Back to Journals » Journal of Pain Research » Volume 17
Effectiveness of Single Intravenous Dexamethasone in Prolongation of Spinal Anesthesia for Postoperative Analgesia in Elective Cesarean Section: A Systematic Review of Randomized Controlled Trials
Authors Abebe M, Alemu B, Teku G, Eshetu O, Wale E , Besha A, Kebede MY, Geta L
Received 10 January 2024
Accepted for publication 24 March 2024
Published 5 April 2024 Volume 2024:17 Pages 1361—1368
DOI https://doi.org/10.2147/JPR.S451595
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rushna Ali
Minda Abebe,1 Belete Alemu,1 Gudeta Teku,1 Oliyad Eshetu,1 Endeshaw Wale,1 Aschalew Besha,1 Mengistu Yinges Kebede,1 Lamesgen Geta2
1Department of Anesthesia, Collage of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia; 2Department of Anesthesia, Collage of Medicine and Health Sciences, Debremarkos University, Debremarkos, Ethiopia
Correspondence: Minda Abebe, Email [email protected]
Background: The analgesic effectiveness of a single perioperative dose of dexamethasone is not clearly defined. The administration of systemic medication like dexamethasone, opioids, and non-steroidal anti-inflammatory drugs has a positive effect on the prolongation of postoperative analgesia after cesarean section under spinal anesthesia. A single-dose administration of dexamethasone with moderate to high dose reduces postoperative pain, reduces opioid consumption, and prolongs spinal anesthesia after cesarean delivery.
Objective: The aim of this systematic review was to investigate the effectiveness of single intravenous dexamethasone in prolongation of spinal anesthesia for postoperative analgesia in elective cesarean section.
Methods: We conducted a search on PubMed, Google Scholar, the Cochrane Library, Hinari, and review articles on the effectiveness of intravenous dexamethasone for extending spinal anesthesia during elective cesarean sections, until June 2023. The searches were conducted by using keyword (IV dexamethasone OR/AND analgesia OR postoperative pain AND cesarean section OR child birth AND prolongation of spinal anesthesia). The articles included describe the analgesic efficacy of dexamethasone for prolongation of spinal anesthesia during cesarean section.
Results: A total of 25,384 papers were found using different searching methodologies from different electronic databases. The EndNote reference manager was used to remove duplicates, and 438 articles were selected for screening. Of those, 57 were included for critical evaluation, and 49 were removed with justification. The effectiveness of IV dexamethasone on the prolongation of spinal anesthesia and postoperative analgesia in women undergoing cesarean delivery is the subject of eight RCT studies on 628 parturients that are presented in the chosen journal articles from various countries.
Conclusion: Intravenous dexamethasone administration immediately after clamping of the umbilical cord prolongs the duration of spinal block in patients undergoing cesarean sections and has a significant impact on reduction of postoperative pain severity, opioid consumption, and other pain requirements. When high-dose dexamethasone is administered intravenously, it can overcome complications that may arise after severe pain and increase patient satisfaction by extending the duration of postoperative analgesia and sensory block.
Keywords: dexamethasone, prolongation, spinal anesthesia, analgesia, caesarean section systematic review
Introduction
Currently, the prevalence of cesarean section is around 21%, and cesarean section is defined as the delivery of a fetus via a uterine incision (hysterotomy) and an open abdominal incision (laparotomy).1 Over-the-counter or injectable medications can be used to manage post-cesarean pain. Based on the level of pain and whether they underwent general, spinal, or epidural anesthesia, the choice of painkillers following a cesarean delivery will vary. Through the injection of a local anesthetic into the subarachnoid space, the lower body is rendered numb through the technique of spinal anesthesia. Local anesthetic is injected into the spinal canal to stop the conduction of nerve impulses, reducing pain sensitivity without unconsciousness. During cesarean delivery it improves outcomes of both the fetus and maternal conditions by avoiding the complication of general anesthesia, and also it has prolonged analgesic effect.1
The approach to treating acute surgical pain has changed to include protocols for improved recovery following cesarean section and a reduction in the use of opioids by administering a combination of various medications with analgesic effect. This method, sometimes referred to as balanced analgesia, aims to obtain additional analgesic benefits while lowering the quantity of opioids needed to minimize side effects, which helps to increase the enhanced recovery from anesthesia and surgery.2
Numerous ailments have been treated with dexamethasone. The chronic use of dexamethasone also inhibits phospholipase, which is required for the inflammatory chain reaction along the cyclooxygenase and lipoxygenase pathways. In addition it blocks the C-fibers of the pain pathway, which contributes to its analgesic effects. The systemic administration of dexamethasone has the potential to lengthen the duration of analgesia and reduce the severity of postoperative pain, opioid consumption, and sleep disturbance during the first night following surgery with minimal adverse effects.2,3
Intraoperative administering of high-dose dexamethasone (greater than 0.2 mg/kg) reduces postoperative pain and spares opioids. After surgical procedures, an effective multimodal pain management strategy using intermediate dose dexamethasone (0.11 to 0.2 mg/kg) has been found to be safe with minimal side effects. During cesarean sections, preoperative IV dexamethasone administration has a stronger impact on postoperative pain management.4
Intravenous dexamethasone prolongs spinal anesthesia during a cesarean section, improving pain control, and lowering postoperative pain and opioid consumption. It also lessens sore throats caused by intubation and reduces opioid consumption. Additionally, there is a decreased incidence of rebound pain when intraoperative IV dexamethasone is administered. Early postoperative administration of a single intravenous dose of dexamethasone 0.1 mg/kg significantly reduces the need for overall analgesic medication and provides a substantial analgesic benefit.5
Administering high-dose dexamethasone intraoperatively (greater than 0.2 mg/kg) reduces postoperative pain and spares opioids. Following surgery, an effective multimodal pain management strategy is the use of intermediate dose dexamethasone (0.11 to 0.2 mg/kg), which is safe and has few side effects. Preoperative IV dexamethasone administration has a stronger impact on postoperative pain control during cesarean delivery.6 Additionally, there is a decreased incidence of rebound pain when intraoperative IV dexamethasone is administered. A single intraoperative dose of dexamethasone 0.1 mg/kg reduces the need for overall analgesics by providing a substantial analgesic benefit in the early postoperative phase.7
This systematic review set out to bring the body of research on dexamethasone’s effectiveness for postoperative analgesia and spinal anesthesia prolongation in women having cesarean deliveries up to date and draw conclusions. Additionally, it offers dosage guidelines for achieving the best possible effects from this medication.
Review Question
We reviewed the literature to gather information on postoperative pain management methods, identifying the most suitable technique of postoperative analgesia modality and the efficacy of dexamethasone as a prolongation of spinal anesthesia and postoperative analgesia in women undergoing cesarean delivery.
Methods and Material
Protocol
We report our systematic review by using preferred reporting of systematic review and meta-analysis (PRISMA).
Eligibility Criteria
Inclusion Criteria
All randomized controlled trials written in English language, full original length article from 2013–2023 that documented the efficacy of intravenous dexamethasone in extending spinal anesthesia for postoperative analgesia following an elective cesarean section were reviewed.
Exclusion Criteria
Study done on the effectiveness of intravenous dexamethasone administration in prolongation of postoperative analgesia after spinal anesthesia but on other than pregnant populations, case reports, studies that only included abstracts, observational studies, and cross-sectional and cohort studies were excluded from the review.
Searching Strategy
Google Scholar, PubMed, and Cochrane databases (Cochrane Central Register of Controlled Trials; Cochrane Database of Abstracts or Reviews of Effects; Cochrane Database of Systematic Reviews) were searched for randomized controlled trials published from 2013 to June 2023. The search was done by using keywords, phrases, and specific subject headings. Articles were searched for IV dexamethasone OR hexadecadrol AND analgesia OR postoperative pain AND cesarean section OR child birth AND prolongation of spinal anesthesia after keywords were extracted.
Generally 25,384 articles were identified through database searching strategies. Eight randomized controlled trials on 628 pregnant women were evaluated for quality and conclusion after the results were extracted and filtered according to the interventions, publication year, outcome, population data, and methodological quality, as well as inclusion and exclusion criteria. Articles included examined the effects of intravenous (IV) dexamethasone on prolongation of spinal anesthesia and reduced postoperative pain scores and analgesic requirements. Based on the quality of evidence referred from the Oxford Centre for Evidence-Based Medicine, a conclusion was reached and recommendations were made.
The quality of evidence was evaluated by the GRADE system (Table 1), and the system incorporates study quality (risk of bias) comparison of effectiveness across study, PICO (population, intervention, comparator, and outcome), and certainty.8
 |
Table 1 Grade of Recommendation and Level of Evidence |
PICO of this systematic review
P: Study done on pregnant women who had spinal anesthesia for elective cesarean section
I: Study that has full article and done on IV dexamethasone administration after spinal anesthesia
C: Those women not taking IV dexamethasone after spinal anesthesia
O: The analgesic prolongation of IV dexamethasone after spinal anesthesia on pregnant women who underwent elective cesarean section
Data Extraction
The data were extracted by three different reviewers using a customized Excel sheet; disagreements or conflicts were resolved by consensus or another means. Information on the patient population’s socio-demographics, sample size, research design with level of evidence, year of publication, main outcome, complications from interventions, and surgical duration were all obtained.
Data Synthesis and Quality Assessment
During the systematic review process, all of the relevant studies were incorporated into the synthesis. For every outcome, P<0.05 was regarded as statistically significant. The Cochrane Collaboration and the risk of bias assessment tool from ROBIN were used to evaluate the methodological quality and bias risk of each included study. The instrument is used to assess research quality along two dimensions: internal validity (evaluating the data collection method, case definition, study instrument, and mode of data collection) and external validity (evaluating the target population, sampling frame, and sampling).
Critical Appraisal
The risk of bias was assessed using the risk of bias assessment technique for RCT trials developed by the Cochrane Collaboration. The methodological quality of all eight RCT studies was assessed using the ROB tool, which has components for selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other bias (as shown in Table 2 below).
 |
Table 2 Risks of Bias Assessments (ROB) |
Results
A total of 25,384 studies were found using different searching methodologies from different electronic databases. The EndNote reference manager was used to remove duplicates, and 438 articles were selected for screening. Of those, 57 were included for critical assessment and 49 were removed with justification (Figure 1). The effectiveness of IV dexamethasone on the prolongation of spinal anesthesia and postoperative analgesia in women undergoing cesarean delivery is the subject of eight RCT studies that are presented in the chosen journal articles from different countries (Table 3).9–12,16
 |
Table 3 Summary of Articles Used for the Development of This Systematic Review |
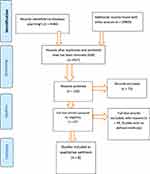 |
Figure 1 PRISMA flow diagram for the searched and used articles. |
Discussion
The results of this review suggest that intermediate to high dose of IV dexamethasone provides a substantial additional benefit to the analgesic’s duration or sensory blockade after intrathecal anesthesia in non-emergency cesarean section. Furthermore, our findings suggest that IV dexamethasone can help reduce cumulative opioid use and postoperative pain. Finally, intravenous dexamethasone does not seem to have any negative effects on the new-born’s Apgar score or cause long-term complications for the mother.
Dexamethasone has been shown in numerous trials to have the ability to increase the duration analgesia of spinal anesthesia, lower postoperative pain levels, and reduce quantity of opioids needed following a procedure. Intravenous dexamethasone and wound site infiltration of local anesthetics agents significantly reduced the need for postoperative analgesics and pain perception for up to one day following a cesarean section, with no negative effects on the surgical site or respiratory tract infection.11 However, few studies have looked at maternal opioid consumption, and pain scores were not decreased by preoperative low to intermediate doses of intravenous dexamethasone administration for cesarean delivery under spinal anesthesia or in conjunction with a multimodal postoperative analgesic regimen that includes intrathecal morphine.13
The efficacy of dexamethasone in treating cerebral edema and the subsequent elevations in intracranial pressure caused by tumors and metastatic lesions has been demonstrated in numerous medical conditions. Its glucocorticoid structure is long and efficient, and its analgesic effects stem from blocking the C-fibers of the pain pathway and inhibiting phospholipase, which is required for the inflammatory chain reaction along the cyclooxygenase and lipoxygenase pathways.5,17
Multiple studies have demonstrated that administration of dexamethasone 8 mg intravenously extends the duration of postoperative analgesia spinal anesthesia after cesarean section and decreases postoperative opioid consumption.9,10,12 Patients who underwent cesarean sections with spinal anesthesia benefit from preoperative dexamethasone 0.1 mg/kg intravenous administration because it reduces pain following surgery (intensity of postoperative pain) by lengthening the sensory receptor block, reduces opioid taken in its entirety on the first postoperative day, and reduces other analgesic requirement; it improves sleep the night after surgery; and a single dose has no impact on the prevalence of surgical site infection or wound healing.5,16
Administering high-dose dexamethasone intraoperatively (greater than 0.2 mg/kg) reduces postoperative pain and spares opioids. Following surgery, an effective multimodal pain management strategy is the use of intermediate dose dexamethasone (0.11 to 0.2 mg/kg), which is safe and has few side effects. Preoperative IV dexamethasone administration has a stronger impact on postoperative pain control during cesarean delivery.6 Furthermore, single dosage intraoperative administration of dexamethasone also decreased incidence of rebound pain by providing a considerable analgesic benefit in the early postoperative phase.7 Additionally administration of dexamethasone lowers 6-h pain at rest as well. Pain with movement was lower among patients who received dexamethasone at 1, 6, 12, and 24 h.15
The majority of strong studies comparing dexamethasone doses by categorizing them into three groups, low dose (0.1 mg/kg), intermediate dose (0.1–0.2 mg/kg), and high dose (more than 0.2 mg/kg), came to the conclusion that a dose of dexamethasone at 0.1 mg/kg is an efficient adjuvant in multimodal approaches to lower opioid use and postoperative pain. Depending on the complexity of the operation and the degree of tissue injury, the dexamethasone dosage may vary. In order to prevent the need for further analgesics and prolong the analgesic effects of spinal anesthesia after surgery, a wide range of doses of intravenous dexamethasone administration is required prior to cesarean delivery.12,18,19
Strengths
The effectiveness of IV dexamethasone in prolongation of intrathecal anesthesia and postoperative analgesia in pregnant women who underwent cesarean section was the focus of the studies included in this review, and all studies were RCT with a high level of evidence.
Conclusion
Dexamethasone prolongs the duration of spinal block and has a positive effect on the early postoperative recovery of patients undergoing CS. The administration of single dose intravenous dexamethasone immediately after clamping of the cord at a dose of 0.1–0.2 mg/kg was effective in lowering postoperative pain, minimizing opioid consumption on the first postoperative day, extending the duration of spinal anesthesia, and delaying the need for initial analgesic medication in patients undergoing cesarean sections under spinal anesthesia.
Generally, the administration of supplemental single dose dexamethasone for non-contraindicated pregnant women who underwent cesarean section under anesthesia improves enhanced recovery from anesthesia and surgery by different aspects. That means when we make the patient pain-free the complications secondary to inability to move because of pain will be decreased.
Abbreviation
ASA, American Society of Anesthesiologists; CS, cesarean section; GCP, good clinical practice; h, hour; HUCSH, Hawassa University Comprehensive Specialized Hospital; IV, intravenous; LA, local anesthetics; LSCS, lower segment cesarean section; MA, meta-analysis; mg, milligram; mg/kg, milligram per kilogram; PICO, population, intervention, control, and outcome; PRISMA, preferred reporting items of systematic review of meta-analysis; RCT, randomized controlled clinical trials; SA, spinal anesthesia; SR, systematic review; WHO, World Health Organization.
Accessibility of Information and Resources
The corresponding author provides data upon reasonable request.
Funding
No funding was received for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Organization WH. Caesarean Section Rates Continue to Rise, Amid Growing Inequalities in Access. Geneva: World Health Organization; 2021.
2. Mkontwana N, Novikova N. Oral analgesia for relieving post‐caesarean pain. Cochrane Database Syst Rev. 2015;(3). doi:10.1002/14651858.CD010450.pub2.
3. Drasner K, Larson MD. 17 Spinal And Epidural Anesthesia. Basics Anesthesia. 2011;252.
4. Roofthooft E, Joshi G, Rawal N, et al. PROSPECT guideline for elective caesarean section: updated systematic review and procedure‐specific postoperative pain management recommendations. Anaesthesia. 2021;76(5):665–680. doi:10.1111/anae.15339
5. Moore SG. Intravenous dexamethasone as an analgesic: a literature review. AANA J. 2018;86(6):488–493.
6. De Oliveira GS, Jung MJ, McCarthy RJ. Discrepancies between randomized controlled trial registry entries and content of corresponding manuscripts reported in anesthesiology journals. Anesthesia Analg. 2015;121(4):1030–1033. doi:10.1213/ANE.0000000000000824
7. Stubbs DJ, Levy N. Role of dexamethasone in reducing postoperative pain. Comment Br J Anaesth. 2021;126:862–871.
8. Bezerra CT, Grande AJ, Galvão VK, Santos D, Atallah ÁN, Silva V. Assessment of the strength of recommendation and quality of evidence: GRADE checklist. A descriptive study. Sao Paulo Med J. 2022;140(6):829–836. doi:10.1590/1516-3180.2022.0043.R1.07042022
9. Shahraki AD, Feizi A, Jabalameli M, Nouri S. The effect of intravenous Dexamethasone on post-cesarean section pain and vital signs: a double-blind randomized clinical trial. J Res Pharmacy Practice. 2013;2(3):99. doi:10.4103/2279-042X.122370
10. Patnaik S, Singh S, Vivekanand D, Singh TP. Evaluation of quality of recovery score in mothers and neonatal outcome assessment after surgery using preoperative dexamethasone for caesarean section. Med J Armed Forces India. 2021;77(2):170–174. doi:10.1016/j.mjafi.2020.03.003
11. Maged AM, Deeb WS, Elbaradie S, et al. Comparison of local and intra venous dexamethasone on post operative pain and recovery after caeseream section. A randomized controlled trial. Taiwanese J Obstetrics Gynecol. 2018;57(3):346–350. doi:10.1016/j.tjog.2018.04.004
12. Shalu PS, Ghodki PS. To study the efficacy of intravenous dexamethasone in prolonging the duration of spinal anesthesia in elective cesarean section. Anesthesia Essays Res. 2017;11(2):321. doi:10.4103/0259-1162.194537
13. Mehdiratta JE, Dominguez JE, Li Y-J, Saab R, Habib AS, Allen TK. Dexamethasone as an Analgesic Adjunct for Postcesarean Delivery Pain: a Randomized Controlled Trial. Anesthesiology Res Practice. 2021;2021:1–9. doi:10.1155/2021/4750149
14. Manohar M, Singhal S. Evaluation of the Effect of Intravenous Dexamethasone on the Duration of Spinal Anaesthesia in Parturient Undergoing Lower Segment Caesarean Section. Cureus. 2023;15(4):e37549. doi:10.7759/cureus.37549
15. Cardoso MMS, Leite AO, Santos EA. Effect of dexamethasone on prevention of postoperative nausea, vomiting and pain after caesarean section. Eur J Anaesthesiol. 2013;30:102–105. doi:10.1097/EJA.0b013e328356676b
16. Melese E, Tesfaye A, Getachew L, Daniel T. Analgesic Effect of Intravenous Dexamethasone Prior to Spinal Anesthesia Among Parturient Undergo Cesarean Section at Gandhi Memorial Hospital, Addis Ababa, Ethiopia, Prospective Cohort Study, 2019. J Anesth Clin Res. 2019;10:12.
17. Stoelting RK, Hillier SC. Pharmacology and Physiology in Anesthetic Practice. Lippincott Williams & Wilkins; 2012.
18. De Oliveira GS, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. J Am Soc Anesthesiologists. 2011;115(3):575–588.
19. Memon N, Bagga J. Effect of single-dose intravenous dexamethasone on postoperative pain and postoperative nausea and vomiting in patients undergoing lower segment cesarean section under spinal anesthesia. Asian J Med Sci. 2022;13(1):31–37. doi:10.3126/ajms.v13i1.40283
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
