Back to Journals » Journal of Blood Medicine » Volume 14
Efficacy of rFIXFc versus N9-GP Prophylaxis in Patients with Hemophilia B: Matching-Adjusted Indirect Comparison of B-LONG and PARADIGM 2 Trials
Authors Mancuso ME, Eriksson D , Falk A, Hakimi Z, Wojciechowski P , Wdowiak M, Klamroth R
Received 16 September 2022
Accepted for publication 16 July 2023
Published 27 July 2023 Volume 2023:14 Pages 427—434
DOI https://doi.org/10.2147/JBM.S389094
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Maria Elisa Mancuso,1,2 Daniel Eriksson,3 Aletta Falk,3 Zalmai Hakimi,3 Piotr Wojciechowski,4,5 Marlena Wdowiak,4 Robert Klamroth6,7
1Center for Thrombosis and Hemorrhagic Diseases, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy; 2Humanitas University, Pieve Emanuele, Milan, Italy; 3Swedish Orphan Biovitrum AB, Stockholm, Sweden; 4Creativ-Ceutical, Krakow, Poland; 5Assignity, Krakow, Poland; 6Department of Internal Medicine, Hemophilia Treatment Center, Vivantes Klinikum im Friedrichshain, Berlin, Germany; 7Institute of Experimental Hematology and Transfusion Medicine, University Hospital Bonn, Medical Faculty, University of Bonn, Bonn, Germany
Correspondence: Robert Klamroth, Department of Internal Medicine – Angiology and Hemostaseology, Center for Vascular Medicine, Haemophilia Treatment Center, Vivantes Klinikum im Friedrichshain, Landsberger Allee 49, Berlin, D-10249, Germany, Tel +49 (0)30 130 231575, Fax +49 (0)30 130 232130, Email [email protected]
Purpose: For patients with hemophilia B, extended half-life factor IX (FIX) products are available for prophylaxis and for treating bleeds. Different methods are used to extend the half-lives of recombinant FIX Fc fusion protein (rFIXFc) and nonacog beta pegol (N9-GP). This affects their biodistribution and plasma FIX levels, although differences do not always correlate with clinical outcomes. A matching-adjusted indirect comparison (MAIC) of prophylaxis with rFIXFc and N9-GP was performed, based on licensed dosing in the European Union.
Patients and Methods: Combined rFIXFc data from the weekly and individualized interval prophylaxis arms of the B-LONG clinical trial, and N9-GP data from the 40 IU/kg once-weekly prophylaxis arm of PARADIGM 2 were used in a MAIC. Individual patient data for rFIXFc (n=87) were matched to aggregated data for N9-GP (n=29). Estimated annualized bleeding rates (ABRs) for rFIXFc were recalculated using a Poisson regression model with adjustment for over-dispersion, and compared with ABRs reported for N9-GP, using incidence rate ratios (IRRs) with 95% confidence interval (CI).
Results: There was no evidence of significant differences in estimated ABRs between prophylaxis with rFIXFc and N9-GP. Analysis of pooled rFIXFc weekly and interval-adjusted dosing compared with N9-GP 40 IU/kg once weekly produced estimated ABRs of 2.59 versus 2.51 (IRR 1.03; 95% CI 0.56– 1.89), as well as 1.34 versus 1.22 (IRR 1.10; 95% CI 0.42– 2.91) and 1.13 versus 1.29 (IRR 0.88; 95% CI 0.47– 1.63) for overall, spontaneous, and traumatic bleeding events, respectively.
Conclusion: The study did not reveal any significant differences in the efficacy of rFIXFc and N9-GP prophylaxis. Given differences in trough levels (rFIXFc dosing was targeted to achieve a trough 1– 3 IU/dL above baseline versus a reported estimated N9-GP mean trough of 27.3 IU/dL), interpreting plasma FIX levels as potential surrogate efficacy markers requires consideration of compound-specific pharmacokinetic profiles.
Keywords: annualized bleeding rate, factor IX deficiency, factor IX Fc fusion protein, nonacog beta pegol, plasma factor IX activity, treatment outcome
Introduction
For patients with hemophilia B, extended half-life (EHL) factor IX (FIX) products are available for both preventing (prophylaxis) and treating bleeds.1 These EHL agents display between 3- and 5-fold extension in mean terminal half-life compared with standard FIX products.2 Consequently, EHL FIX-based therapies offer the chance of prolonging dosing intervals between injections, to reduce treatment-related burden while improving bleed protection, thus satisfying most individual patients’ needs with favorable short- and long-term outcomes.3 This can benefit patients’ quality of life.4,5
Recombinant FIX (rFIX) Fc fusion protein (rFIXFc) is human coagulation FIX covalently linked to the Fc domain of human immunoglobulin G1.6 The molecule binds to the neonatal Fc receptor, which is expressed on many cell types throughout life, including endothelial cells lining the vasculature.7 The interaction between rFIXFc and the neonatal Fc receptor delays lysosomal degradation, enabling recycling back into the circulation and prolonging the product half-life.7 Another therapeutic alternative, nonacog beta pegol (N9-GP), is a human rFIX molecule with a 40 kDa polyethylene glycol (PEG) moiety attached to the activation peptide.8 PEGylation reduces the efficiency of various physiologic elimination processes thus favoring the persistence of the molecule in the bloodstream over prolonged time periods.9
The different methods used to extend the half-lives of these products affect their biodistribution. Under normal physiologic conditions the majority of FIX is present in the extravascular space.10 FIX binds to collagen IV in the subendothelial basement membrane, is in dynamic equilibrium with FIX in plasma, and may contribute to hemostasis from its extravascular location.10 Recent consensus recommendations on management of hemophilia B have highlighted the importance of considering the extravascular distribution of therapeutic products.11 As for unmodified, wild-type FIX,12 rFIXFc has a high volume of distribution,12 in line with an extravascular presence, which may be a consequence of the widespread occurrence of cells bearing Fc receptors.13 In contrast, likely as a result of conjugation with a PEG moiety,14 N9-GP has a low volume of distribution.8 This indicates that it is mainly limited to the plasma compartment.13 Consequently, N9-GP provides higher measurable FIX plasma levels than rFIXFc.13 Although FIX plasma levels are commonly used as a surrogate marker of efficacy in hemophilia B, there is considerable variability in inter-patient pharmacokinetic parameters.1,15 Levels of plasma FIX activity, which may differ between EHL FIX products, do not always correlate with clinical outcomes and assessment of clinical parameters should be used to help guide clinical decision-making.1,13
This study compares rFIXFc with N9-GP based on the licensed dosing for the two products in the European Union.6,8 Both products are approved for treatment and prophylaxis of bleeding in patients with hemophilia B, although use of N9-GP is restricted to patients aged 12 years and above.6,8 The recommended starting regimens for prophylaxis with rFIXFc are either 50 IU/kg once weekly (with the dose adjusted based on individual response), or 100 IU/kg once every 10 days (with the interval adjusted based on individual response) for those ≥12 years of age.6 More frequent or higher doses of rFIXFc may be required in younger individuals.6 When N9-GP is used for prophylaxis, 40 IU/kg once weekly is recommended.8 Adjustment of dosing and administration intervals of N9-GP may be considered based on FIX levels and individual bleeding tendency.8 Different dosing schedules necessarily affect consumption and therefore treatment costs. In addition to clinical outcomes, economic burden is a key aspect of hemophilia B treatment.16
There are no head-to-head trials directly comparing clinical outcomes in rFIXFc- and N9-GP-treated patients. Also, there are no studies with the same reference arms, precluding indirect comparison through a common comparator. In the absence of these, indirect comparison of trial data using methods for population-adjusted comparison of disconnected intervention, such as simulated treatment comparison17 or matching-adjusted indirect comparison (MAIC),18 can be used to assess relative efficacy. In the absence of studies with randomized comparisons against the same treatment or with common comparators, and as disconnected evidence was available, MAIC was identified as the most appropriate technique for this comparison. Using MAIC, the aim of this study was to indirectly compare the efficacy of rFIXFc and N9-GP using efficacy data from two pivotal Phase 3 clinical trials evaluating these products, B-LONG12 and PARADIGM 2,19 respectively.
Materials and Methods
Data Sources and Sample Selection
Data for the comparison of rFIXFc with N9-GP were obtained from the two pivotal, multicenter, Phase 3 trials, B-LONG (ClinicalTrials.gov identifier: NCT01027364) and PARADIGM 2 (ClinicalTrials.gov identifier: NCT01333111), respectively, which have been fully reported elsewhere.12,19 Both studies involved previously treated male patients (≥12 or ≥13 years of age in B-LONG and PARADIGM 2, respectively), with factor IX levels ≤2 IU/dL.
B-LONG was a non-randomized, open-label study in which 123 patients were enrolled and assigned to one of four rFIXFc treatment groups:12 weekly prophylaxis (n=63); individualized interval prophylaxis (n=29); on-demand (episodic) treatment (n=27); perioperative management (n=12). Patients assigned to weekly prophylaxis initially received rFIXFc 50 IU/kg, with the dose adjusted as needed. Those assigned to individualized interval prophylaxis initially received rFIXFc 100 IU/kg every 10 days, with the interval adjusted as needed. The dose or interval was adjusted, according to pharmacokinetic data, to maintain a trough level 1–3 IU/dL above baseline, or higher, if clinically necessary.
PARADIGM 2 was a randomized study in which 74 patients received N9-GP either as prophylaxis (fixed dose of 10 IU/kg once weekly [n=30] or 40 IU/kg once weekly [n=29]) or on-demand treatment (n=15).19 Pharmacokinetic assessments (at initiation and after 12 to 44 weeks of prophylaxis) and the estimation of predose FIX trough levels took place during the study.19
Based on the licensed dosing regimens for the two products, as described in the respective Summaries of Product Characteristics,6,8 the current analysis compares rFIXFc combined data from the weekly and individualized interval prophylaxis arms of B-LONG study with N9-GP data from the 40 IU/kg once-weekly prophylaxis arm of PARADIGM 2.
Outcome Assessment
The efficacy of prophylactic treatment with either rFIXFc or N9-GP was evaluated in terms of estimated annualized bleeding rates (ABRs), considered for any (ie, overall) spontaneous and traumatic bleeds.
Data Analysis
As the trials evaluating rFIXFc and N9-GP have included neither randomized comparisons against the same treatment nor evaluations involving common comparators, neither Bucher’s standard methodology for indirect comparison20 nor network meta-analysis21,22 were feasible for comparing the two products. Consequently, MAIC was used in accordance with methodologic guidelines for population-adjusted comparison issued by the National Institute for Health and Care Excellence Decision Support Unit (NICE DSU).17
MAIC is a method of choice for comparing disconnected evidence from different sources.17 It requires access to patient-level data from at least one of the sources.17 For this study, (anonymized) individual patient data from B-LONG were available.12 Aggregated data from the comparator source(s) were collated.17,18 In this case, the publicly available patient baseline characteristics and efficacy estimates, quantified in terms of ABR, from PARADIGM 219 were used.
When performing MAIC, to adjust the comparison for population differences across trials, the individual patient data are reweighted.17,18 Patients with characteristics closer to the aggregated data may be assigned a higher weight, such that the mean characteristics in the reweighted population match the aggregated baseline characteristics of the patients in the comparator trial(s).17 In this analysis participants receiving rFIXFc prophylaxis were assigned weights to balance between-trial differences for the following characteristics available for PARADIGM 2:19 age, body weight, the proportions of patients who had prior prophylaxis, white race and Asian ethnicity (which, as the most frequently recorded race/ethnicity classifications in PARADIGM 2, indirectly adjust for others), and the presence of target joints at baseline.
The effects of rFIXFc in the B-LONG study were then recalculated with the use of weights assigned in the previous step, thereby facilitating estimation of the efficacy of rFIXFc had it been administered to the patients in whom the efficacy of N9-GP 40 IU/kg once weekly was determined in PARADIGM 2. In B-LONG, ABRs had originally been determined using negative binomial regression,12 whereas, in PARADIGM 2 ABRs were estimated using a Poisson regression model, with dose as a factor.19 To ensure that the method of estimation was fully consistent with PARADIGM 2,19 ABRs from B-LONG were recalculated using a Poisson regression model accounting for over-dispersed data. The recalculated effects of rFIXFc were compared with the estimated ABRs reported for N9-GP. To facilitate this comparison, incidence rate ratios (IRRs) with 95% confidence interval (CI) were used: logarithmic values of the ABRs for rFIXFc and N9-GP were determined, the difference between these was calculated, and the exponent (power) of this difference was then assessed. If the 95% CI did not include 1.0, statistical significance was considered apparent. Statistical comparisons were performed using R software version 3.5.5 (https://www.r-project.org/).
Results
Patient Baseline Characteristics
Ninety-two patients were included in the weekly and individualized interval prophylaxis arms of the B-LONG study. Matching of baseline characteristics with the PARADIGM 2 trial was performed only for those who were included in the B-LONG efficacy analysis (n=87). Of the patients not included in the analysis, five patients were excluded as they did not receive adequate dosing to permit evaluation of rFIXFc prophylaxis, in the weekly prophylaxis arm, one patient did not receive rFIXFc and one received only one dose, and from the individualized interval prophylaxis arm, two received an alternative formulation of rFIXFc and one received only one dose of rFIXFc.12
Baseline characteristics of the 87 patients in the rFIXFc prophylaxis arms of the B-LONG study before and after matching with those for the 29 patients assigned to receive N9-GP 40 IU/kg once weekly in PARADIGM 2 are shown in Table 1. Treatment duration was not included in the MAIC evaluation. ABRs were determined over a median of 49.26 weeks in B-LONG and, as defined in the trial design, 52 weeks in PARADIGM 2, time periods may be regarded as a proxy for treatment duration.
 |
Table 1 Balance of Baseline Characteristics and Effective Sample Size Following Matching of Patients in the B-LONG12 and PARADIGM 219 Phase 3 Studies |
At baseline, patients receiving rFIXFc prophylaxis in the B-LONG study had a mean age of 32.8 versus 30.0 years, and a mean body weight of 76.0 versus 70.4 kg compared to individuals assigned prophylaxis with N9-GP 40 IU/kg once weekly in PARADIGM 2. White and Asian patients comprised 62.1% and 16.1%, respectively, of the B-LONG cohort. Corresponding values for PARADIGM 2 were 72.4% and 17.2%, respectively. A numerically lower proportion of the B-LONG (51.7%) than PARADIGM 2 patients had received prior prophylaxis (58.6%), and the proportions with target joints were 49.4% versus 51.7%, respectively.
After adjustment for demographic and disease-related factors, as well as history of treatment, the patient characteristics in both studies were well balanced, and in the current study the effective sample size (ESS) was 59, which corresponded to 68% of the overall population (Figure 1).
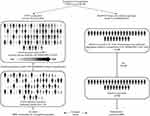 |
Figure 1 Matching-adjusted indirect comparison of prophylaxis with rFIXFc versus N9-GP, based on data from the B-LONG12 and PARADIGM 219 clinical trials, respectively. *Combined weekly and individualized groups. †N=92; five patients were excluded as they did not receive adequate dosing to permit evaluation of rFIXFc prophylaxis. Abbreviations: ABRs, annualized bleeding rates; N9-GP, nonacog beta pegol; rFIXFc, recombinant factor IX Fc fusion protein. |
Annualized Bleeding Rates
Population-adjusted comparison did not provide evidence of differences in estimated ABRs between prophylaxis with rFIXFc and N9-GP 40 IU/kg once-weekly prophylaxis. Analysis of pooled rFIXFc weekly and interval-adjusted dosing compared with N9-GP 40 IU/kg once weekly produced estimated ABRs of 2.59 versus 2.51 (IRR 1.03; 95% CI 0.56–1.89), as well as 1.34 versus 1.22 (IRR 1.10; 95% CI 0.42–2.91) and 1.13 versus 1.29 (IRR 0.88; 95% CI 0.47–1.63) for any, spontaneous, and traumatic bleeding events, respectively (Figure 2). Additional analyses considering rFIXFc weekly and interval-adjusted prophylaxis separately also showed ABRs to be comparable with N9-GP 40 IU/kg once weekly, with no statistically significant differences shown for IRRs (Supplementary Table 1).
 |
Figure 2 Comparison of estimated annualized bleeding rates for any, spontaneous and traumatic bleeds in patients receiving prophylaxis with rFIXFc versus N9-GP, after matching for baseline variables. Data compare the pooled arms for prophylaxis with rFIXFc (weekly and individualized regimens) in the B-LONG12 study, adjusted for age, weight, prior prophylaxis, White race, Asian ethnicity and the presence of target joints at baseline, to match those assigned weekly prophylaxis with N9-GP 40 IU/kg in PARADIGM 2.19 Abbreviations: ESS, effective sample size; CI, confidence interval; IRR, incidence rate ratio; N9-GP, nonacog beta pegol; rFIXFc, recombinant factor IX Fc fusion protein. |
Discussion
The results of the current indirect treatment comparison, which accounted for baseline differences in the age and weight of the patients in the compared studies, as well as race/ethnicity, any prior prophylaxis, and the presence of target joints, showed no significant difference in efficacy between prophylaxis with rFIXFc and N9-GP, administered as per the licensed dosing.6,8 Efficacy, evaluated in terms of the frequency of any, spontaneous, and traumatic bleeding, as measured by estimated by ABRs, was similar for the two products despite differences in trough levels. The target trough levels for rFIXFc prophylaxis were 1–3 IU/dL above baseline,12 whereas for the N9-GP prophylaxis the observed mean trough activity was estimated at 27.3 IU/dL.19 As the trough levels for the patients receiving rFIXFc prophylaxis were not a measured endpoint, a more direct comparison of trough levels achieved during the rFIXFc and N9-GP prophylaxis was not possible.
Previously reported product comparison via pharmacokinetic modeling23 suggests that N9-GP may be well described by a one-compartment model, with the drug remaining mostly in the plasma, while rFIXFc data fit a two- or three-compartment model. This is consistent with the differences in the volumes of distribution between the two products, and in line with rFIXFc exhibiting a similar vascular distribution to wild-type FIX, with an extravascular presence.14,23 While the extravascular contribution of rFIXFc remains to be definitively determined, our efficacy results show the value of using clinical outcomes in addition to plasma FIX activity to help guide clinical decision making. Plasma levels of FIX should be interpreted in the light of the pharmacokinetic characteristics of the therapeutic product used.
With an absence of direct, head-to-head, assessment of clinical outcomes, and as there are no randomized clinical trials in which rFIXFc and N9-GP have been evaluated against the same treatment, to compare the efficacy of these two products, a MAIC18 was performed. This is an established methodology that has been used for comparing other hemophilia treatments, both for hemophilia A24–29 and for hemophilia B,30 but it does have limitations. In the current analysis, the adjusted patient baseline characteristics across the two studies were perfectly matched. However, it is not possible to adjust for all differences, including for unpublished variables of potential relevance, and there is thus the possibility of residual confounding. This is a general feature of such analyses, but with appropriate adjustment the methodology can still be valid. Furthermore, reweighting necessarily decreases the ESS. An adequate sample size is required in the study from which the individual patient data are obtained. In the current study, with a range of key baseline variables reweighted, the ESS for the adjusted B-LONG patient population remained above the sample size of the PARADIGM 2 cohort used in the comparison (59 and 29, respectively). The ESS of 59 was 68% of the initial sample size after matching. This result was an acceptable loss of data with a relatively low risk of bias.17 Analysis of the patients receiving rFIXFc prophylaxis involved a pooled population of patients receiving both weekly and individualized interval prophylaxis arms. This sample was compared with receipt of N9-GP 40 IU/kg once-weekly prophylaxis. The treatment arms considered in the B-LONG and PARADIGM 2 studies may be regarded as reflective of the recommended dosing strategies for rFIXFc and N9-GP, respectively. Additional analyses considering rFIXFc weekly and interval-adjusted prophylaxis separately also did not reveal any significant differences compared with N9-GP 40 IU/kg once-weekly prophylaxis, although the rFIXFc ESS was necessarily smaller. This may raise some concerns regarding the statistical power of these comparisons. In addition, the current analysis restricted outcome assessment to ABR. It was not possible to compare ABR by previous treatment regimen as the relevant patient characteristics were not reported for PARADIGM 2. Beyond ABR, it would have been valuable to include additional outcomes, such as product consumption, although again, relevant data to facilitate this were not available.
Despite the limitations of the current analysis, this indirect treatment comparison provides useful information about the relative efficacy of prophylaxis with rFIXFc and N9-GP in patients with hemophilia B.
Conclusions
Population-adjusted comparison did not reveal any difference in the efficacy of rFIXFc prophylaxis, weekly/individualized interval, and N9-GP 40 IU/kg once-weekly prophylaxis, regarding the frequency of bleeding events assessed in terms of ABR. Interpretation of plasma FIX levels (1–3 IU/dL targeted with rFIXFc, and an estimated mean of 27.3 IU/dL measured with N9-GP) as potential surrogate markers of efficacy requires consideration of compound-specific pharmacokinetic profiles.
Abbreviations
ABR, annualized bleeding rate; CI, confidence interval; EHL, extended half-life; ESS, estimated sample size; FIX, factor IX; IRR, incidence rate ratio; MAIC, matching-adjusted indirect comparison; N9-GP, nonacog beta pegol; NICE DSU, National Institute for Health and Care Excellence Decision Support Unit; PEG, polyethylene glycol; rFIX, recombinant FIX; rFIXFc, recombinant FIX Fc fusion protein.
Compliance with Ethics Guidelines
Ethical approval was not required for this analysis as it was based on data from two previously published Phase 3 trials, B-LONG and PARADIGM 2. These studies had been performed in line with the Declaration of Helsinki and local regulations, with protocols approved by the authorities and the institutional review board/ethics committee at each participating site. In the original trials, participating patients, or their guardians, provided written informed consent. Further informed consent was not required as the current analysis used de-identified individualized patient-level data from B-LONG (with permission from the dataset owner) and anonymized, previously published data from PARADIGM 2 (with no direct contact with the dataset owner).
Acknowledgments
The project was funded by Sobi. Medical writing and editorial support, funded by Sobi, was provided by Andy Lockley, PhD and Hayley Owen, PhD, Bioscript Medical, Macclesfield, UK. Sobi and Sanofi reviewed and provided feedback on the manuscript.
Author Contributions
All authors made a significant contribution to the reported work: conception; study design and execution; data acquisition, analysis and interpretation; or all of these. The authors all participated in drafting, revising and critically reviewing the article, approving the final version to be published, having agreed on the journal to which the article has been submitted. The authors agree to be accountable for all aspects of the work.
Disclosure
M.E. Mancuso has acted as paid consultant/speaker and/or advisor for Bayer, Biomarin, CSL Behring, Grifols, Kedrion, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Sobi, Spark Therapeutics, Takeda and UniQure. D. Eriksson, A. Falk and Z. Hakimi are employees of Sobi. When the analyses described in this manuscript were performed, P. Wojciechowski and M. Wdowiak were both employees of Creativ-Ceutical, a consultancy company that received funding from Sobi for this research. R. Klamroth reports research funding and honoraria for consulting and lectures from Bayer, Biomarin, Biotest, CSL Behring, Chugai, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Sobi, Takeda/Shire and UniQure. The authors report no other conflicts of interest in this work.
References
1. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia. Haemophilia. 2020;26 Suppl 6:1–158.
2. Lambert T, Benson G, Dolan G, et al. Practical aspects of extended half-life products for the treatment of haemophilia. Ther Adv Hematol. 2018;9(9):295–308.
3. Castaman G. The benefits of prophylaxis in patients with hemophilia B. Expert Rev Hematol. 2018;11(8):673–683.
4. Astermark J, Hermans C, Ezzalfani M, et al. rFIXFc prophylaxis improves pain and levels of physical activity in haemophilia B: post hoc analysis of B-LONG using haemophilia-specific quality of life questionnaires. Haemophilia. 2022;28(1):18–26.
5. Chowdary P, Kearney S, Regnault A, Hoxer CS, Yee DL. Improvement in health-related quality of life in patients with haemophilia B treated with nonacog beta pegol, a new extended half-life recombinant FIX product. Haemophilia. 2016;22(4):e267–74.
6. European Medicines Agency. Alprolix® Summary of Product Characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/alprolix-epar-product-information_en.pdf.
7. Mancuso ME, Mannucci PM. Fc-fusion technology and recombinant FVIII and FIX in the management of the hemophilias. Drug Des Devel Ther. 2014;8:365–371.
8. European Medicines Agency. Refixia Summary of Product Characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/refixia-epar-product-information_en.pdf.
9. Hamidi M, Azadi A, Rafiei P. Pharmacokinetic consequences of pegylation. Drug Deliv. 2006;13(6):399–409.
10. Mann DM, Stafford KA, Poon MC, Matino D, Stafford DW. The Function of extravascular coagulation factor IX in haemostasis. Haemophilia. 2021;27(3):332–339.
11. Hart DP, Matino D, Astermark J, et al. International consensus recommendations on the management of people with haemophilia B. Ther Adv Hematol. 2022;13:20406207221085202.
12. Powell JS, Pasi KJ, Ragni MV, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313–2323.
13. Iorio A, Fischer K, Blanchette V, et al. Tailoring treatment of haemophilia B: accounting for the distribution and clearance of standard and extended half-life FIX concentrates. Thromb Haemost. 2017;117(6):1023–1030.
14. Preijers T, Bukkems L, van Spengler M, Leebeek F, Cnossen M, Mathôt R. In silico comparison of pharmacokinetic properties of three extended half-life factor IX concentrates. Eur J Clin Pharmacol. 2021;77(8):1193–1200.
15. Marchesini E, Morfini M, Valentino L. Recent Advances in the Treatment of Hemophilia: a Review. Biologics. 2021;15:221–235.
16. Li N, Sawyer EK, Maruszczyk K, et al. Adult lifetime cost of hemophilia B management in the US: payer and societal perspectives from a decision analytic model. J Med Econ. 2021;24(1):363–372.
17. Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ NICE DSU Technical Support Document 18: methods for population-adjusted indirect comparisons in submission to NICE. Available from: http://nicedsu.org.uk/wp-content/uploads/2017/05/Population-adjustment-TSD-FINAL.pdf.
18. Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947.
19. Collins PW, Young G, Knobe K, et al. Recombinant long-acting glycoPEGylated factor IX in hemophilia B: a multinational randomized phase 3 trial. Blood. 2014;124(26):3880–3886.
20. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691.
21. Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607–617.
22. Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. 2002;21(16):2313–2324.
23. Escuriola Ettingshausen C, Hegemann I, Simpson ML, et al. Favorable pharmacokinetics in hemophilia B for nonacog beta pegol versus recombinant factor IX-Fc fusion protein: a randomized trial. Res Pract Thromb Haemost. 2019;3(2):268–276.
24. Batt K, Gao W, Ayyagari R, et al. Matching-adjusted indirect comparisons of annualized bleeding rate and utilization of BAY 94-9027 versus three recombinant factor VIII agents for prophylaxis in patients with severe hemophilia A. J Blood Med. 2019;10:147–159.
25. Bonanad S, Núñez R, Poveda JL, et al. Matching-Adjusted Indirect Comparison of Efficacy and Consumption of rVIII-SingleChain Versus Two Recombinant FVIII Products Used for Prophylactic Treatment of Adults/Adolescents with Severe Haemophilia A. Adv Ther. 2021;38(9):4872–4884.
26. Hakimi Z, Santagostino E, Postma MJ, Nazir J. Recombinant FVIIIFc Versus BAY 94-9027 for Treatment of Patients with Haemophilia A: comparative Efficacy Using a Matching Adjusted Indirect Comparison. Adv Ther. 2021;38(2):1263–1274.
27. Klamroth R, Wojciechowski P, Aballéa S, et al. Efficacy of rFVIIIFc versus emicizumab for the treatment of patients with hemophilia A without inhibitors: matching-adjusted indirect comparison of A-LONG and HAVEN Trials. J Blood Med. 2021;12:115–122.
28. Pocoski J, Li N, Ayyagari R, et al. Matching-adjusted indirect comparisons of efficacy of BAY 81-8973 vs two recombinant factor VIII for the prophylactic treatment of severe hemophilia A. J Blood Med. 2016;7:129–137.
29. Vashi P, Batt K, Klamroth R, et al. Indirect Treatment Comparison of Damoctocog Alfa Pegol versus Turoctocog Alfa Pegol as Prophylactic Treatment in Patients with Hemophilia A. J Blood Med. 2021;12:935–943.
30. Astermark J, Wojciechowski P, Aballéa S, Hakimi Z, Nazir J, Klamroth R. Efficacy of rFIXFc versus rIX-FP for the Treatment of Patients with Hemophilia B: matching-Adjusted Indirect Comparison of B-LONG and PROLONG-9FP Trials. J Blood Med. 2021;12:613–621.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
