Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Expression of Hedgehog Signaling Pathway Proteins in Basal Cell Carcinoma: Clinicopathologic Study
Authors Deng LJ, Jia M, Luo SY, Li FZ, Fang S
Received 19 September 2022
Accepted for publication 21 October 2022
Published 2 November 2022 Volume 2022:15 Pages 2353—2361
DOI https://doi.org/10.2147/CCID.S389551
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Li-Jia Deng, Meng Jia, Si-Yu Luo, Feng-Zeng Li, Sheng Fang
Department of Dermatology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Correspondence: Sheng Fang, Department of Dermatology, The First Affiliated Hospital of Chongqing Medical University, No. 1, Youyi Road, Yuzhong District, Chongqing, 400016, People’s Republic of China, Email [email protected]
Objective: Basal cell carcinoma (BCC) is the most common type of cancer with a growing incidence rate over recent decades. The increasing economic burden and incidence of BCC have generated major interest in developing targeted therapies for this disease. The critical role of the Hedgehog (Hh) pathway in the pathogenesis of BCC has become evidently demonstrated. The purpose of this study was to observe the expression of PTCH1 and Gli1 in BCC and further evaluate their relationship with clinicopathological features.
Methods: This retrospective study included 84 patients with BCC. Information of 84 patients with pathologically diagnosed BCC (including location, sex, tumor size, pathological type, and depth of invasion) were collected, and tissue paraffin blocks were collected for immunohistochemical staining. Western blot analysis for PTCH1 and Gli1 were also performed. The staining intensity and percentage of stained cells were expressed as a histochemical score (HSCORE).
Results: PTCH1 and Gli1 were overexpressed in BCC compared with adjacent normal epidermis. Our study found that the expression of PTCH1 and Gli1 in BCC in exposed sites was significantly higher than in non-exposed sites. Moreover, no significant difference was observed in sex, Breslow thickness, tumor size or pathological type (P> 0.05).
Conclusion: PTCH1 and Gli1 were overexpressed in BCC. Higher PTCH1 and Gli1 expression were in exposed sites lesions. Our study suggests that UV radiation may be associated with aberrant activation of the Hh-PTCH1-Gli1 intercellular signaling pathway in BCC. The molecular mechanism of UV-related PTCH1 and Gli1 differential expression deserves more rigorous research in the future.
Keywords: basal cell carcinoma, Hh pathway, UV radiation, PTCH1, Gli1
Introduction
Basal cell carcinoma (BCC) is the most common skin malignancy, and its incidence has been steadily increasing, imposing a heavy burden on patients and healthcare systems.1,2 The pathological and clinical features of BCC are complex and diverse with basic histological types including superficial, nodular, infiltrating, and micronodular, etc.3
The pathogenesis of BCC has not been fully elucidated. BCC mostly occurs in sun-exposed areas such as the head and neck, followed by the trunk and extremities.4 But there is no consensus on the pathogenesis of BCC in non-exposed sites, suggesting that there may be differences in the pathogenesis between exposed and non-exposed sites.5 Among the molecular signals involved in the development of BCC, recent studies suggest that the activation of Hh signaling pathway may play a critical role.6 The Hh pathway critical components could be targeted by potential anti-basal cell carcinoma drugs.7 However, no study has clearly reported whether the expression of PTCH1 and the activation of downstream signaling pathways are consistent in different clinical features and pathological types of BCC.
To shed light on this issue, this study compared the expression of PTCH1 and Gli1 (signaling molecules downstream of the Hh signaling pathway) in different pathological and clinical features like the pathological types, depth of tumor invasion, tumor site, tumor size, and patient’s gender, aimed to further clarify the correlation between the activation of Hh signaling pathway and the clinicopathological characteristics and to provide suggestions for the clinical application of drugs targeting this signaling pathway.
Materials and Methods
This study was approved by the Medical Ethics Committee, the First Affiliated Hospital of Chongqing Medical University. Our analysis was a retrospective design using fully anonymized data, and thus, the IRB waived the requirement for informed consent. We conduct a retrospective analysis for a total of 84 paraffin-embedded BCC tissue samples provided by the department of dermatology of the First Affiliated Hospital of Chongqing Medical University in 2021. The samples represented BCC patients who underwent surgical resection between January 2016 and June 2021. We also collected clinicopathological data from these patients’ medical charts and medical records systems. The clinicopathological data of 84 patients with BCC were collected, including sex, age, location, size, Breslow thickness, and pathological classification. We divided the locations into exposed sites (head, face, neck, V-neck, outer forearms, dorsum of the hand, and lower legs) and non-exposed sites.
Immunohistochemistry
All the enrolled cases underwent routine treatment and paraffin embedding. Cut 4 μm micron sections of tumor tissue from the paraffin block, immerse the slides in xylene I for 15 minutes, then immerse in xylene II for dewaxing, and hydrate these sections in different ethanol concentration gradient solutions. Subsequently, these tissues were preheated in the sodium citrate epitope repair solution, then cooled to room temperature for 1 hour after being treated with microwave at medium high heat for 20 minutes. After washing with PBS (Triton X-100 washing solution) for 3×5 minutes. To counteract the endogenous peroxidase activity, the cells were blocked with 3% H2O2 for 10 min and washed in PBS for 3×5 minutes. The sections were blocked with 3% bovine serum albumin for 10 minutes. Then incubated with PTCH1, Gli-1 antibody (1:200 dilution, PTCH1:53715 / Gli1:217326; Abcam, Cambridge, UK) for 60 min, washed in PBS for 3 × After 5 min, sections were incubated with secondary antibodies for 40 min. And finally washed three times again. The sections were treated with DAB chromogenic agent for 3–5 min, counterstained with hematoxylin, and then dehydrated with alcohol to remove the tissue. The staining results were observed by a pathology scanner.
Western Blot
Tissue samples for Western blot analysis were frozen in liquid nitrogen soon after excision and stored at − 80°C until use. The samples include BCC and normal skin tissue. And Patients were offered informed consent documents before surgery. The cytoplasm of fresh tumor biopsy specimens was lysed with phosphatase inhibitor (Roche) in RIPA buffer to release the target proteins, which were separated into SDS-PAGE gels. The proteins in the gel were moved into nitrocellulose membranes and enclosed with 5% BSA in TBS-T (20 mM Tris, pH 7.6, 130 mM NaCl and 0.1% Tween 20) solution for 60 min. Then, incubate overnight with PTCH1 and Gli-1 antibodies (1:1000 dilution, PTCH1: 53715 / 0.1–0.5 μg/ mL concentration, Gli1: 217326; Abcam, Cambridge, UK). respectively. The secondary antibody was recovered after incubation and chemiluminescence developed. Signals were measured with an Odyssey fluorescence scanner (Li-cor Bioscience, Inc., Lincoln, NE, USA).
Evaluation of PTCH1 and Gli1 Expression
As a result, the HSCORE was calculated using the formula: HSCORE=∑Pi(i+1), where i is the staining intensity of 0,1,2,3(0, no staining; 1, light yellow; 2, dark yellow; 3, brown or dark brown), and Pi is the percentage of cells at each level ranging from 0% to 100% (0.00–1.00). Three senior dermatopathologists evaluated the staining intensity of tumor cells and the percentage of stained cells. 5 high magnification fields (×400) were randomly selected for each section, and the number of positive cells in 100 cells was counted in each field, and the average result was recorded as the percentage of positive cells. HSCORE ranged from a minimum of 0 in unstained cases to a maximum of 4.0 for the highest staining intensity of tumor cells in all cases. Due to abnormal distributions, data were presented as median(M) and interquartile range (p25, p75) to describe HSCORE.
Statistical Analysis
Statistical analysis was performed using SPSS for Windows software (version 25.0; SPSS Inc.). The expression of PTCH1 and Gli1 at different sites and gender were analyzed using the nonparametric Mann–Whitney. Kruskal–Wallis test was used to check if there were differences in protein expression between different pathological subtypes of BCC, as well as the Spearman rank correlation test was used to evaluate the degree of relationship in Breslow thickness, and tumor size. Correlations and differences were considered significant if the two-tailed p-value was <0.05. Conversely, if P>0.05, it was determined as having no statistical significance.
Results
General Data
The general data regarding the clinical and pathological characteristics of 84 cases of BCC are presented in Table 1. Among them, 45 cases (53.6%) were male and 39 cases (46.4%) were female, with no significant bias with respect to sex. The age of the patients ranged from 28 to 94 years, with a mean age of 68.5 years. BCC was most commonly found in exposed areas, predominately the face (47.6%), followed by the trunk (23.8%), head and neck (22.6%), and the limbs (6%). 60 cases (71.4%) were located in exposed sites (ES), while 24 cases (28.6%) were in non-exposed sites (NES). Most of the tumors were less than 1 cm in diameter (41 cases), about 30 cases were 1–3 cm in diameter, and 13 cases were larger than 3 cm in diameter. BCC could be classified as nodular (24 cases), superficial (24 cases), micronodular (10 cases), infiltrative (10 cases), adenoid (10 cases), and morpheaform (6 cases) in our research. In most cases, the depth of invasion of the tumor was no more than 4 mm.
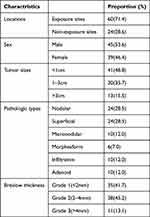 |
Table 1 Clinicopathological Features of Patients |
The Expression of PTCH1, Gli1 in BCC and Normal Epidermis
PTCH1 and Gli1 were positively expressed in BCC and normal skin epidermis, apocrine glands, eccrine glands, hair follicles, and sebaceous glands located in both the nucleus and cytoplasm. However, the expression of PTCH1and Gli1 was higher in BCC compared to adjacent normal skin. Differences in the expression of PTCH1 and Gli1 between the normal epidermis (HSCORE=1.21 (1.07, 2.80) and HSORE=2.07 (1.56, 2.34) respectively) and BCC (HSCORE=2.35 (1.97, 2.77) and HSCORE=2.78 (2.13, 3.41) respectively) were remarkable (P<0.001).
The Expression of PTCH1 in Different Clinicopathological Features
The correlations between PTCH1 immunostaining and tumor clinicopathological features (tumor location, pathological variants, sex, size, and Breslow thickness) are summarized in Table 2. In different clinical variants, lesions of 60 cases of BCC were located on exposed sites, while the lesions of 24 cases were located on non-exposed sites. We found that PTCH1 expression was increased in exposed sites (HSCORE =2.58 (2.37, 3.56)) compared with non-exposed sites (HSCORE =1.56 (1.46, 2.40)) (P <0.001)(Figure 1). These findings were in accordance with the results of Western blot analysis (Figure 2). Kruskal–Wallis test was used to show the difference in expression of BCC in the five different pathological types. The relative expression levels of PTCH1 in BCC were not significantly correlated with the pathological variants (P>0.05), but HSCORE values for superficial BCC (HSCORE =2.61 (2.14, 3.39)) were relatively higher. There was no statistically significant correlation between PTCH1 expression and Breslow thickness, sex or tumor size (P>0.05).
 |
Table 2 PTCH1 Expression in Different Clinicopathological Features of Basal Cell Carcinoma |
The Expression of Gli1 in Different Clinicopathological Features
Glioma-associated oncogene 1 (Gli1) acts as a target and mediator of the hedgehog pathway. We next evaluated the level of Gli1 expression in different clinicopathological features including pathological types, Breslow thickness, tumor site and tumor size (Table 3). The results showed that, similar to the expression of PTCH1, there was no significant difference in the expression of Gli1 among different pathological types and Breslow thicknesses (P > 0.05). Gli1 was expressed in the cytoplasm and nucleus of all the studied cases, and the expression in the exposed site cases (HSORE=2.82 (2.37, 3.52)) was higher than that in the non-exposed site (HSORE=2.05 (1.62, 2.49)) with statistical significance. (P < 0.01) (Figure 3). These findings were in accordance with the results of Western blot analysis (Figure 2). Similar to PTCH1, there was no significant correlation between BCC tumor size, patient sex, and Gli1 expression.
 |
Table 3 Gli1 Expression in Different Clinicopathological Features of Basal Cell Carcinoma |
Discussion
BCC is the most common keratinocyte carcinoma worldwide and one of the most common non-melanoma skin tumors.8 Although BCC rarely metastasizes, it can destroy local tissue and recur.9 It occurs mainly due to the accumulation of ultraviolet radiation in sunlight.10–12 Previous studies have found that almost all BCCs are constitutively activated by the Hedgehog (Hh) signaling pathway, whereas intermittent UV radiation plays a dominant role in the carcinogenesis of BCC.13 The Hh/Gli signaling pathway was originally discovered in Drosophila. This pathway consists of a series of ligands (Shh, Ihh and Dhh), transmembrane receptors (PTCH1 and PTCH2), transcription factors (Gli1-3) and signaling regulators (SMO, etc.).14 Previous studies have shown that aberrant activation of the Hh signaling pathway leads to a variety of tumors, such as small cell lung cancer, gastric cancer, upper gastrointestinal cancer, pancreatic cancer, and prostate cancer.15 More than 75% of BCC cases may be accompanied by mutations in PTCH1.16 This suggests that the Hh signaling pathway plays an important role in the pathogenesis of human malignancies. In this study, we evaluated PTCH1 and Gli1 expression in BCC; furthermore, we compared PTCH1 and Gli1 expression in BCC with different clinicopathological features.
Our study found that the expression of PTCH1 and Gli1 in BCC in exposed sites was significantly higher than in non-exposed sites, which suggests that UV radiation may be involved in the abnormal activation of the Hh-PTCH1-Gli1 intercellular signaling pathway. Previous studies have shown that abnormal activation of the Hh signaling pathway can lead to skin photoaging, while inhibition of the Hh signaling pathway can significantly reduce experimental photoaging, also demonstrating a close relationship between UV and Hh signaling pathways.17 Inhibition of smoothened signaling has also been found to prevent UV induced basal cell carcinoma by regulating Fas expression and apoptosis.18 Sporadic PTCH1 mutations have been described in BCCs from otherwise normal individuals, some of which are UV-mutations with CT or CCTT changes.19 Wanyeon Kim’s research suggests that Hh ligands are up-regulated by UV irradiation, resulting in increased expression of Gli1 and Gli2, which affects MAPK (p38, ERK, and JNK) activation and inflammatory responses (upregulation of COX-2, IL-1b, IL-6, and TNF-a).17 Whole-exome gene sequencing analyzed mutations in BCCs and revealed PTCH1 mutation as a very important dysfunctional mutation.20 PTCH1 is a tumor suppressor gene located in the cell membrane and plays an important role in promoting cell differentiation and proliferation. Functional mutation of PTCH1 eliminates the deterrent effect on the subsequent molecule SMO, which triggers the Hh signaling pathway.21 Binding of PTCH1 to its ligand (Shh) releases SMO, leading to the initiation of a signal transduction cascade and subsequent activation of the transcription factor Gli1 to facilitate Gli1 localization in the nucleus.22 There is a feedback loop between PTCH1 and Gli1, and increased Gli1 expression further enhances PTCH1 mRNA encoding non-functional PTCH1 protein upon activation of the pathway,23 which may initially explain the upregulation of PTCH1 as an oncogene in BCC lesions.
In terms of depth of invasion of the tumor, Vornicescu et al believe that Breslow thickness>2mm proved to be predictive for recurrence, in order to explore the significance of Gli1 predicting recurrence in BCC, by comparing the Gli1 immunohistochemical staining intensity of 8 recurrences and 38 non-recurrent BCC, the result shows that Gli1 has little impact on predicting tumor recurrence.24 Our study also showed that PTCH1 and Gli1 expression did not correlate with Breslow thickness. Although Gli1 is strongly positive in BCC, there is no statistical difference in expression in different pathological types. Kim et al used real-time PCR and immunohistochemical analysis to show that Gli1 molecule is highly expressed in BCC, and there is no difference in expression between different pathological types, which is also consistent with our results.25 Also, Almomani et al found that the correlation between low-risk or high-risk BCC subtypes and the PTCH1 expression level was not statistically significant.26 We found that the intensity of IHC staining for PTCH1 was higher in superficial BCC than other subtypes, although this difference was not significant. We speculate that this may be related to the anatomical location of superficial BCC, where more superficial tumors have a higher mutational burden or are more likely to accumulate UV-associated mutations. These differences suggest that the pathophysiology of BCC may vary between anatomical sites and histological subtypes. Besides, this study also showed that PTCH1 and Gli1 expression did not correlate with gender and tumor size.
Surgery is not always the first choice of treatment for patients with advanced BCC, such as those with recurrence and metastasis. Currently, drugs that inhibit the Hh signaling pathway inhibition (HPI) are now available in advanced BCC patients, such as vismodegib and sonidegib, which act as SMO inhibitors to block Gli1 under partial activation of the canonical pathway, thereby inhibiting tumor evolution.27 In contrast, while SMO inhibitors are not highly effective in clinical observations, recent studies in colorectal cancer have shown that suppression of Gli1, which is activated by a non-canonical pathway (KRAS-BRAF), is more sensitive to decrease cellular proliferation and induce apoptosis.28 Gli1 is currently considered the primary effector of Hh signaling. Our study showed that Gli1 expression was significantly elevated in BCC, especially in exposed sites, compared with normal epidermis indicating its potential for use as a therapeutic target for this disease.
The limitations of our study can be summarized as follows. The small samples size of this study may lead to unavoidable bias. Since this study was retrospective, accurate patient prognostic information could not be obtained. Further experimental evidence is needed to confirm the underlying mechanism by which UV radiation activates the Hh signaling pathway leading to BCC.
In conclusion, we examined the level of PTCH1 and Gli1 expression in BCC and evaluated the relationship between the clinicopathological characteristics. Our study demonstrated that PTCH1 and Gli1 were overexpressed in BCC and that higher expression is associated with exposed site lesions. No significant difference in PTCH1 and Gli1 expression were observed in cases with different Breslow thickness, sex, age or pathological type. Our study may help in the development of targeted and immunotherapy treatments against PTCH1 and Gli1 according to different clinical and pathological conditions.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Statement of Ethics
Ethical approval was given by the Medical Ethics Committee, the First Affiliated Hospital of Chongqing Medical University (2022-K424). This study was conducted in accordance with the Declaration of Helsinki.
Funding
Chongqing medical scientific research project (Joint project of Chongqing Health Commission and Science and Technology Bureau), [2022QNXM014].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Saldanha G, Fletcher A, Slater D. Basal cell carcinoma: a dermatopathological and molecular biological update. Br J Dermatol. 2003;148(2):195–202. doi:10.1046/j.1365-2133.2003.05151.x
2. Lang BM, Balermpas P, Bauer A, et al. S2k guidelines for cutaneous basal cell carcinoma–part 2: treatment, prevention and follow-up. JDDG. 2019;17(2):214–230.
3. Arits A, Schlangen M, Nelemans P, Kelleners-Smeets N. Trends in the incidence of basal cell carcinoma by histopathological subtype. J Eur Acad Dermatol Venereol. 2011;25(5):565–569. doi:10.1111/j.1468-3083.2010.03839.x
4. Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147(1):41–47. doi:10.1046/j.1365-2133.2002.04804.x
5. Takahashi H. Non-ulcerative basal cell carcinoma: arising on the genitalia. J Dermatol. 2000;27(12):798–801. doi:10.1111/j.1346-8138.2000.tb02285.x
6. Ju S, Fan W, Rokohl AC, Guo Y, Kakkassery V, Heindl LM. Genetic factors underlying basal cell carcinoma risk: a narrative review. Front Oral Maxillofac Med. 2021. doi:10.21037/fomm-21-70
7. Choudhury MIM, Shabnam N, Ahsan T, et al. Cutaneous malignancy due to arsenicosis in Bangladesh: 12-year study in tertiary level hospital. Biomed Res Int. 2018;2018:4678362. doi:10.1155/2018/4678362
8. Riihilä P, Nissinen L, Kähäri VM. Matrix metalloproteinases in keratinocyte carcinomas. Exp Dermatol. 2021;30(1):50–61. doi:10.1111/exd.14183
9. Correia de Sa TR, Silva R, Lopes JM. Basal cell carcinoma of the skin (part 2): diagnosis, prognosis and management. Future Oncol. 2015;11(22):3023–3038. doi:10.2217/fon.15.245
10. Pfister H, Schegget JT. Role of HPV in cutaneous premalignant and malignant tumors. Clin Dermatol. 1997;15(3):335–347. doi:10.1016/S0738-081X(96)00162-9
11. Stern SR, Preston M. Nonmelanoma cancers of the skin. N Engl J Med. 1992;327(23):1649–1662. doi:10.1056/NEJM199212033272307
12. Wollenberg A, Peter R, Przybilla B. Multiple superficial basal cell carcinomas (basalomatosis) following cobalt irradiation. Br J Dermatol. 1995;133(4):644–646. doi:10.1111/j.1365-2133.1995.tb02722.x
13. Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303–317. doi:10.1016/j.jaad.2018.03.060
14. Armas-López L, Zúñiga J, Arrieta O, Ávila-Moreno F. The hedgehog-GLI pathway in embryonic development and cancer: implications for pulmonary oncology therapy. Oncotarget. 2017;8(36):60684–60703. doi:10.18632/oncotarget.19527
15. Evangelista M, Tian H, de Sauvage FJ. The hedgehog signaling pathway in cancer. Clin Cancer Res. 2006;12(20 Pt 1):5924–5928. doi:10.1158/1078-0432.CCR-06-1736
16. Harwood CA, Attard NR, O’Donovan P, et al. PTCH mutations in basal cell carcinomas from azathioprine-treated organ transplant recipients. Br J Cancer. 2008;99(8):1276–1284. doi:10.1038/sj.bjc.6604665
17. Kim W, Kim E, Yang HJ, et al. Inhibition of hedgehog signalling attenuates UVB-induced skin photoageing. Exp Dermatol. 2015;24(8):611–617. doi:10.1111/exd.12735
18. Athar M, Li C, Tang X, et al. Inhibition of smoothened signaling prevents ultraviolet B-induced basal cell carcinomas through regulation of fas expression and apoptosis. Cancer Res. 2004;64(20):7545–7552. doi:10.1158/0008-5472.CAN-04-1393
19. Ehrhart JC, Gosselet FP, Culerrier RM, Sarasin A. UVB-induced mutations in human key gatekeeper genes governing signalling pathways and consequences for skin tumourigenesis. Photochem Photobiol Sci. 2003;2(8):825–834. doi:10.1039/b302281a
20. Jayaraman SS, Rayhan DJ, Hazany S, Kolodney MS. Mutational landscape of basal cell carcinomas by whole-exome sequencing. J Invest Dermatol. 2014;134(1):213–220. doi:10.1038/jid.2013.276
21. Athar M, Li C, Kim AL, Spiegelman VS, Bickers DR. Sonic hedgehog signaling in basal cell nevus syndrome. Cancer Res. 2014;74(18):4967–4975. doi:10.1158/0008-5472.CAN-14-1666
22. Sigafoos AN, Paradise BD, Fernandez-Zapico ME. Hedgehog/GLI signaling pathway: transduction, regulation, and implications for disease. Cancers. 2021;13(14):3410. doi:10.3390/cancers13143410
23. Bonifas JM, Epstein EH Jr, Pennypacker S, et al. Activation of expression of hedgehog target genes in basal cell carcinomas. J Invest Dermatol. 2001;116(5):739–742. doi:10.1046/j.1523-1747.2001.01315.x
24. Vornicescu C, Șenilă SC, Bejinariu NI, et al. Predictive factors for the recurrence of surgically excised basal cell carcinomas: a retrospective clinical and immunopathological pilot study. Exp Ther Med. 2021;22(5):1–10. doi:10.3892/etm.2021.10771
25. Kim HS, Kim YS, Lee C, Shin MS, Kim JW, Jang BG. Expression profile of sonic hedgehog signaling-related molecules in basal cell carcinoma. PLoS One. 2019;14(11):e0225511. doi:10.1371/journal.pone.0225511
26. Almomani R, Khanfar M, Bodoor K, et al. Evaluation of patched-1 protein expression level in low risk and high risk basal cell carcinoma subtypes. Asian Pac J Cancer Prev. 2019;20(9):2851–2857. doi:10.31557/APJCP.2019.20.9.2851
27. Zhu H, Lewis DJ. Genetic alterations conferring resistance to hedgehog inhibitors in basal cell carcinoma. Expert Opin Drug Saf. 2022;21(4):581–582. doi:10.1080/14740338.2022.2037884
28. Schneider P, Bayo-Fina JM, Singh R, et al. Identification of a novel actin-dependent signal transducing module allows for the targeted degradation of GLI1. Nat Commun. 2015;6:8023. doi:10.1038/ncomms9023
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



