Back to Journals » Journal of Blood Medicine » Volume 14
Flow Cytometric Enumeration of Peripheral Blood CD34+ Cells Predicts Bone Marrow Pathology in Patients with Less Than 1% Blasts by Manual Count
Authors Jelic TM, Estalilla OC, Vos JA, Harvey G, Stricker CJ, Adelanwa AO, Khalid AA, Plata MJ
Received 16 April 2023
Accepted for publication 6 September 2023
Published 21 September 2023 Volume 2023:14 Pages 519—535
DOI https://doi.org/10.2147/JBM.S417432
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Tomislav M Jelic,1 Oscar C Estalilla,1 Jeffrey A Vos,2 Gary Harvey,1 Caitlin J Stricker,1 Ayodele O Adelanwa,2 Ahmed A Khalid,3 Milton J Plata1
1Department of Pathology and Laboratory Medicine, Charleston Area Medical Center, Charleston, WV, USA; 2Department of Pathology, Anatomy and Laboratory Medicine, West Virginia University School of Medicine, Morgantown, WV, USA; 3Cancer Center, Charleston Area Medical Center, Charleston, WV, USA
Correspondence: Tomislav M Jelic, Charleston Area Medical Center, Department of Pathology and Laboratory Medicine, 3200 MacCorkle Avenue, Charleston, WV, 25304, USA, Tel +1 304 546 6714, Email [email protected]
Background and Aims: Significance of absolute number of CD34+ cells in the peripheral blood of patients with less than 1% myeloblasts by manual differential count is unknown and our aim is to study its relevance in clinical practice.
Methods: We studied 138 peripheral bloods flow cytometric analyses in patients with less than 1% myeloblasts by manual differential, when CD34+ events were present in the gate that encompassed lymphocytes, monocytes, stem cells, and blasts.
Results: The average absolute number of CD34+cells in the peripheral blood was 11 CD34+cells/μL ranging from less than 1 cell/μL to 147 cells/μL. The average absolute number of CD34+ cells in patients with an abnormal expansive process involving bone marrow (metastases, myelodysplasia, granulomas, marrow infections) or if bone marrow biopsy not performed, presumed expansive marrow process was 25 cells/μL, and in patients without an expansive marrow process (or presumed negative) was 4 cells/μL (P< 0.00007). Cutoff 12 CD34+ cells/μL had 93% positive predictive value for bone marrow involvement by an expansive process and 78% negative predictive value.
Conclusion: Flow cytometric testing of the peripheral blood is extremely sensitive method for enumerating CD34+ cells and can detect fewer than one CD34+ cell/μL. The absolute number of CD34+ cells in the peripheral blood is a useful parameter in determining marrow involvement by an expansive process and may provide guidance with respect to the necessity for bone marrow biopsy.
Keywords: flow cytometry, CD34 positive cells, peripheral blood, myeloblasts, bone marrow biopsy
Introduction
Although numerous advances in characterization of myeloid neoplasms largely derived from gene expression analysis and next generation sequencing are now available, the percentage of myeloblasts in the bone marrow and peripheral blood remains the most important parameter in diagnosing acute myeloid leukemia, myelodysplastic syndrome, and myeloproliferative neoplasms.1 When myeloblasts are detected by microscopic examination of the peripheral blood smear, a subsequent bone marrow study is typically necessary to determine the significance of this finding. Microscopic examination of the peripheral blood with a differential count of 100 white blood cells, as a method of detecting blasts, is of limited accuracy and sensitivity. For example, one percent myeloblasts in a patient with a white blood cell count of 7,000 WBC/µL (white blood cells/microliter of blood) indicates that there are already 70 myeloblasts/µL in the peripheral blood. The sensitivity at such low percentages may only be improved upon by counting increasing numbers of cells. Flow cytometric analysis of peripheral blood usually counts 20,000 leukocytes (ie, events) and is thus about 200 times more sensitive than manual counting for detecting the low-level presence of myeloblasts in the peripheral blood. Enumeration of the absolute number of CD34+ cells in the peripheral blood by flow cytometry has been performed for more than 28 years.2 Basal levels of CD34+ cells in peripheral blood differ between individuals and are typically stable for 18 months.3 The CD34+ cells, which most frequently represent myeloblasts co-expressing CD34 and CD33, are present in the circulation in about one-tenth the concentration seen in the bone marrow (0.2% vs 1.8%).4 According to the medical literature3,5 and our study, flow cytometric analysis of the peripheral blood can accurately detect and enumerate even less than one myeloblast/µL.
At the time of this study, there are no reports in the medical literature concerning the absolute number of CD34+ cells in the peripheral blood in patients with various peripheral blood hematological abnormalities and with less than 1% myeloblasts obtained by manual count on glass slide smear. Our study determined the significance of enumerating these low-level myeloblast percentages in the peripheral blood by comparing them to the bone marrow pathology. We found that the absolute number of CD34+ cells (as well as percentage) determined from these flow cytometry studies of peripheral blood, may provide a reasonable guide in determining the need for bone marrow biopsy.
Materials and Methods
Flow Cytometry
We analyzed 138 peripheral blood flow cytometric studies (133 patients, 62 women and 71 men) in whom CD34+ cells were recorded in a mononuclear gate that encompassed lymphocytes, monocytes, stem cells, and blasts on right angle scatter/forward scatter dot plot. Institutional Review Board approval for this study was obtained. Inclusion criterium was the presence of CD34+ events (cells) forming distinct cloud or cluster of bright dots comprising at least 0.1% of events on CD34/CD19 dot plot in a mononuclear gate. Patients in whom such CD34+ events were not detected were excluded from the study.
The lowest percentage of CD34+ cells in the targeted gate that our Beckman Coulter Navios flow cytometer was able to detect was 0.1%. Peripheral blood for flow cytometric study was collected in citric acid medium. Forward-light scatter versus side scatter (FSC vs SSC) gating strategy was used to select region that encompasses all mononuclear leukocytes (Figure 1) including stem cells, lymphocytes, blasts, and monocytes.6 This gate ensured that blasts and other progenitor cells that vary in size from small lymphocytes to monocytes would be included and that nonviable cells and residual nonlysed erythrocytes will be excluded. The absolute number of CD34+ cells in the peripheral blood was then obtained by using the same calculation sequence (formula) as is typically employed to determine the absolute number of T-lymphocytes in human immunodeficiency virus (HIV) infected patients.7 Multiparameter (six-color) flow cytometric analysis of the peripheral blood was carried out on the Beckman Coulter Navios flow cytometer. The CD34 antibody was conjugated with the allophycocyanin (APC) fluorochrome and CD34 positive events were regarded as real only when they formed a cloud or cluster of dots in the mononuclear gate and if their percentage was at least 0.1% (Figure 1). Scattered CD34+ dots were regarded as nonspecific staining (dust-like artifacts) and such finding was regarded as negative for the presence of CD34+ cells and excluded from the study. The CD1a+ (coupled with APC fluorochrome) events in the targeted gate were also examined in all patients to further exclude artifacts of nonmyeloblast/stem cell events. All patients included in our study were required to show essentially no CD1a+ co-expressing events. This selection process was used, rather than attempting to subtract CD1a+ cells from CD34+ cells, to prevent the introduction of false positive data. Patients with T-lymphoblastic leukemia (T-ALL) were excluded from our study because they all had more than 1% blasts by manual count on the peripheral blood smear. Patients with other types of acute leukemia were also excluded because they all had at least 1% of blasts in the peripheral blood at the time when flow cytometric analysis of the peripheral blood was performed. Our panel for flow cytometric study of the peripheral blood used markers with fluorochromes as follows: Tube 1: Isotype IgG1 (FITC fluorochrome), Isotype IgG1 (Pe fluorochrome), isotype IgG1 (ECD fluorochrome), isotype IgG1 (Pc5.5 fluorochrome), CD45 (Pc7 fluorochrome), isotope IgG1 (APC fluorochrome); Tube 2: CD5 (FITC fluorochrome), CD10 (Pe fluorochrome), CD19 (ECD fluorochrome), CD20 (Pc5.5 fluorochrome), CD45 (Pc7 fluorochrome), CD22 (APC fluorochrome); Tube 3: CD Kappa (FITC fluorochrome), CD Lambda (Pe fluorochrome), CD19 (ECD fluorochrome), CD20 (Pc 5.5 fluorochrome), CD45 (Pc7 fluorochrome), CD34 (APC fluorochrome); Tube 4: CD33 (FITC fluorochrome), CD10 (Pe fluorochrome), CD13 (ECD fluorochrome), CD117 (Pc5.5 fluorochrome), CD45 (Pc7 fluorochrome), CD34 (APC fluorochrome); Tube 5: CD7 (FITC fluorochrome), CD15 (Pe fluorochrome), CD14 (ECD fluorochrome), CD HLA-DR (Pc 5.5 fluorochrome), CD45 (Pc7 fluorochrome), CD1a (APC fluorochrome), Tube 6: CD8 (FITC fluorochrome), CD4 (Pe fluorochrome), CD2 (ECD fluorochrome), CD56 (Pc5.5 fluorochrome), CD45 (Pc7 fluorochrome), CD3 (APC fluorochrome). All of these antibody (CD) markers are ASRs (analyte specific reagents) manufactured by Beckman Coulter. Analysis was performed by Beckman Coulter Navios software.
Peripheral Smear Evaluation and Complete Blood Count (CBC) Analysis
Peripheral blood smears were stained by standard Wright/Giemsa stain. A manual differential count on 100 WBC was performed by either of two hematopathologists (OCE, TMJ) with the Olympus microscope at 600× magnification. The percentage of cells was compared to that obtained by automated hematology analyzer (Beckman Coulter DxH 900) and if discrepancy was present, recounting was performed.
Whole blood was collected in EDTA medium and CBC analysis was performed by automated hematology analyzer (Beckman Coulter DxH 900). The leukocyte count obtained from this analysis was subsequently used in the calculation of circulating CD34+ cells.
Enumeration of CD34+ Cells
The absolute number of CD34+ cells was calculated by utilizing a dual platform-based method consisting of data obtained from the flow cytometry study, number of leukocytes in the peripheral blood determined by CBC analysis, and sum of the percentage of lymphocytes and monocytes obtained by a manual differential count. The calculation method is presented here first by mathematical equation and then in detail step by step. Absolute number of CD34+ cells = (absolute number of mononuclear cells) × (percentage of CD34+ cells in mononuclear gate)/100.
For patient number (#) 42 in Table 1, Figure 1, a 62-year-old-man with presumed marrow involvement by mantle cell lymphoma, the absolute number of CD34+ cells in the peripheral blood was obtained as follows: a manual differential count yielded: 69% neutrophils, 2% bands, 2% eosinophils, 1% LGL (large granular lymphocytes), 20% small lymphocytes, and 6% monocytes. The sum of mononuclear cells (percentage of lymphocytes + percentage of monocytes) was 27% and with 13,600 WBC/µL, it yielded 3672 (lymphocytes + monocytes)/µL. Flow cytometric study showed that in the lymphocytic-monocytic gate (mononuclear gate) the percentage of CD34+ cells was 1.1% (Figure 1). This means that 1.1% of 3672 is the absolute number of CD34+ cells in the peripheral blood or 40.392 cells/µL (1.1 × 3672/100) or 40 CD34+ cells/µL. The percentage of CD34+ cells in the peripheral blood is 0.297% of WBC (40.392 × 100/13,600).
 |
Table 1 Patients with Bone Marrow Involvement or Presumed Involvement by an Expansive Process (Excluding Simple Hyperplasia) |
The comprehensive clinical and laboratory data of 138 studies are presented in Table 1 and Table 2. Table 1 lists data from 48 patients (49 studies) with bone marrow involvement or presumed involvement by an expansive process (nonreactive), while Table 2 lists data from 85 patients (89 studies) in which marrow is not or supposedly not involved by an expansive process.
 |
Table 2 Patients with No Bone Marrow Involvement or Presumed Uninvolvement by an Expansive Process (Excluding Simple Hyperplasia) |
For one patient flow cytometric study of the peripheral blood was performed on three occasions (Table 2 #46, #47, #67), and in three patients on two occasions each (#56, #57) and (#61, #72) Table 2 and in Table 1 (#5, #12).
Bone Marrow Evaluation and Clinical Information
Clinical and demographic information for each patient in this study, to include age, gender, and clinical diagnosis, was obtained via review of the electronic health record. In addition, the bone marrow pathology was reviewed, when available, as well as the corresponding cytogenetic data. Based on the sum of the clinicopathologic data, patients were classified into two groups as having either “expansive” or “nonexpansive” bone marrow process. In our study, expansive marrow process, refers to marrow involvement by an expansive abnormal pathological process such as metastases, lymphoma, leukemia, myelodysplasia, myeloproliferative processes, sarcoidosis, granulomatous, and inflammatory processes secondary to marrow involvement by infection. Myeloid hyperplasia due to pneumonia, erythroid hyperplasia due to B12 deficiency, or megakaryocytic hyperplasia in immune thrombocytopenia are not considered expansive marrow processes (atypically expansive) by our definition.
Statistical Analysis
Student’s t-test function for independent samples was used for statistical analysis. Relationships demonstrating a P-value of 0.05 or less were considered statistically significant. Positive predictive value was calculated according to standard formula:
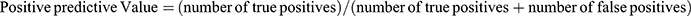 . Negative predictive value was calculated according to standard formula:
. Negative predictive value was calculated according to standard formula:
Results
Detailed characteristics of the patients, their diagnoses, hematological parameters, and results of flow cytometric studies are presented in Table 1 and Table 2. The essential findings are as follows: the average absolute number of CD34+ cells in the peripheral blood in patients in whom these cells were detectable (138 flow cytometric studies) by flow cytometric method was 11 CD34+ cells/µL varying in a wide range (standard deviation 17.264) from less than 1 cell/µL to 147 cells/µL. The average absolute number of CD34+ cells in patients (49 flow cytometric studies) with an expansive (or presumed expansive) process (neoplastic or inflammatory) in the marrow was 25 cells/µL, and in patients (89 flow cytometric studies) without an expansive marrow process (or presumed negative) was 4 cells/µL (P<0.00007). The average percentage of CD34+cells was 0.098% of leukocytes ranging from 0.01% to 0.715%. The average percentage of CD34+ cells in patients with bone marrow involved with expansive process or presumably involved was 0.171% and in patients with marrow not involved (or presumably not involved) was 0.057% (P<0.00008).
The lowest absolute number of CD34+ cells in our study was 0.38 cells/µL or 38 CD34+ cells per 100 µL of blood or 0.021% of leukocytes. The patient was a 60-year-old woman with pancytopenia and 1,800 leukocytes/µL (patient #1 Table 2). The highest absolute number was 147 CD34+ cells/µL or 0.705% of leukocytes. The patient was an 83-year-old man with primary myelofibrosis (JAK-2 positive) and 20,800 leukocytes/µL (#49 Table 1). The lowest percentage of CD34+ cells was 0.01% of peripheral blood leukocytes. The patient was a 59-year-old man with less than 1 CD34+ cell/µL who had had stem cell transplantation five years earlier because of precursor B cell acute lymphoblastic leukemia (#3 Table 2). The highest percentage was 0.715%, in an 88-year-old-woman with non-Hodgkin lymphoma (#37 Table 1). In a 26/27-year-old woman with hidradenitis suppurativa, flow cytometric study of the peripheral blood was performed three times in a 2-year period (studies #46, #47, and #67 Table 2). The absolute numbers of CD34+ cells were 3 cells/µL, 3 cells/µL, and 5 cells/µL, respectively. In a 65/67-year-old woman with microcytic erythrocytes and minimal lymphocytosis, flow cytometric analysis of the peripheral blood documented four CD34+ cells/µL on both occasions in a two-year period (studies #56 and #57 Table 2). In a 75/76-year-old woman with refractory anemia with excess blasts-2 and monosomy-7 (studies #5 and #12, Table 1), flow cytometric analysis of the peripheral blood documented three CD34+ cells/µL and five CD34+ cells/µL in 2-month period respectively. A 46-year-old woman with bicytopenia (hemoglobin 6.9 g/dL, 2500 leukocytes/µL) had by manual count on the peripheral blood smear 1% myeloblasts (she was not included in our study), thus 25 myeloblasts/µL. Her flow cytometric study of the peripheral blood documented 18 CD34+ cells/µL.
A comprehensive analysis of patients’ clinical findings, hematological parameters, and bone marrow findings enabled us to separate patients into two distinct groups. One group of patients (48 patients, 49 testings) had bone marrow involvement by an expansive process (proved by bone marrow biopsy) or presumed involvement (if bone marrow was not performed) on the basis of clinical picture, imaging studies, hematological parameters, and nature of the patient’s disease. The other group of patients (85 patients, 89 testings) had marrow free of expansive process (excluding reactive marrow hyperplasia) or presumed free of expansive process. The average absolute number of CD34+ cells in the patients with an expansive process or presumed expansive process in the marrow was 25 cells/µL, while in the group without expansive processes it was four cells/µL. The difference was statistically significant (P<0.00007). Cut-off 12 CD34+ cells/μL had 93% positive predictive value and 78% negative predictive value in differentiating patients with bone marrow involvement or presumably bone marrow involvement by an expansive process, versus patients with bone marrow noninvolvement by an expansive or presumably expansive process.
Discussion
Our analysis of 138 studies in 133 patients (62 women, 71 men) with less than 1% of myeloblasts in the peripheral blood by standard manual count on glass slide smears demonstrated that flow cytometric analysis can accurately obtain the absolute number and percentage of the CD34+ positive cells in the peripheral blood. Our methodology adds to those previously described showing that flow cytometry provides a robust, accurate, and sensitive means of enumerating CD34+ cells in the peripheral blood and that this value within an individual remains rather constant over time.3 The stability of this value further validates the use of flow cytometry to accurately obtain and apply these data toward a clinical endpoint.
Several methods of quantification of CD34+ cells in the peripheral blood have been described (Milan-Mulhouse, ISHAGE, Sihon, Procount),8 including guidelines from the International Society of Hematotherapy and Graft Engineering (ISHAGE Guidelines)9 as well as modifications of this protocol.5 However, these protocols were primarily designed to quantify CD34+ cells in bone marrow transplant patients. Variations of flow cytometric methods included alterations in gating strategy, mainly CD45 versus right angle side light scatter was used, number of fluorochromes, and/or standardized beads with the intent of enumerating the number of progenitor cells within a donor product.10,11 Our method is conceptually similar but not identical to the Milan-Mulhouse method and the ISHAGE suggested method. However, our method employs a simpler gating strategy that allows for testing and subsequent calculations to be more easily performed. Our gate drawn on the forward scatter vs side scatter dot plot excluded cell detritus and was broad enough not to miss CD34+ cells that may vary in size or might not express enough of CD45 to be gated on CD45/side scatter dot plot. Nonspecific binding of CD34 to cell dusts was eliminated since only cluster or cloud of CD34 brightly positive events were regarded as real and used for quantification of CD34+ cells. Moreover, since no CD3+ lymphocytes, CD19+ lymphocytes or CD14+ monocytes co-expressed CD34 coupled with APC were counted, and since CD1a coupled with APC was 0%, it is unlikely that CD34+ events in the targeted gate were contaminated by CD34+ nonspecific and/or nonmyeloblast/stem cell events. The CD45 versus right-angle light scatter gating is the most used approach to blast isolation and quantification in bone marrow.12,13 However, according to the literature and our findings, forward-angle light scatter versus right angle-light scatter gating strategy is ideal for peripheral blood analysis.14
Our results are remarkably similar to those obtained by other authors using different but similar methods. There is a consensus that, in normal individuals, the percentage of CD34+ cells in the peripheral blood is very low, ranging from 0.01% to 0.1%, or an absolute count of 0.6 to 6 CD34+ cells/µL in a patient with a WBC count of 6,000 for example.5,9 The median number of CD34+ cells in the peripheral blood determined by an ISHAGE-guided flow cytometric study was 2.3 cells/µL in control subjects and 114 cells/µL in patients with primary myelofibrosis.15 Our findings similarly showed a low-level of circulating CD34+ cells in the patient group that, while not normal had nonexpansive bone marrow disorders but had hematological abnormalities that necessitated a flow cytometric study (average absolute number of four and mode of two CD34+ cells/µL).
Our study showed that determining the absolute number of CD34+ cells in the peripheral blood predicted whether the bone marrow would likely be involved by an expansive process or not. The difference between the average absolute numbers of CD34+ cells in each of group of patients was statistically significant (P<0.00007), with little overlap between the two populations. For example, in a 70-year-old patient with small cell lung cancer (patient #33 Table 1) in whom bone marrow biopsy was not performed and who had 22 CD34+ cells/µL, we can conclude that his bone marrow was involved by metastases. We can reach the same conclusion for a 63-year-old man with prostate cancer (patient #32 Table 1) who had 18 CD34+ cells/µL. By the same token we can conclude that a 70-year-old man with low normal number of platelets (patient #18 Table 2) who had had nephrectomy for renal cell carcinoma three years ago did not have bone marrow metastases since he had two CD34+ cells/µL in the peripheral blood. The absolute number of CD34+ cells in the peripheral blood of normal adult volunteers according to literature2 varied from about one to five CD34+ cells/µL. According to our study, in a context of clinical data and laboratory parameters, the absolute number of CD34+ cells in the peripheral blood may therefore have a significant impact on the decision to perform bone marrow biopsy which is a painful, invasive, and costly procedure. For example, our data show that in a patient with carcinoma in whom bony metastasis is suspected, a bone marrow biopsy may be avoided, and patient presumed to have metastasis if the number of CD34+ cells is elevated (12 or greater CD34+ cells/µL), so long as these data support the clinical and radiologic impression. In addition to avoiding a procedure for the patient, another perceived advantage of using this method of determining bone marrow involvement is the inevitable possibility of sampling error when the lesion is focal and may be missed on a limited core biopsy. The absolute number of CD34+ in the peripheral blood in certain clinical settings might serve as additional information for cancer staging purposes; however, additional studies to validate our data would clearly be necessary.
To gain a better understanding of the factors that influence the numbers of peripheral blood blasts, we evaluated the relationship between the flow cytometry results, the white blood cell count and the number of bone marrow blasts. A positive correlation was identified between the number of leukocytes and absolute number of CD34+ cells in the peripheral blood (Pearson's coefficient correlation=0.6971). This relationship seems reasonable as the cytokines which participate in the recruitment of leukocytes resulting in leukocytosis do so, in part, by acting on bone marrow cells in the same way administered cytokines act to mobilize CD34+ stem cells for allograft and auto transplantations. However, in settings where no clear association to a cytokine-driven process is evident, other pathologic mechanisms may be present, such as the expansive processes described in our study. Whether physical or biochemical in nature, these pathologic processes may alter the microenvironment of the bone marrow, allowing the inappropriate release of immature cells into the peripheral blood. The 74-year-old man (#41 Table 1) with severe lung infection with Cryptococcus also had Cryptococcus meningitis with numerous Cryptococci in histiocytes in the cerebrospinal fluid. In his peripheral blood were 23,800 WBC/µL and 38 CD34+ cells/µL. Since the percentage of his CD34+ cells was 0.16% of leukocytes, similar to the average percentage in patients with marrow involvement by an expansive process (0.171%), we inferred that the increased number of CD34+ cells (38 cells/µL) was due to bone marrow involvement by infection (inflammatory tumor) with Cryptococcus (bone marrow was not done) and not only to leukocytosis per se. Thus, percentage of CD34+ cells in the peripheral blood is also a useful parameter. The average percentage of CD34+ cells in the peripheral blood of our 138 studies was 0.098% ranging from 0.01% to 0.715%. The average percentage of patients with expansive marrow process or presumably having expansive process was 0.171% and of patients with marrow not involved or presumably not involved by an expansive process was 0.057% (P<0.00008). Interestingly, the absolute number of CD34+ cells in the peripheral blood did not correlate with the percentage of myeloblasts in the bone marrow (coefficient correlation was –0.23219). These data suggest the number of circulating blasts is less reflective of the bone marrow differential and more dependent upon the presence of pathology that may disrupt the regulatory processes which govern the release of cells into the peripheral blood.
We have chosen cutoff value of 12 CD34+ cells/µL in the peripheral blood to differentiate patients with marrow involvement with expansive versus nonexpansive processes because its positive predictive value was 93% and negative predictive value 78%. However, several outliers were discovered in our study. In a small number of patients, the absolute number of CD34+ cells in the peripheral blood overlapped between the groups in our study. For example, several outliers with low absolute number of CD34+ cells in the peripheral blood had marrow involved by an expansive process. However, fewer numbers of patients showed the converse (only two out of 89 patients) without marrow involvement (or presumably without involvement) had slightly increased numbers of CD34+ cells. Patient #1 Table 1 (a 28-year-old woman with anaplastic large cell lymphoma) and one CD34+ cell/µL had only four malignant cells in the bone marrow, not obvious in the hematoxylin and eosin-stained core biopsy but demonstrated by an immunoperoxidase stain for CD30. In her case we can suggest that the minuscule amount of expansive process was related to low absolute number of CD34+ cells in the peripheral blood. We have no explanation why patient #6 Table 1 (79-year-old man) who had a 95% marrow replacement by B cell chronic lymphocytic leukemia cells had only three CD34+ cells/µL while patient #45 Table 1 (72-year-old man) with the same disease had 80 CD34+ cells/µL. Patient #8 Table 1 (65-year-old man with pancytopenia and myelodysplastic syndrome with multilineage dysplasia) had three CD34+ cells/µL while patient #31 Table 1 (48-year-old woman) with the same disease had 17 CD34+ cells/µL.
Here are four possible mechanisms for (outliers) discrepancy between the expansive or nonexpansive marrow status and absolute number of CD34+ cells in the peripheral blood. (1) Minimal bone marrow involvement by tumor prevents significant disruption in the normal release of CD34+ cells and leads to falsely low result. (2) Treatment-related clearance of CD34+ cells might also lead to false low result. (3) Unrecognized pathology in the bone marrow (for example biopsy missed neoplastic lesion) may result in unexpectedly high absolute number of CD34+ cells in the peripheral blood but be actually correct. (4) Treatment-related damage to the bone marrow environment might lead to inappropriate release of CD34+ cells and false positive results.
Patient #36 Table 1, a 66-year-old man with mantle cell lymphoma involving 70% of the marrow core biopsy, had 24 CD34+ cells in the peripheral blood and after treatment there were no detectable CD34+ cells in the peripheral blood. We could conclude that the clearance of CD34+ cells from the peripheral blood after treatment, as detected by flow cytometry may be indicative of response to the treatment and that marrow became free of involvement by mantle cell lymphoma. Bone marrow biopsy was not performed. Since there were no CD34+ cells in the peripheral blood, this patient’s second study was not included in this research or our statistics. Given these findings, the number of CD34+ cells should not be regarded as absolutely decisive, but rather as another data point that should be integrated in the patient’s whole clinical, laboratory, and radiological picture. With knowledge of these limitations, we recommend that determination of the absolute number and percentage of CD34+ cells obtained by flow cytometric analysis of the peripheral blood be performed with intent of using these values to predict bone marrow pathology and ultimately help in the determination of the necessity for bone marrow biopsy.
We have found that multiparameter flow cytometric testing by simple gating mononuclear cells can accurately quantify the absolute number of CD34+ cells in the peripheral blood. The method is extremely sensitive and can detect fewer than one CD34+ cells/µL. Quantification of the CD34+ cells in the peripheral blood by flow cytometric analysis is a useful parameter that may predict whether the bone marrow is involved or not by an expansive process, when evaluated in concert with the clinical picture, laboratory data, and imaging findings. Based on the results of our study, flow cytometry of peripheral blood is recommended as these data may provide a useful threshold in the decision-making process to perform a bone marrow biopsy. As part of the initial evaluation, flow cytometric enumeration of peripheral CD34+ cells may play an important role in directing the hematologic evaluation of patients when circulating blasts are not seen on peripheral blood smear. The absolute number of 12 or more CD34+ cells/µL in the peripheral blood favors marrow involvement by an expansive process despite a negative bone marrow biopsy, suggesting possibility of inadequate sampling procedure which may have missed the lesion.
Conclusions
- Flow cytometric method is very sensitive, can accurately detect in the peripheral blood less than one CD34+ cells/µL, and in our study it demonstrated that the average absolute number of CD34+ cells in the peripheral blood in patients with less than 1% blasts by manual count was 11 CD34+ cells/µL.
- The average absolute number of CD34+ cells in the peripheral blood in patients with expansive pathologic process (or presumed expansive) is 25 CD34+ cells/µL. It is significantly higher (P<0.00007) than in patients without marrow pathologic expansive processes, four CD34+ cells/µL, and thus, absolute number of CD34+ cells can help make diagnosis regarding marrow involvement.
- The absolute number CD34+ cells in the peripheral blood can help to decide whether or not to do bone marrow biopsy.
- The absolute number of 12 or more CD34+ cells/µL in the peripheral blood might suggest bone marrow involvement despite a negative bone marrow biopsy for cancer staging purpose which, may have missed focal lesion.
- We suggest that other clinicians use our simple method of counting absolute number of CD34+ cells in the peripheral blood by flow cytometry and confirm our findings. The aims, data and analysis of our study support the conclusion, essentially that this method offers insight into the likelihood of a significant underlying bone marrow process, and with this additional (rapidly available) information, clinicians may be able to determine the appropriateness of bone marrow biopsy in the management of their patients.
Ethical Approval
Research has been carried out within an appropriate ethical framework in accordance with COPE guidelines approved by (Charleston Area Medical Center, Charleston West Virginia/West Virginia University) CAMC/WVU Charleston Division Institutional Review Board (IRB), approval number 20-680 on June 23, 2020. Regarding human participants a waiver of consent was requested at the time of study application and CAMC/WVU Charleston Division IRB granted (approval number 20-680) an exemption from requiring written informed consent, a waiver of consent 45 CFR 46.116 (f), as it was determined the use of retrospective data posed “not greater than minimal risk” to participants, and the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Arber DA, Orazi A, Hassserijan RP, et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140(11):1200–1228. doi:10.1182/blood.2022015850
2. Sutheralnd DR, Keating A, Nayar R, Anania S, Stewart AK. Sensitive detection and enumeration of CD34 positive cells in peripheral blood and cord blood by flow cytometry. Exp Hematol. 1994;22(10):1003–1010.
3. Eidenschink L, DiZerega G, Rodgers K, Bartlett M, Wells DA, Loken MR. Basal levels of CD34 positive cells in peripheral blood differ between individuals and are stable for 18 months. Cytometry B Clin Cytom. 2012;82(1):18–25. doi:10.1002/cyto.b.20611
4. Bender JG, Unverzagt KL, Walker DE, et al. Identification and comparison of CD34-positive cells and their subpopulations from normal peripheral blood and bone marrow using multicolor flow cytometry. Blood. 1991;77(12):2591–2596. doi:10.1182/blood.V77.12.2591.2591
5. Kikuchi-Taura A, Soma T, Matsuyama T, Stern DM, Taguci A. A new protocol for quantifying CD34+ cells in peripheral blood of patients with cardiovascular disease. Tex Heart Inst J. 2006;33(4):427–429.
6. Siena S, Bregni M, Brando B, et al. Flow cytometry for clinical estimation of circulating hematopoietic progenitors for autologous transplantation in cancer patients. Blood. 1991;77(2):400–409. doi:10.1182/blood.V77.2.400.400
7. Kutok JL, Roma AO, Lemire SJ, Dorfman DM. Four-color flow cytometric immunophenotypic determination of peripheral blood CD4+ T-lymphocyte counts: a comparison of validity and cost-effectiveness with a two-color method. Am J Clin Pathol. 1998;110(4):465–470. doi:10.1093/ajcp/110.4.465
8. Gratama JW, Orfao A, Barmett D, et al. Flow cytometric enumeration of CD34+ hematopoietic stem and progenitor cells. European Working Group on Clinical Cell Analysis. Cytometry. 1998;34(3):128–142. doi:10.1002/(SICI)1097-0320(19980615)34:3<128::AID-CYTO3>3.0.CO;2-D
9. Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Jee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5(3):213–226. doi:10.1089/scd.1.1996.5.213
10. Gajkowska A, Oldak T, Jastrzewska M, et al. Flow cytometric enumeration of CD34+ positive hematopoietic and progenitor cells in leukapheresis product and bone marrow for clinical transplantation: a comparison of three methods. Folia Histochem Cytobiol. 2006;44(1):53–60.
11. Sutherland DR, Nayyar R, Acton E, Giftakis A, Dean S, Mosiman VL. Comparison of two single-platform ISHAGE-based CD34 enumeration protocols on BD FACSCalibur and FACSCanto flow cytometers. Cytotherapy. 2009;11(5):595–605. doi:10.1080/14653240902923161
12. Kroft SH, Karandikar NJ. Flow cytometric analysis of acute leukemias, myelodysplastic syndromes, and myeloproliferative disorders. In: Carey JL, McCoy JP, Keren DF, editors. Flow Cytometry in Clinical Diagnosis.
13. Harrington AM, Olteanu H, Kroft SH. A dissection of the CD45/side scatter “blast gate”. Am J Clin Pathol. 2012;137(5):800–804. doi:10.1309/AJCPN4G1IZPABRLH
14. Sun T, Sangaline R, Ryder J, et al. Gating strategy for immunophenotyping of leukemia and lymphoma. Am J Clin Pathol. 1997;108(2):152–157. doi:10.1093/ajcp/108.2.152
15. Passamonti F, Vanelli L, Malabarba L, et al. Clinical utility of the absolute number of circulating CD34-positive cells in patients with chronic myeloproliferative disorders. Haematologica. 2003;88(10):1123–1129.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.