Back to Journals » Journal of Pain Research » Volume 17
Efficacy of Electroacupuncture Combined with Chinese Herbal Medicine on Pain Intensity for Chronic Sciatica Secondary to Lumbar Disc Herniation: Study Protocol for a Randomised Controlled Trial
Authors Xia JC , Huang YC, Wu K, Pang J , Shi Y
Received 17 November 2023
Accepted for publication 9 March 2024
Published 8 April 2024 Volume 2024:17 Pages 1381—1391
DOI https://doi.org/10.2147/JPR.S448631
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Jing-Chun Xia,1,2,* Yu-Cheng Huang,1,2,* Ke Wu,1,2 Jian Pang,1,2 Ying Shi1,2
1Shi’s Center of Orthopedics and Traumatology, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 2Institute of Traumatology & Orthopedics, Shanghai Academy of Traditional Chinese Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jian Pang; Ying Shi, Email [email protected]; [email protected]
Purpose: Chinese herbal medicine and electroacupuncture (EA) have been used to control pain for many decades in China. We aim to explore the efficacy of intervening patients whose discogenic sciatica symptoms lasting longer than 3 months with these conservative treatments.
Patients and Methods: This is a single-center, parallel-group, patient-unblinded Randomized Controlled Trial (RCT) with blinded outcome assessment and statistician. One hundred and twenty-four patients will be assigned randomly into 2 groups including conservative treatment group (Shenxie Zhitong capsule combined with EA treatment) and Nonsteroidal Anti-inflammatory Drugs (Nonsteroidal Anti-inflammatory Drugs, NSAIDs) control group (Celecoxib) in a 1:1 ratio. The trial involves a 4-week treatment along with follow-up for 6 months. The primary outcome is the leg pain intensity measured by the visual analogue scale (VAS) at 6 months after randomization. Secondary outcomes include leg pain intensity at other time points, back pain intensity, leg pain and back pain frequency, functional status, quality of life, return to work status and satisfaction of patients. Adverse events will also be recorded.
Strengths and Limitations of This Study: Through this study, we want to observe the efficacy of electroacupuncture combined with Chinese herbal medicine on pain intensity for chronic sciatica secondary to Lumbar Disc Herniation. If the final results are favorable, it is expected to be a safe, economical, and effective treatment for patients. The study design has the following limitations: the setup of control group was less than perfect; patients and doctors could not be blinded in this trial; we skipped the feasibility study. We have tried our best to minimize adverse impacts.
Trial Registration: ChiCTR2300070884 (Chinese Clinical Trial Registry, http://www.chictr.org.cn, registered on 25th April 2023).
Keywords: sciatica of lumbar disc herniation, shenxie zhitong capsule, electroacupuncture treatment, celecoxib, single-center randomized controlled trial, protocol
Introduction
Sciatica is characterized by radiating leg pain extending from the buttock down the course of the sciatic nerve and accompanied by back pain and neurological deficits sometimes. Symptoms of sciatic pain are common with the highest incidence of 40%, and the pain can easily progress to a chronic and recurrent stage, as sciatica can be caused by interference anywhere in the sciatic nerve.1,2 Of all patients with sciatica, 85% of cases are associated with a disc disorder.3–5 The prevalence of sciatica varies between 1.6% and 43%, with a significantly higher incidence in developing countries.6 Thus, sciatica is thought to be a major health issue worldwide. In addition to severe disability and work absenteeism, it has a significant impact on medical costs.7 Surgical treatment has short-term benefits for patients with acute sciatica, but the long-term benefits are not obvious.8–11 Besides its high cost is often prohibitive. Conservative treatments (eg, exercise and spinal manipulation) are more acceptable for cost-efficiency and their few adverse reactions. However, the efficacy of them is controversial.12–14 Based on the above analysis, safe, economical, and effective treatments are an unmet clinical need for patients with chronic sciatica.
Acupuncture combined with Chinese herbal medicine is a widely utilized approach in China. In ancient traditional Chinese medicine, acupuncture and Chinese herbal medicine were believed to balance Yin and Yang, harmonize qi and blood, and unblock meridian and collateral.15 In recent years, acupuncture combined with Chinese herbal medicine has made great progress in treating motor neuron disease,16 angina pectoris of coronary heart disease,17 non-alcoholic fatty liver disease,18 type 2 diabetes mellitus,19 osteoporosis,20 Gouty Arthritis21 and so on. Shi’s orthopedics and traumatology academic school, one of Traditional Chinese medicine (TCM) schools, also has done some researches in this area. We have found it effective in treating acute ankle sprain and steroid-induced avascular necrosis of the femoral head.22,23
Electroacupuncture (EA) is a therapy that involves electrical stimulation in conjunction with acupuncture and has gained widespread recognition for its efficacy in treating musculoskeletal disorders. Notably, it has demonstrated effectiveness even in cases where conventional acupuncture treatment has proven ineffective.24 It has been recognized as an effective treatment by the American Pain Society and the National Center for Complementary and Alternative Medicine and is used by millions of people to reduce pain and block inflammation.25–27 Shenxie Zhitong capsule is officially recognized by Shanghai Food and Drug Administration Bureau, which is a traditional Chinese medicine preparation developed according to experience of Shi’s orthopedics and traumatology. As a compound preparation of blood-activating and stasis-resolving medicinal (Sanqi) and medicinal insects (Ground beetle, Scorpion, Centipede), Shenxie Zhitong capsule can improve blood rheology and conform to the theory of dissipating blood stasis, activating blood, resolving stasis, freeing the collateral vessels, and relieving pain of traditional Chinese medicine with no adverse reactions such as liver and kidney damage so far. Prior to this study protocol, Shi’s Center of Orthopedics and Traumatology has completed many clinical studies on Shenxie Zhitong capsule. The research results and clinical experience have showed that Shenxie Zhitong capsule has a good effect on knee osteoarthritis, lumbar disc herniation, cervical spondylosis in pain relieving with no obvious adverse reactions.28–33 However, sufficient evidence of EA combined with Shenxie Zhitong capsule for chronic sciatica secondary to Lumbar Disc Herniation is lacking. Therefore, we will conduct a randomised controlled trial to evaluate the effectiveness and safety of it.
Methods/Design
Study Aims
This study is a single-center RCT study aimed at investigating the efficacy and safety of the combination of Shenxie Zhitong capsule and EA treatment for discogenic sciatica. In terms of effectiveness, we will mainly focus on the advantages of Shenxie Zhitong capsule combined with EA treatment, compared with Celecoxib, in pain reduction. Besides, we will also evaluate the effectiveness of Shenxie Zhitong capsule combined with EA treatment in improving lumbar dysfunction and daily living ability.
Study Design
The detailed study process is illustrated in Figure 1. We design this single-center, parallel-group, patient-unblinded RCT with blinded outcome assessment and statistician following the Consolidated Standards of Reporting Trials (CONSORT) and the Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) guidelines.34,35 The recruiting time is from 2023-05-01 to 2023-12-31 and the study execute time is from 2023-05-01 to 2024-04-01. The trial will be conducted in outpatient departments of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine in China. IRB of Shuguang Hospital affiliated with Shanghai University of TCM has approved the study protocol (version 2.0, 6 April 2023) with an approval number of 2023-1305-72-01. It was registered at the Chinese Clinical Trial Registry (http://www.chictr.org.cn) on 25th April 2023 with the registration number of ChiCTR2300070884. The protocol is reported in accordance with the Standard Protocol Items (SPIRIT).36,37 For details, please see Additional File 1.
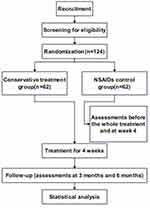 |
Figure 1 Flowchart of the trial procedure. Notes: Conservative treatment group, Shenxie Zhitong capsule combined with EA treatment; NSAIDs control group, Celecoxib. |
Patients
Shi’s Center of Orthopedics and Traumatology in Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine will conduct this study to ensure recruitments where many patients visit doctors for sciatica of Lumbar Disc Herniation (LDH) every month. Eligible patients will be recruited from outpatient clinics through advertisements on posters and WeChat. To promote recruitment and compliance, all treatments during the study period will be provided free of charge.
Inclusion Criteria
The study will include both male and female patients (1) aged between 18 and 75 years; (2) having unilateral radiculopathy, including static and progressive neurological deficit, diagnosed as unilateral radiating leg pain with positive straight-leg raise test at <70°; (3) having findings on magnetic resonance imaging (MRI) of posterolateral herniation of the disk between the fourth and fifth lumbar vertebrae (L4-L5) or in the lumbosacral junction (L5-S1) on the appropriate side, with compression of the corresponding nerve root; (4) leg pain intensity for 40 mm to 70 mm measured by the VAS;38 (5) having sciatica symptoms lasted more than 3 months and less than 12 months; (6) voluntarily participated in this trial and signed the informed consent form.
Exclusion Criteria
Patients will be excluded if they meet any of the following criteria: (1) patients whose radiculopathy secondary to foraminal stenosis; (2) patients whose radiculopathy secondary to intra-foraminal or far lateral disc herniation; (3) patients whose radiculopathy secondary to lumbar central or lateral recess stenosis; (4) patients who have undergone surgery for lumbar disc herniation; (5) patients who have received other conservative treatments except Chinese herbal medicine and acupuncture within 2 weeks; (6) patients who have received NSAIDs (eg, aspirin) within 2 weeks; (7) patients who have lumbar spondylolisthesis or lateral listhesis at level of disc herniation; (8) patients who have received epidural injection or subarachnoid root block at the involved level; (9) patients who have severe coexisting diseases (eg, cancer); (10) patients who are pregnant, breastfeeding, or planning to conceive during the trial; (11) patients who have severe cardiovascular, cerebrovascular system diseases, renal insufficiency or are undergoing cardiovascular disease intervention (surgery or medication); (12) patients who have allergic history in Shenxie Zhitong capsule or Celecoxib; (13) patients without permanent home address and contact details; (14) patients who have drug abuse; (15) patients continually taking antidepressant medication; (16) patients who have alcohol abuse; (17) patients who are unable to comprehend the questionnaires correctly or sign the informed consent form.
Dropout Criteria
Patients will be removed if they are unwilling to continue participation. The reasons for dropouts will be recorded.
Randomization
An independent researcher that is blinded to the study protocol will generate the random sequence by the central block randomization method using IBM SPSS Statistics, version 26.0 (International Business Machines Corporation, China) to assign eligible patients randomly into conservative treatment group (Shenxie Zhitong capsule combined with EA treatment) and NSAIDs control group (Celecoxib) in a 1:1 ratio. After the sequence number is generated, the researcher will make allocation cards and seal each card in an opaque envelope. The envelope will be kept by the same researcher responsible for sequence generation. According to the timing sequence of the patients visiting the hospital, another researcher will arrange the patients into different groups and inform the clinician of the group assignments. Before randomization, each eligible patient will be informed trial information. Those who are willing to participate will write the informed consent form.
Blinding
Outcome assessors and statistician will be blinded to the assignment.
Interventions
All patients will receive health education on daily living for managing sciatica of LDH, such as remaining active, using a hard bed, or losing weight. And on this basis they will receive conservative treatment or take Celecoxib. During the study period, any adverse events and any drugs taken by the patients that are not part of the study will be recorded in detail.
Conservative treatment group
Shenxie Zhitong capsule combined with EA treatment will be provided in this group. Patients will take 3 capsules twice a day and receive 8 sessions of 30-min EA treatments over 4 weeks at the same time (ideally 2 times a week with an interval of 2 or 3 days). Obligatory acupoints are bilateral Jiaji (Ex-B2) corresponding to LDH, Huangtiao (GB30), Weizhong (BL40) and Yaotu (empirical acupoint). Locations shown in Table 1 and Figure 2. Huatuo Brand disposable stainless steel acupuncture needles (0.30×40/75 mm, Suzhou Medical Appliance Factory in China, CL) and a SDZ-III electric stimulator (Suzhou Medical Appliance Factory in China, CL) will be used in the EA treatment. A trained clinician with more than 2 years of experience with acupuncture manipulation will perform EA. When patients are in a prone position, after strict disinfection of the skin around acupoints and acupuncturists’ hands using 75% alcohol, the needle will be vertically inserted rapidly into JiaJi (Ex-B2), Weizhong (BL40) and Yaotu (empirical acupoint) deeply to 40 mm and will be inserted into Huangtiao (GB30) to a depth of 70 mm. After performing twirling, lifting, and thrusting manipulations for about 10s, the patients are expected to experience soreness and distension transmitted to the leg. Then the electric apparatus is applied to the JiaJi (Ex-B2) acupoints with a dilatational wave using a 50 Hz frequency and a comfortably tolerated maximum current intensity. All needles will be retained for 30 min.
 |
Table 1 Location of Acupoints |
 |
Figure 2 Locations of acupoints. |
Shenxie Zhitong capsule is a kind of hospital preparation in Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (drug batch number: Z04100919), which is composed of Sanqi, Scorpion and so on. It has the effect of activating blood circulation, dredging meridians and collaterals and relieving pain. The indications are neck pain, upper-extremity radicular symptoms and arthrodynia caused by other degenerative diseases. By far no adverse reactions have been reported.
The efficacy and safety of acupoints (Jiaji, Huantiao, Weizhong, Yaotu) in the treatment of lumbar disc herniation has been widely verified in clinic. Adverse events such as fainting, local pain, bleeding and so on may occur occasionally.
During the trial, the use of analgesic drugs or other treatments was not permitted.
NSAIDs control group
Celecoxib (drug batch number: H20203357, Sichuan Guowei Pharmaceutical Co. Ltd.) will be provided in this group. Patients will take 1 capsule twice a day.
The indications of Celecoxib are osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, acute pain in adults, arthrodynia and postoperative pain. Common adverse events are gastrointestinal problems, such as nausea, emesis, diarrhea or inappetence. Headache, dizziness, tiredness, rash, gastrointestinal bleeding and the ulcerous perforation of digestive tract may occur sometimes. In addition, there may be an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke.
Outcomes Measures
The primary outcome is defined as visual analogue scale for intensity of leg pain (0–10: 0 = no pain, 10 worst pain) measured at 6 months following the initiation of study treatment. The VAS presents as a 0–100 mm ruler with 0 representing no pain and 100 representing unbearable pain. The specific score will be determined by the distance from 0 to the patient’s mark.
Secondary outcomes include VAS for intensity of leg pain and low back pain at other time points (0–10: 0 = no pain, 10 worst pain), VAS for frequency of leg pain and low back pain at other time points (0–10: 0= never, 10 constant), Oswestry Disability Index (ODI),39 36-item Short-Form Health Survey (SF-36),40 Return to Work Status and expectations of patients. The ODI plays a role in giving a subjective percentage score of function level for patients with low back pain through examining perceived disability in 10 activities of daily living (0–100: A higher score in the ODI indicates more serious dysfunction). SF-36 is a questionnaire for measuring health status, which includes eight subscales to measure eight aspects of health status (Physical Function, Role Physical, Role Emotional, Bodily Pain, Vitality, Mental Health, Social Functioning, and General Health) and a single item (Reported Health Transition) to provide an indication of perceived change in health. Each item was scored from 0 to 100, with a higher score indicating a better health state. The Return to Work Status is defined to assess the work status of patients when they return to work. The expectations of patients refer to general expectations about our treatment. Because patients may not know the exact efficacy of Chinese herbal medications used in this study plus electroacupuncture, or NSAIDs in treating sciatica before enrollment, we set this index to observe whether patients’ expectations can affect the clinical efficacy or not. Before beginning treatment, we will ask patients: “What results do you expect from your treatment?” related to complete relief from symptoms (pain, numbness, weakness, and instability). Patients will use a five-point Likert scale anchored with “definitely yes” to “definitely not” to indicate their general expectation for the treatment. After the whole treatment, patients will complete another questionnaire in follow-up period considering the question: “Are your expectations for treatment met?” and rating this using a Likert scale ranging from “not at all” to “totally”.41,42
Time points for collection of secondary outcome measures are study enrolment, 4 weeks, 3 months and 6 months following the initiation of study treatment. Detailed arrangements of every outcome are shown in Figure 3.
 |
Figure 3 Detailed arrangements of every outcome.(SPIRIT). Abbreviations: w, week; m, month; VAS, visual analogue scale; ODI, Oswestry Disability Index; SF-36, 36-item Short-Form Health Survey. |
Sample Size Calculation
The VAS for intensity of leg pain (0–10: 0 = no pain, 10 worst pain) measured at 6 months following the initiation of study treatment will be the primary outcome measure for sample size calculation. Taking into consideration of at least 2 points of between-group difference, a two-sided significance level of 5% (α=0.05) and a test power of 90% (β=0.10), we refer to the following sample size calculation formula to estimate, the required number of patients is 74 in total (37 patients in each group).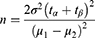
Meanwhile, considering the importance of ODI measured at 6 months following the initiation of study treatment, 98 patients will be required for the sample size at least (49 patients in each group) to provide 90% power to detect a difference of 10 points between the groups with a two-sided 5% level of significance.
In addition to that, considering a 20% dropout rate, the sample size is 62 patients in each group. Thus, a minimum of 124 patients will be necessary for the sample size.
Statistical Analysis
All results will be analyzed in accordance with the intention-to-treat (ITT) principle in the research.
We will calculate basic summary statistics (including average, percentage, standard deviation). We will perform a mixed model analysis of repeated measurements, with treatment, time, and baseline points as fixed variables and random variables as objects. The study will report the estimated mean, standard errors and 95% confidence intervals for the mean treatment effect at each follow-up time point. Differences between two treatments will be assessed by the main therapeutic effect and the interaction of time and treatment. Generalized estimating equation will be used in category variance outcome. The therapeutic effect will be estimated as the difference in estimated proportions. We will plot each estimated result score by time and by group to illustrate changes over time. There is no adjustment for multiple comparisons. An interim analysis will be performed when 50% of patients have been enrolled to ensure safety.
Discussion
Shi’s orthopedics and traumatology academic school is well known throughout China, with a history of 150 years. The current school inheritor, Professor Yin-Yu Shi, was awarded the honorary title of national famous doctor of TCM by the Chinese government. Shenxie Zhitong capsule was also developed based on Professor Shi’s clinical experience.
Shenxie Zhitong capsule is a compound preparation that contains Sanqi and medicinal insects (Ground beetle, Scorpion, Centipede). Sanqi is a kind of stasis-resolving hemostatic medicinal for the treatment of blood syndrome. Insects in medicine have a long history of application in China. About 300 insect species, belonging to 13 orders, were officially written into more than 1700 classical Chinese herbal prescriptions, which are still used by modern Chinese people. Ground beetle has the effect of breaking blood and expelling stasis. Pharmacological studies have shown that it can reduce blood viscosity and fibrinogen and inhibit platelet aggregation. Scorpion and Centipede can free the collateral vessels and relieve pain by inhibiting thrombosis.43,44
EA treatment is more appreciated for its controlled and quantifiable stimulation frequency and intensity in recent years.45 Moreover, its effect is superior to manual acupuncture for relieving pain and improving paresthesia and dysfunction.46 Several studies have reported that EA may be effective in treating neuropathic pain and relieving sciatica symptoms with few adverse events.47–49
NSAIDs are used in sciatica commonly, given their analgesic and anti-inflammatory mechanisms of action and recommended in clinical practice guideline.50 In clinical trials, 50–60% of enrolled patients were taking an NSAID at baseline.51,52 Therefore, we selected it as the control group.
The trial has certain limitations. Firstly, the setup of control group was less than perfect. Given ethical review, we did not set the sham acupuncture control group, but chose NSAIDs as control group according to clinical practice guideline. Secondly, according to practical conditions, patients and doctors could not be blinded in this trial. Besides, we skipped the feasibility study which is important in estimation of important parameters that are needed to design the main study.
Based on those limitations, we have tried our best to minimize these impacts. Before official recruitment, every research participant is required to obtain good clinical practice (GCP) certificates authorized by the China State Food and Drug Administration (SFDA) in this project, which is to ensure that all aspects of research are standardized and to reduce bias during the implementation.
Clinical Implications
The results of this study will contribute to a better understanding of the effectiveness of the combination of Shenxie Zhitong capsule and EA in patients with lumbar disc-induced sciatica with symptoms lasting longer than 3 months. This study will also contribute to clinical practice by providing evidence that will help guide decisions about the appropriate treatment of patients with discogenic sciatica. Besides, the relationship between patients’ general expectations about our treatment and clinical efficacy will help to assess the necessity of verbal suggestion in this therapy. It is expected that patients will benefit from this study, especially those in developing countries. Because it may contribute to alleviating the problems of “difficulty and high cost of getting medical service”. The results will be published once the study is completed.
Trial Status
Recruitment of this study (protocol version 3.0, 11 April 2023) began on May 1, 2023, and will be completed on December 31, 2023. Execution of the study began on May 1, 2023, and will be completed on April 1, 2024. It goes according to plan.
Abbreviations
EA, Electroacupuncture; RCT, Randomized Controlled Trial; NSAIDs, Nonsteroidal Anti-inflammatory Drugs; VAS, Visual Analogue Scale; TCM, Traditional Chinese medicine; CONSORT, Consolidated Standards of Reporting Trials; STRICTA, The Standards for Reporting Interventions in Clinical Trials of Acupuncture guidelines; SPIRIT, The Standard Protocol Items; LDH, Lumbar Disc Herniation; MRI, Magnetic Resonance Imaging; ODI, Oswestry Disability Index; SF-36, 36-item Short-Form Health Survey; ITT, Intention to Treat; GCP, Good Clinical Practice; SFDA, State Food and Drug Administration.
Data Sharing Statement
Data sharing does not apply to this article as no data sets were generated or analyzed during the current study.
Ethics Approval and Consent to Participate
IRB of Shuguang Hospital affiliated with Shanghai University of TCM has approved the study protocol (version 2.0, 6 April 2023) with an approval number of 2023-1305-72-01. The methods are performed in accordance with the Declaration of Helsinki. Written informed consent will be obtained from all enrolled patients before randomization in this study. Any modifications to the protocol will be reported. Treatment and consultations related to sciatica will be offered freely to patients during the study. When the study is completed, an additional individual advice will be given based on their conditions. Besides, a transportation subsidy of 150 yuan will be offered to each patient who completes the treatment. Personal information about participants will be collected by questionnaires, shared and maintained in our research center. Researchers of the study will have access to the final trial dataset.
Acknowledgments
The authors would like to thank Dr Yin-Yu Shi who contributed to the treatment concept of LDH in TCM.
Funding
This work was supported by the three-year action plan of speeding up the inheritance, innovation and development of TCM supported by Shanghai(2021-2023) (ZY (2021-2023)-0209-02); Shanghai Municipal Clinical Research Center for Chronic Musculoskeletal Conditions (no. 20MC1920600); Shanghai High-Level Local University Innovation Team (SZY20220315); Shanghai clinical specialty “traditional Chinese medicine orthopaedic traumatology” (shslczdzk03901).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lindblom K, Rexed B. Spinal nerve injury in dorso-lateral protrusions of lumbar disks. J Neurosurg. 1948;5(5):413–432. doi:10.3171/jns.1948.5.5.0413
2. Konstantinou K, Dunn KMJS. Sciatica: review of Epidemiological Studies and Prevalence Estimates. Spine;2008;33.
3. Deyo RA, Mirza SK. CLINICAL PRACTICE. Herniated Lumbar Intervertebral Disk. New Engl J Med. 2016;374(18):1763–1772. doi:10.1056/NEJMcp1512658
4. Porchet F, Wietlisbach V, Burnand B, Daeppen K, Villemure JG, Vader JP. Relationship between severity of lumbar disc disease and disability scores in sciatica patients. Neurosurgery. 2002;50(6):1253–1259. doi:10.1097/00006123-200206000-00014
5. Ropper AH, Zafonte RD. Sciatica. New Engl J Med. 2015;372(13):1240–1248. doi:10.1056/NEJMra1410151
6. Valat JP, Genevay S, Marty M, Rozenberg S, Koes B. Sciatica. Best Pract Res. 2010;24(2):241–252. doi:10.1016/j.berh.2009.11.005
7. Parreira P, Maher CG, Steffens D, Hancock MJ, Ferreira ML. Risk factors for low back pain and sciatica: an umbrella review. Spine j. 2018;18(9):1715–1721. doi:10.1016/j.spinee.2018.05.018
8. Peul WC, van den Hout WB, Brand R, Thomeer RT, Koes BW. Prolonged conservative care versus early surgery in patients with sciatica caused by lumbar disc herniation: two year results of a randomised controlled trial. BMJ. 2008;336(7657):1355–1358. doi:10.1136/bmj.a143
9. Peul WC, van Houwelingen HC, van den Hout WB, et al. Surgery versus prolonged conservative treatment for sciatica. New Engl J Med. 2007;356(22):2245–2256. doi:10.1056/NEJMoa064039
10. Osterman H, Seitsalo S, Karppinen J, Malmivaara A. Effectiveness of microdiscectomy for lumbar disc herniation: a randomized controlled trial with 2 years of follow-up. Spine. 2006;31(21):2409–2414. doi:10.1097/01.brs.0000239178.08796.52
11. Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006;296(20):2441–2450. doi:10.1001/jama.296.20.2441
12. Cuenca-Zaldívar JN, Fernández-Carnero J, Sánchez-Romero EA, et al. Effects of a Therapeutic Exercise Protocol for Patients with Chronic Non-Specific Back Pain in Primary Health Care: a Single-Group Retrospective Cohort Study. J Clin Med. 2023;12(20):6478. doi:10.3390/jcm12206478
13. Martínez-Pozas O, Sánchez-Romero EA, Beltran-Alacreu H, et al. Effects of Orthopedic Manual Therapy on Pain Sensitization in Patients With Chronic Musculoskeletal Pain: an Umbrella Review With Meta-Meta-analysis. Am j Phys Med Rehabilitation. 2023;102(10):879–885. doi:10.1097/PHM.0000000000002239
14. Pangarkar SS, Kang DG, Sandbrink F, et al. VA/DoD Clinical Practice Guideline: diagnosis and Treatment of Low Back Pain. J Gen Intern Med. 2019;34(11):2620–2629. doi:10.1007/s11606-019-05086-4
15. Gui M, Chen M. Acupuncture combined with Chinese herbal medicine for 32 cases of acute gouty arthritis. Huan J Traditional Med. 2014;30(12):74–75.
16. Peng S, Chang W, Tian Y, et al. Herbal medicine and acupuncture relieved progressive bulbar palsy for more than 3 years: a case report. Medicine. 2022;101(45):e31446. doi:10.1097/MD.0000000000031446
17. Wang S, Liu AD, Wang Z, Zhang Y. Efficacy and safety of acupuncture combined with Chinese herbal medicine in the treatment of angina pectoris of coronary heart disease (CHD): a protocol for systematic review and meta analysis. Medicine. 2021;100(52):e28450. doi:10.1097/MD.0000000000028450
18. Ma Y, Sun K, Cao J, et al. Acupuncture combined with Chinese herbal medicine on non-alcoholic fatty liver disease: a protocol for systematic review and meta-analysis. Medicine. 2021;100(51):e28083. doi:10.1097/MD.0000000000028083
19. Bao P, Mi J, Yu Z, et al. Efficacy and safety of acupuncture combined with Chinese herbal medicine in the treatment of type 2 diabetes mellitus: a protocol for a systematic review and meta-analysis. Medicine. 2021;100(43):67.
20. Long P, Ju S, Wang J. Efficacy and safety of acupuncture in combination with Chinese herbal medicine in dealing with osteoporosis: a protocol for a systematic review and network meta-analysis. Medicine. 2022;101(52):e32441. doi:10.1097/MD.0000000000032441
21. Liang H, Wu Y, Zhang W, et al. Efficacy and Safety of Acupuncture Combined with Herbal Medicine in Treating Gouty Arthritis: meta-Analysis of Randomized Controlled Trials. Evidence-Based Complementary Alternative Med. 2021;2021:8161731. doi:10.1155/2021/8161731
22. Ji W, Zhao YF, Guo HL, Li T, Zhang XC. Clinical Trail of Mid-term Efficacy of Shi’s Acupuncture Combined with Plaster for Treating Acute Ankle Injury. Chinese J Trad Med Traum Orthop. 2018;26(04):12–17.
23. Wu HF, Wang J, Zhang B, Zhan HS, Shi YY, Du J. Experience with differentiation and treatment of steroid-induced femoral head necrosis in SHI’s traumatology. CJTCMP. 2019;34(08):3541–3543.
24. Comachio J, Oliveira Magalhães M, Nogueira Burke T, et al. Efficacy of acupuncture and electroacupuncture in patients with nonspecific low back pain: study protocol for a randomized controlled trial. Trials. 2015;16(1):469. doi:10.1186/s13063-015-0850-7
25. Ulloa L, Quiroz-Gonzalez S, Torres-Rosas R. Nerve Stimulation: immunomodulation and Control of Inflammation. Trends Mol Med. 2017;23(12):1103–1120. doi:10.1016/j.molmed.2017.10.006
26. Zhang R, Lao L, Ren K, Berman BM. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120(2):482–503. doi:10.1097/ALN.0000000000000101
27. Zhang Q, Zhou M, Huo M, et al. Mechanisms of acupuncture-electroacupuncture on inflammatory pain. Molecular Pain. 2023;19:17448069231202882. doi:10.1177/17448069231202882
28. Wang HS, Gu XF, Liu T, et al. A clinical study on oral application of Shenxie Zhitong capsule for treatment of knee osteoarthritis. J Trad Chin Orthop Trauma. 2015;27(03):22–26.
29. An JW, Tang YJ, Wu HB, et al. Analysis of the experience of treating the pain of neck and back and legs with the Shen-xie-chong-fenfang in Shi’s Center of Orthopedics and Traumatology. Clin j Traditional Chine Med. 2018;30(04):653–655.
30. Chen YC, Pang J, Shi YY, et al. Clinic Research on Knee Osteoarthritis Treatment Based on Shi’s Unique Knee Osteoarthritis Therapy. Chine J Trad Med Traum Orthop. 2016;24(06):9–12.
31. Wang HH, Shen ZB, Zhan HS. Zhan Hongsheng’s experience in treating chronic diseases related to sinews and bones by resolving stasis and unblocking collaterals method. Shanghai J Traditional Chin Med. 2021;55(12):40–43.
32. Yang J, Gu W, Zhu WJ, Wang XZ, Sun J. Effect study on Shenxie Zhitong capsule of knee osteoarthritis after total knee arthroplasty. Shaanxi J Traditional Chine Med. 2022;43(12):
33. Li SS, Tan Z, Zhan HS, Chen DY. Therapeutic efficacy of Shi’s traumatology Shen-Xie-Chong powder decoction combined with adenosylcobalamin on lumbar disc herniation. Mod J Integr Traditional Chinese and Western Med. 2023;32(15):2099–2103.
34. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Obstetrics Gynecol. 2010;115(5):1063–1070. doi:10.1097/AOG.0b013e3181d9d421
35. MacPherson H, Altman DG, Hammerschlag R, et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. PLoS Med. 2010;7(6):e1000261. doi:10.1371/journal.pmed.1000261
36. Mann H. SPIRIT 2013. Evidence-Based Dentistry. 2013;14(4):120. doi:10.1038/sj.ebd.6400973
37. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Internal Med. 2013;158(3):200–207. doi:10.7326/0003-4819-158-3-201302050-00583
38. Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72(1–2):95–97. doi:10.1016/S0304-3959(97)00005-5
39. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271–273.
40. Patrick DL, Deyo RA, Atlas SJ, Singer DE, Chapin A, Keller RB. Assessing health-related quality of life in patients with sciatica. Spine. 1995;20(17):1899–1908. doi:10.1097/00007632-199509000-00011
41. Bishop MD, Bialosky JE, Cleland JA. Patient expectations of benefit from common interventions for low back pain and effects on outcome: secondary analysis of a clinical trial of manual therapy interventions. J Manual Manipulative Therapy. 2011;19(1):20–25. doi:10.1179/106698110X12804993426929
42. Sánchez Romero EA, Lim T, Villafañe JH, et al. The Influence of Verbal Suggestion on Post-Needling Soreness and Pain Processing after Dry Needling Treatment: an Experimental Study. Int J Environ Res Public Health. 2021;18(8):4206. doi:10.3390/ijerph18084206
43. Zhong H, Zhao J. Clinical application of insect drugs. J Traditional Chine Med. 2003;23(4):257–259.
44. Feng Y, Zhao M, He Z, Chen Z, Sun L. Research and utilization of medicinal insects in China. Spine:2009;39(5):313–316.
45. Pang T, Lu C, Wang K, et al. Electroacupuncture at ST25 Inhibits Cisapride-Induced Gastric Motility in an Intensity-Dependent Manner. Evid Based Complement Alternat Med. 2016;2016:3457025. doi:10.1155/2016/3457025
46. Zhang X, Wang Y, Wang Z, Wang C, Ding W, Liu Z. A Randomized Clinical Trial Comparing the Effectiveness of Electroacupuncture versus Medium-Frequency Electrotherapy for Discogenic Sciatica. Evid Based Complement Alternat Med. 2017;2017:9502718. doi:10.1155/2017/9502718
47. Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine j. 2008;8(1):8–20. doi:10.1016/j.spinee.2007.10.005
48. Asche CV, Kirkness CS, McAdam-Marx C, Fritz JM. The societal costs of low back pain: data published between 2001 and 2007. J Pain Palliative Care Pharmacother. 2007;21(4):25–33.
49. Zhang Z, Hu T, Huang P, et al. The efficacy and safety of acupuncture therapy for sciatica: a systematic review and meta-analysis of randomized controlled trails. Front Neurosci. 2023;17:1097830. doi:10.3389/fnins.2023.1097830
50. Group COAoSSGCOAoOR. Clinical practice guideline for diagnosis and treatment of lumbar disc herniation. Chin J Orthop. 2020;40(8):477–487.
51. Cummins J, Lurie JD, Tosteson TD, et al. Descriptive epidemiology and prior healthcare utilization of patients in the Spine Patient Outcomes Research Trial’s (SPORT) three observational cohorts: disc herniation, spinal stenosis, and degenerative spondylolisthesis. Spine. 2006;31(7):806–814. doi:10.1097/01.brs.0000207473.09030.0d
52. Bronfort G, Hondras MA, Schulz CA, Evans RL, Long CR, Grimm R. Spinal manipulation and home exercise with advice for subacute and chronic back-related leg pain: a trial with adaptive allocation. Ann Internal Med. 2014;161(6):381–391. doi:10.7326/M14-0006
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
