and Low Birth Weight Among Live Births in West Wollega Hospitals, 2019
In the study area, 31 mothers (10.6%), comprising 17 cases (23.3%) and 14 controls (6.4%), had a history of pregnancy-induced hypertension, 44 (15%) had a history of anemia, and only eight (3%) had a history of malaria during the current pregnancy (Table 2). Using the HFIAS, all mothers who participated in this study were assessed for their household food insecurity status. A significant proportion of mothers reported that their household did not have enough food, 26 cases (35.6%) and 38 controls (17.4%). None of them reported going day and night without eating anything because of not having enough food. Based on this, 95 mothers (32.5%) were food insecure, 39 cases (53.4%) and 56 controls (25.6%). More than half, 55 (57.9%), were mildly food insecure, 19 cases (48.7%) and 36 controls (64.3%); 32 (33.7%) were moderately food insecure, 13 cases (33.3%) and 19 controls (34%); and eight (8.4%) (18% cases and 1.8% controls) were severely food insecure (Table 3).
 |
Table 2 Maternal Exposure to Addictive Substances, Mothers’ Health Conditions, and Their Respective Newborns’ Birth Weight in Selected Hospitals in West Wollega, Ethiopia, 2019 |
 |
Table 3 Food Insecurity Status of Mothers and Their Respective Newborns’ Birth Weight in Selected Hospitals in West Wollega, Ethiopia, 2019 |
Determinant Factors of Low Birth Weight Among Live Births in West Wollega Hospitals, 2019
Bivariate analysis was performed between each variable and newborns with LBW, and selected candidate variables with a p-value of less than 0.25 were considered candidate variables for the final multivariate model. Based on this, the educational status of the mother, birth interval, age at first birth, number of ANC visits, first ANC initiation month, hypertension, anemia, MUAC of the mother, and food security status of the mother were further analyzed using multiple logistic regression analysis to determine their independent effect on the LBW. The results of multiple logistic regression showed that the educational status of mothers was statistically significantly associated with LBW (AOR [95% CI] = 5 [1.8–14]). The odds of LBW was four times higher among women who had first given birth at an age younger than 18 years and five times higher than among mothers who reported having a birth interval of less than 24 months between the current and previous deliveries (AOR [95% CI] = 4 [1.1–15] and AOR [95% CI] = 4.6 [1.2–18], respectively).
Late antenatal visit initiation after the first trimester was positively associated with LBW compared to those who started their first ANC visit earlier during the first trimester of pregnancy (AOR [95% CI] = 4.4 [1.3–15.7]), whereas pregnancy-related hypertension was a maternal medical condition that was identified as a predictor of LBW (AOR [95% CI] = 3.6 [1.03–12.9]). The odds of LBW was about three times (AOR [95% CI] = 2.9 [1.05–8]) higher among newborn babies born to mothers of food-insecure households compared to mothers from food-secure households. A maternal MUAC of less than 23 cm was also positively associated with LBW (AOR [95% CI] = 11 [4–35]) (Table 4).
 |
Table 4 Multiple Logistic Regression Results of Determinant Factors Associated with Low Birth Weight Among Newborns in West Wollega, Ethiopia, 2019 |
Discussion
This study aimed to identify the predictors of LBW using an unmatched case–control study design among live-birth newborns. The study found that predictors of LBW among live newborns in West Wollega hospitals were: household food insecurity, maternal MUAC, late initiation of ANC visit, birth interval of less than 24 months between the current and previous births, and age at first delivery. Therefore, securing maternal nutrition and resolving food insecurity, early ANC visit initiation, and delaying the age of the first pregnancy could avert LBW.
The odds of LBW were about three times higher in mothers found to be in household food insecurity than those in food security. This finding is in line with studies conducted in Addis Ababa15 and Afar, Ethiopia,16 rural Haiti17 and Pakistan,18 which stated that food-insecure women had an increased risk of delivering an LBW newborn. This may be because food insecurity among women during pregnancy ranges up to 90% in underdeveloped and developing countries.19 Since there is an increased demand for nutrients during pregnancy,20 pregnant women must have adequate quality and quantity of food. Food-insecure mothers have limited or uncertain availability of nutritionally adequate food, which prevents them from meeting the guidelines for healthy eating during pregnancy and leads to their infants having LBW.
Maternal MUAC was the other factor determinant of LBW in the study area, where the odds of LBW was higher among mothers with MUAC measurement of less than 23 cm compared to those with greater than 23 cm. This finding is consistent with studies conducted in Addis Ababa14 and the Oromia region of Ethiopia,21 and Ibadan in Nigeria,22 which pointed out that MUAC is an indicator of maternal nutritional status and birth outcome. Because food insecurity is a significant nutrition-sensitive factor associated with low MUAC21 and, in this study area, food insecurity was an independent predictor of LBW, poor nutritional status in pregnancy is expressed as the percentage with low MUAC, which is high in Ethiopian women.23
This study revealed that a birth interval of less than 24 months between the current and previous deliveries was a risk factor for LBW. According to this finding, neonates born at an interval of less than 24 months had a 4.6 times increased risk of LBW. The finding is similar to studies conducted in Bale Zone and North-west Ethiopia, which indicated that a short interpregnancy interval of less than 24 months was associated with LBW.24,25 This may be because the close interval of pregnancy and period of lactation worsens the mother’s nutritional status and there is inadequate time for the mother to recover from the psychological stress of the last pregnancy.25
Giving birth before the age of 18 years increased the risk of having LBW neonates as compared to their counterparts. The result was consistent with research conducted in India.14,26 This may be because the body of the adolescent is not sufficiently well developed to nourish and accommodate the fetus. In this study area, the odds of giving LBW babies was higher among uneducated mothers compared to educated mothers. This is in line with findings from the Ethiopian Demographic and Health Survey 201627 and North Shewa,28 which showed that infants born to mothers with no education are more likely to have LBW compared with those born to educated women. This may be because educated women can gain access to job opportunities that enable them to find better medical services, such as early prenatal care, and obtain treatment for other medical problems. Educated women may also take better care of themselves and make more informed decisions on the care of the unborn baby.
Hypertension was a maternal medical problem associated with LBW. Mothers who had a history of hypertension during pregnancy had about 3.6 times increased risk of having LBW compared with those without hypertension during pregnancy. This concurs with previous reviews of LBW in Ethiopia,29 Nigeria,30 and South Asia.31 This could be because high blood pressure during pregnancy may affect the development of the placenta, causing the nutrient and oxygen supply to the baby to be limited.32 This can lead to early delivery, LBW, placental separation (abruption), and other complications for the baby.32
Early initiation of ANC visits is one of the most important means of maintaining fetal and maternal well-being. The study showed that late initiation of ANC after the first trimester increases the risk of LBW. This finding is in line with a study conducted in Oman, which revealed that late initiation of ANC is associated with an increased risk of LBW.33 This is because pregnant women who did not start to attend ANC services as early as possible may have missed the full package of prenatal care services, where a lack of nutrition counseling during pregnancy and a lack of iron/folic acid supplementation during pregnancy were associated with LBW.34
Despite these important findings, the study had some limitations. First, the sources of both cases and controls were hospitals, and home delivery was considered neither for cases nor for controls. Secondly, this study design cannot calculate absolute risk. Thirdly, there may be recall bias for some variables and social desirability bias for variables such as household food security.
Conclusion
The study showed that household food insecurity was a determinant factor of LBW. Maternal education, a birth interval of <24 months, age at first birth of <18 years, late initiation of first ANC services, pregnancy-induced hypertension, and maternal MUAC of <23 cm were also determinants of LBW in the study area. The government and local stakeholders should focus on and give aid to pregnant mothers in households with food insecurity.
In addition to this community, health care providers and health systems should promote pregnant mothers for early screening and detection of medical and obstetric problems as well as maternal nutritional status. A further prospective cohort study should be undertaken to confirm the clear association between maternal nutrition or household food security and pregnancy outcome in this study area.
Abbreviations
ANC, antenatal care; AOR, adjusted odds ratio; CI, confidence interval; FANTA, Food and Nutrition Technical Assistance Project; HFIAS, Household Food Insecurity Access Scale; IUGR, intrauterine growth restriction; LBW, low birth weight; MUAC, mid-upper arm circumference; USAID, United States Agency for International Development.
Data Sharing Statement
The corresponding author can make the required data and material available whenever needed, upon reasonable request.
Ethical Considerations
This study was conducted in line with the Declaration of Helsinki on health research.35 Ethical clearance was obtained from Wollega University, School of Graduate Studies ethical review committee. Permission for conducting the study was secured from each hospital. Written consent was obtained from all the study participants after they had been briefed about the objectives and the aim of the research. The interviewees were assured of the confidentiality of the information gathered.
Acknowledgments
The authors would like to thank Wollega University, Institute of Health Science, Department of Public Health, for giving them this chance and for their support in this research activity, and West Wollega Zonal Health Department for their cooperation in providing the necessary information. Special thanks to the heads of each hospital and the department heads for their cooperation during the data collection. Last but not least, we would like to thank all data collectors and respondents for their cooperation.
Author Contributions
MB is the principal investigator, who initiated and was involved in the proposal development, analysis of the data, interpretation of the data, report writing, and manuscript preparation. BT and HB were involved in the analysis, report writing, and preparation of the manuscript, and reviewing the paper. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest regarding the publication of the paper.
References
1. International Federation of Red Cross and Red Crescent Societies. Nutrition Matters. Guidance for Nutrition Programming. Geneva: International Federation of Red Cross and Red Crescent Societies; 2016.
2. United Nations Children’s Fund and World Health Organization. Low Birth Weight, Country, Regional and Global Estimates. New York: UNICEF; 2014.
3. WHO. Global Nutrition Monitoring Framework Operational Guidance for Tracking Progress in Meeting Targets for 2025. WHO; 2017.
4. WHO. Comprehensive Implementation Plan on Maternal, Infant, and Young Child Nutrition. WHO; 2014.
5. UNHCR. Operational guidelines on improving newborn health in refugee operations; 2014. Available from: https://www.unhcr.org/54bd0dc49.pdf.
6. Bililign N, Legesse M, Akibu M. A review of low birth weight in Ethiopia: socio-demographic and obstetric risk factors. Glob J Res Rev. 2018;5(1):4.
7. Ethiopian Federal Ministry of Health. National Strategy for Newborn and Child Survival. Addis Ababa, Ethiopia: Ethiopian Federal Ministry of Health; 2015.
8. Stylianou-Riga P, Kouis P, Kinni P, et al. Maternal socioeconomic factors and the risk of premature birth and low birth weight in Cyprus: a case-control study. Reprod Health. 2018;15:157. doi:10.1186/s12978-018-0603-7
9. Chowdhury M, Dibley MJ, Alam A, Huda TM, Raynes-Greenow C. Household food security and birth size of infants: analysis of the Bangladesh demographic and health survey 2011. Curr Dev Nutr. 2018;2(3):nzy003. doi:10.1093/cdn/nzy003
10. USAID. Feed the future, the future Ethiopia growth through nutrition activity feed the future Ethiopia growth through nutrition activity; 2021. Available from: https://www.usaid.gov/ethiopia/agriculture-and-food-security/feed-future.
11. Grilo SA, Earnshaw VA, Lewis JB, et al. Food matters: food insecurity among pregnant adolescents and infant birth outcomes. J Appl Res Child. 2015;6(2):4.
12. ACC/SCN. ACC/SCN low birth weight: report of a meeting in Dhaka, Bangladesh on 14−17 June 1999. In Pojda J, Kelley L, editors. Nutrition Policy Paper #18. Geneva: ACC/SCN in collaboration with ICDDR, B; 2000.
13. World Health Organization. Global nutrition targets 2025: low birth weight policy brief. World Health Organization; 2014. Available from: https://apps.who.int/iris/handle/10665/149020.
14. Vilanova CS, Hirakata VN, de Souza Buriol VC, et al. The relationship between the different low birth weight strata of newborns with infant mortality and the influence of the main health determinants in the extreme south of Brazil. Popul Health Metrics. 2019;17:15. doi:10.1186/s12963-019-0195-7
15. Sahlu D, Deyessa N, Firdu N, Asfaw S. Food insecurity and other possible factors contributing to low birth weight: a case-control study in Addis Ababa, Ethiopia. Asian Pac J Reprod. 2020;9(4):174–181. doi:10.4103/2305-0500.288585
16. Abdu J, Kahssay M, Gebremedhin M. Household food insecurity, underweight status, and associated characteristics among women of reproductive age group in Assayita District, Afar Regional State, Ethiopia. J Environ Public Health. 2018;2018:8. doi:10.1155/2018/7659204
17. Richterman A, Raymonville M, Hossain A, et al. Food insecurity as a risk factor for preterm birth: a prospective facility-based cohort study in rural Haiti. BMJ Global Health. 2020;5:e002341. doi:10.1136/bmjgh-2020-002341
18. Saeed A, Naqvi M, Javed A. Effects of maternal food insecurity on birth weight of neonates: a prospective cohort. Ann King Edward Med Univ. 2017;23(4):524–530. doi:10.17582/journal.akemu/2017/23
19. Ramalho AA, Martins FA, Koifman RJ. Food insecurity during the gestational period and factors associated with maternal and child health. J Nutr Health Food Eng. 2017;7(4):
20. Laraia BA, Siega-Riz AM, Gundersen C, Dole N. Psychosocial factors and socioeconomic indicators are associated with household food insecurity among pregnant women. J Nutr. 2006;136(1):177–182. doi:10.1093/jn/136.1.177
21. Ghosh S, Spielman K, Kershaw M, et al. Nutrition-specific and nutrition-sensitive factors associated with mid-upper arm circumference as a measure of nutritional status in pregnant Ethiopian women: implications for programming in the first 1000 days. PLoS One. 2019;14(3):e0214358. doi:10.1371/journal.pone.0214358
22. Lawoyin TO, Oyediran AB. A prospective study on some factors which influence the delivery of low birth weight babies in a developing country. Afr J Med Med Sci. 1992;21(1):33–39.
23. Tang AM, Chung M, Dong K, et al. Determining a Global MidUpper Arm Circumference Cutoff to Assess Malnutrition in Pregnant Women. Washington, DC: FHI 360/Food and Nutrition Technical Assistance III Project (FANTA); 2016.
24. Demelash H, Motbainor A, Nigatu D, Gashaw K, Melese A. Risk factors for low birth weight in Bale zone hospitals, South-East Ethiopia: a case-control study. BMC Pregnancy Childbirth. 2015;15:264. doi:10.1186/s12884-015-0677-y
25. Aman Y, Fikre E, Seifu H, Mokonnen A. Effect of interpregnancy interval on low birth weight in Gondor and Bahir Dar referral Hospital: a case control study from North West Ethiopia. J Health Med Nurs. 2016;31:2422–8419.
26. Gogoi N. Socio-demographic determinants of low birth weight in the northeastern city, India. Int J Intg Med Sci. 2018;5(3):587–591. doi:10.16965/ijims.2018.103
27. Central Statistical Agency (CSA) [Ethiopia] and ICF. Ethiopia Demographic and Health Survey 2016. Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF; 2016.
28. Gizaw B, Gebremedhin S. Factors associated with low birthweight in North Shewa zone, Central Ethiopia: case-control study. Ital J Pediatr. 2018;44:76. doi:10.1186/s13052-018-0516-7
29. Getaneh T, Negesse A, Dessie G, et al. The impact of pregnancy-induced hypertension on low birth weight in Ethiopia: systematic review and meta-analysis. Ital J Pediatr. 2020;46:174. doi:10.1186/s13052-020-00926-0
30. Ndu IK, Edelu BO, Uwaezuoke S, Chinawa JC, Ubesie A. Maternal risk factors associated with low birth weight neonates: a multi-centre, cross-sectional study in a developing country. J Neonatal Biol. 2015;4:190. doi:10.4172/2167-0897.1000190
31. Ediriweera DS, Dilina N, Perera U, et al. Risk of low birth weight on adulthood hypertension - evidence from a tertiary care hospital in a South Asian country, Sri Lanka: a retrospective cohort study. BMC Public Health. 2017;17(1):358. doi:10.1186/s12889-017-4268-x
32. Cleveland Clinic’s Ob/Gyn & Women’s Health Institute. How is high blood pressure (hypertension) during pregnancy different from high blood pressure at other times?; 2021. Available from: https://my.clevelandclinic.org/health/diseases/4497-high-blood-pressure-hypertension-during-pregnancy.
33. Islam MM, ElSayed MK. Pattern and determinants of birth weight in Oman. Public Health. 2015;129(12):1618–1626. doi:10.1016/j.puhe.2015.07.011
34. Girma S, Fikadu T, Agdew E, et al. Factors associated with low birth weight among newborns delivered at public health facilities of Nekemte town, West Ethiopia: a case-control study. BMC Pregnancy Childbirth. 2019;19:220. doi:10.1186/s12884-019-2372-x
35. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.