Back to Journals » Clinical Interventions in Aging » Volume 19
Glial Fibrillary Acidic Protein as a Potential Indicator for Symptomatic Intracranial Hemorrhage in Acute Ischemic Patients Undergoing Endovascular Thrombectomy
Authors Li M, Liu H, Xu M, Yu B, Guo M, Wang X, Shi G, Zhou R
Received 4 November 2023
Accepted for publication 17 January 2024
Published 22 January 2024 Volume 2024:19 Pages 123—132
DOI https://doi.org/10.2147/CIA.S448180
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Minghao Li,1,2,* Hua Liu,1,3,* Mingyang Xu,1,3 Baiyang Yu,3,4 Minwang Guo,1,3 Xiaorong Wang,1,3 Guomei Shi,1,3 Rujuan Zhou1,3
1Stroke Center, Taixing People’s Hospital, Taixing, Jiangsu, People’s Republic of China; 2Department of Vascular Surgery, Taixing People’s Hospital, Taixing, Jiangsu, People’s Republic of China; 3Department of Neurology, Taixing People’s Hospital, Taixing, Jiangsu, People’s Republic of China; 4Department of Neurology, Taixing Clinical College of Bengbu Medical College, Bengbu, Anhui, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guomei Shi; Rujuan Zhou, Stroke Center, Taixing People’s Hospital, No. 1 Changzheng Road, Taixing, Jiangsu Province, 225400, People’s Republic of China, Tel +86-15240200702 ; +86-13951158499, Email [email protected]; [email protected]
Background: The correlation between glial fibrillary acidic protein (GFAP) and symptomatic intracranial hemorrhage (sICH) in acute ischemic stroke (AIS) patients undergoing endovascular thrombectomy (EVT) treatment remains uncertain. We aimed to assess the association between levels of GFAP in the bloodstream and the occurrence of sICH.
Methods: Between June 2019 and May 2023, 142 consecutive AIS patients undergoing EVT at Stroke Center and 35 controls from the Physical Examination Center were retrospectively included. The levels of GFAP in the bloodstream were quantified using enzyme-linked immunosorbent assay prior to endovascular treatment (T1) and 24 h after the procedure (T2). The identification of sICH was based on the Heidelberg Bleeding Classification.
Results: Serum GFAP levels at T1 in AIS patients were significantly higher than those in the controls (0.249 [0.150– 0.576] versus 0.065 [0.041– 0.110] ng/mL, p = 0.001), and there was a notably elevation in GFAP levels at T2 compared to T1 (3.813 [1.474, 5.876] versus 0.249 [0.150– 0.576] ng/mL, p = 0.001). Of the 142 AIS patients, 18 (14.5%) had sICH after EVT. Serum GFAP levels at T2 showed significant associations with sICH in both the unadjusted model (OR 1.513, 95% CI 1.269– 1.805, p = 0.001) and multivariable adjusted model (OR 1.518, 95% CI 1.153– 2.000, p = 0.003). Furthermore, the addition of GFAP at T2 to conventional model resulted in a significant enhancement of risk reclassification for sICH (integrated discrimination improvement [IDI] 0.183, 95% CI 0.070– 0.295, p = 0.001).
Conclusion: Serum GFAP levels were notably increased in AIS patients 24 h after EVT. Elevated GFAP levels were correlated to an elevated risk of sICH. GFAP could potentially serve as a dependable indicator for sICH in AIS individuals who treated with EVT.
Keywords: glial fibrillary acidic protein, stroke, symptomatic intracranial hemorrhage, indicator, endovascular thrombectomy
Introduction
Stroke has been identified as a standout cause of both mortality and morbidity in China, and it also contributes significantly to healthcare expenses.1,2 The implementation of endovascular thrombectomy (EVT) has led to notable enhancements in clinical outcomes for acute ischemic stroke (AIS) patients involving occlusion of large vessels.3,4
Symptomatic intracranial hemorrhage (sICH) is a highly concerning complication following interventional procedures among AIS individuals, with reported incidence rates ranging from 4.4% to 16.0%.3,5 Previous studies have consistently demonstrated that sICH is associated with an elevated risk of unfavorable clinical outcomes and a diminished benefit-to-risk ratio of EVT accordingly.6–8 Hence, timely and precise identification of sICH following EVT holds importance for enhancing perioperative management and clinical prognosis.
Neuroinflammation is a pervasive phenomenon in the pathological progression of brain ischemic.9 Astrogliosis, a pivotal aspect of neuroinflammation, is distinguished by the substantial elevation of glial fibrillary acidic protein (GFAP) expression. The excessive expression of GFAP has been documented in diverse neurological disorders, including dementia, autoimmune encephalitis, multiple sclerosis (MS), traumatic brain injury, and malignant brain tumors.10–14 Recently, GFAP has been proposed as a reliable biomarker for distinguishing AIS from intracerebral hemorrhage.15,16 Moreover, elevated GFAP levels were intimately related to unfavorable prognosis at 90 days in patients after EVT for large vessel occlusion.17 Furthermore, patients with higher GFAP levels at 24 h after stroke had a 2.51-fold increased risk for parenchymal hemorrhage.18 Nevertheless, the relationship between GFAP and sICH in AIS patients undergoing EVT had not been investigated.
Herein, the study aimed to clarify whether GFAP is in relation to sICH in AIS patients undergoing EVT.
Materials and Methods
Study Participants
This was a retrospective cohort study based on prospectively collected data of AIS patients admitted to Taixing People’s Hospital between June 2019 and May 2023. Inclusion criteria were patients who presented with large vessel occlusion in the anterior circulation and underwent EVT utilizing contemporary thrombectomy techniques. Conversely, patients diagnosed with concomitant conditions such as aneurysm, vasculitis, arterial dissection, arteriovenous malformation, Moyamoya syndrome, or hematological system diseases were excluded from the study. The control group consisted of participants without a history of stroke from the Physical Examination Center. Approval for this study was obtained from the Ethics Committee of Taixing People’s Hospital (No. LS2021017). Based on a retrospective design of the study, the Ethics Committee of Taixing People’s Hospital waived informed consent and de-identified patient information.
Data Collection
Demographics (age and gender), vascular risk factors, subtype of stroke, blood pressure, baseline National Institutes of Health Stroke Score (NIHSS), baseline Alberta Stroke Program Early CT Score (ASPECTS), procedural characteristics (occlusion site, interval time, collateral status, recanalization status, and passes with retriever) as well as laboratory data were collected as previously described.19,20 The subtype of stroke was classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria, specifically as large-artery atherosclerosis, cardio-embolism or other subtypes.21 The collateral status was assessed using the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) grading system.22 Recanalization status was categorized using the modified Thrombolysis in Cerebral Infarction (mTICI) score, wherein a score of mTICI 2b or 3 indicated successful reperfusion.23 Age, gender, and vascular risk factors were collected as clinical characteristics from the control group.
Definition of sICH
The evaluation of any hemorrhagic transformation was conducted using a follow-up computed tomographic 24 h after EVT or whenever there was a deterioration in clinical condition. We defined sICH as any intracranial hemorrhage based on the Heidelberg Bleeding Classification with NIHSS score increase ≥ 4 points in the total score or ≥ 2 points in one category from baseline within 72 h after EVT.24,25 The diagnose of sICH was independently conducted by two certified neuroradiologists blinded to clinical data. When disagreement occurred, a third neuroradiologist was consulted to make a final judgment.
Measurement of Serum GFAP Levels
Blood samples were collected from both the control group and all patients with AIS prior to EVT (T1) and 24 h after the procedure (T2). The collected specimens were subjected to centrifugation at a speed of 1,000 times the force of gravity for a duration of 15 minutes at a temperature of 4°C. The resulting serum was then transferred to cryotubes and stored at a temperature of −80°C until further analysis. The levels of serum GFAP levels were quantified using an enzyme-linked immunosorbent assay kit (Catalog No. RK09302, ABclonal), following the instructions provided by the manufacturer. The minimum detectable level of GFAP was determined to be 0.1 ng/mL. The coefficients of variation for GFAP, both within and between assays, were found to be 10.0% and 15.0%, respectively.
Statistical Analysis
Statistical analyses were conducted by R 4.1.3 (http://www.R-project.org/) and GraphPad Prism 9.3.1 (GraphPad Software, San Diego, CA, USA). Categorical variables were represented as numbers accompanied by percentages, while continuous variables were expressed as means accompanied by standard deviations or medians accompanied by quartiles. Univariate analyses were performed utilizing the t-test or Mann–Whitney U-test for continuous variables, while the Pearson’s chi-square test was employed for categorical variables, as deemed suitable In order to assess the independent risk factors associated with sICH, multivariable logistic regression analyses were adjusted for age, gender and variables with a p-value < 0.1 in the univariate analysis. Furthermore, the C-statistic, net reclassification index (NRI) and integrated discrimination improvement (IDI) were computed to evaluate the discriminatory power as well as predictive value of GFAP when incorporated into conventional models.26,27 Statistical analyses were conducted with two-tailed testing, and p-values < 0.05 were deemed to be statistically significant.
Results
Baseline Characteristics
A total of 149 patients diagnosed with AIS and treated with endovascular therapy were initially screened for inclusion in this study. Seven patients were excluded for the following reasons: hematological system diseases (n=3), aneurysm (n=2), arterial dissection (n=1), and Moyamoya syndrome (n=1). Finally, 142 patients were recruited for analysis. The average age of the included patients was 70.9 ± 10.1 years, with 64.1% being male (Table 1). The baseline median NIHSS score was 15 (interquartile range [IQR], 12–18), and the baseline median ASPECTS was 9 (IQR 8–9). Poor collateral status was observed in 77 patients (54.2%), while successful recanalization was achieved in 118 patients (83.1%). The median GFAP at T1 and T2 were 0.249 (IQR 0.150–0.576) and 3.813 (IQR 1.474–5.876) ng/mL, respectively.
 |
Table 1 Clinical Characteristics of Participants According to with and without sICH in Patients Undergoing EVT |
In addition, 35 control individuals were enrolled (24 males and 9 females), with an average age of 67.8 ± 7.2 years. The baseline characteristics of the control group and all patients with AIS were described in Table 2. Significant differences were found between AIS patients and controls in respect of age (70.9 ± 10.1 versus 67.8 ± 7.2, p = 0.038) and GFAP (0.249 [0.150–0.576] versus 0.065 [0.041–0.110] ng/mL, p = 0.001). Although the controls were younger, similar results were obtained when GFAP levels were compared based on the age-adjusted model (p = 0.001).
 |
Table 2 Clinical Characteristics of the Control Group and Patients with AIS (at Baseline Before EVT) |
Figure 1 showed the violin plot of serum Log GFAP levels in AIS patients and controls. The average Log GFAP levels in controls were 4.2 ± 0.6 pg/mL. Compared to controls, a significant elevation was observed in patients with AIS at T1 (5.7 ± 1.0 pg/mL) or T2 (8.0 ± 0.9 pg/mL). Besides, Log GFAP levels were higher at T2 than at T1 (p = 0.001).
Correlation Between GFAP and sICH
Eighteen patients (14.5%) were diagnosed with sICH. As depicted in Table 1, patients with sICH exhibited higher baseline NIHSS (p = 0.045), lower baseline ASPECTS (p = 0.001), poorer collateral status (p = 0.004), more passes with retriever (p = 0.047), higher levels of FBG (p = 0.002) and GFAP at T2 (p = 0.001), compared to those without sICH.
After multivariable adjustment for age, gender, and potential confounders mentioned above in univariate analysis, patients with higher GFAP levels at T2 had an increased risk of sICH (OR 1.518, 95% CI 1.153–2.000, p = 0.003) (Table 3). Adding GFAP at T2 to models containing conventional risk factors did not significantly enhance the C-statistics (p = 0.283) for sICH. However, the risk reclassification for sICH was modestly improved by adding GFAP at T2 to convention models (IDI 0.183, 95% CI 0.070–0.295, p = 0.001, Table 4).
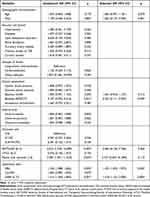 |
Table 3 Logistic Regression Analyses for the Related Factors Associated with sICH in Patients Undergoing EVT |
 |
Table 4 Reclassification of GFAP for sICH in Patients Undergoing EVT |
Discussion
To our knowledge, this study represents the initial investigation into the potential correlation between GFAP and sICH in AIS patients who underwent EVT. Our findings demonstrated a significant increase in serum GFAP levels 24 h after EVT, with higher levels correlating to an elevated risk of sICH. Furthermore, the inclusion of GFAP in conventional models resulted in improved risk reclassification for sICH. These results suggested that GFAP might serve as a dependable prognostic indicator for sICH following EVT in AIS patients.
In our cohort, the prevalence of sICH after EVT was 14.5%, which was in parallel with the rate reported in the ACTUAL registry (16.0%),5 but remarkably higher than that reported in the ESCAPE-NA1 trial (4.0%)28 and MR CLEAN registry (5.9%).7 The inclusion criteria employed in the randomized controlled trials were more stringent compared to those applied in real-world clinical practices.5,7,28 In contrast to prior extensive randomized controlled trials,5,7,28 the expansion of clinical indications, less favorable treatment conditions, and inadequate experience among neurointerventionists were more prevalent in real-world clinical practices. These may be significant contributors to an increased risk of sICH in our study. Meanwhile, our research uncovered that sICH patients had higher baseline NIHSS score, lower initial ASPECTS score, poorer collateral status, more passes of retriever as well as higher levels of FBG, which were coherent with literature data.25,29,30 Contrary to previously published findings, our study did not establish a substantial correlation between advanced age, elevated blood pressure, longer procedure duration and sICH.30,31 This discrepancy may be primarily caused by variations in study design, participants, regions, and the definition and timing of assessing sICH.
GFAP is a pivotal protein that specifically exists in astroglial intermediate filaments. When glial is damaged with astrocyte disintegration, this protein would be released from intracellular compartment into cerebrospinal fluid and bloodstream.32 In our cohort, the serum GFAP levels were elevated in AIS patients, and a subsequent continuous increase was observed 24 h after EVT in AIS patients. The findings indicated that GFAP levels reflect astrocytic dysfunction and blood-brain barrier disruption. Elevated GFAP levels have also been detected in neurodegenerative disorders.12–14 In a single-center cross-sectional study with 129 MS patients, serum GFAP was proposed as a potentially interesting marker to distinguish primary progressive MS and relapsing remitting MS.13 Another recent-published study with 282 Italian frontotemporal lobar degeneration patients observed a significant association between serum GFAP concentrations and disease intensity as well as severity.14 Moreover, the expression of GFAP is preferentially increased in intracerebral hemorrhage, which showed clinical significance in distinguishing between ischemic stroke and intracerebral hemorrhage, even during the initial stage.15,16 However, studies concerning the value of GFAP in EVT-treated AIS patients are relatively scarce. According to the study conducted in Sweden, serum GFAP predicted unfavorable functional outcome 3-month after EVT.17 In a recent clinical study involving 60 Taiwanese recanalized AIS patients, increased GFAP was linked to a greater risk of parenchymal hemorrhage.18 The present study found a strong association between elevated levels of GFAP at 24 h following EVT and an increased risk of sICH, alone or combined with other potential confounding factors. Our research built upon the prior discoveries by suggesting that serum GFAP could also serve as a promising predictor of sICH in AIS patients following EVT. In addition, our study also found that incorporation of GFAP alongside traditional risk factors resulted in a more accurate risk reclassification for sICH. This finding implies that GFAP has the potential to be integrated into existing factors to construct a predictive model for identifying EVT patients who are at a heightened risk of developing sICH.5,25,33 Consequently, this model could provide valuable clinical insights and help neurologists in identifying high-risk individuals.
Several limitations deserve comment. First, the presence of selection bias was unavoidable due to the retrospective nature of this study conducted in a single facility with a relatively limited number of participants, potentially diminishing the statistical strength. Second, the levels of GFAP might be influenced by a pre-existing neurological condition before the occurrence of a stroke, and the GFAP levels prior to the onset of stroke remained unknown. Third, several parameters, which may be potential confounders for sICH in previous study,34–38 were not measured, such as prior utilization of anticoagulants, early increase in body temperature, white matter hyperintensity, cerebral microbleeds, and fluctuations in blood pressure. Fourth, owing to the cross-sectional observational design of the study, it was challenging to establish a causal relationship between GFAP and sICH. In addition, the findings of our study were not corroborated in an external cohort. To further validate and expand the findings of our study, additional studies in external cohorts with larger sample sizes are necessary.
Conclusions
To summarize, this study indicated that higher levels of GFAP were independently linked to a higher risk of sICH in AIS patients following EVT. Despite its inherent limitations, our study presented preliminary evidence supporting the potential of serum GFAP as a valuable biomarker for diagnosing and predicting sICH. We cautiously proposed that incorporating GFAP into the sICH risk model for AIS patients undergoing EVT could enhance its reliability. Further prospective studies with larger sample sizes should be performed to comprehensively validate these preliminary results and to further explore the exact mechanism linking GFAP and sICH.
Data Sharing Statement
The data that support the findings of this study are available from Guomei Shi upon reasonable request.
Ethics Approval
The study was approved by the Ethics Committee of Taixing People’s Hospital (No. LS2021017) and conducted according to the Declaration of Helsinki. The fully de- identified data on the participants enrolled in the study and its virtue of retrospective study design enables this study conducted under a waiver of informed consent by the Ethics Committee of Taixing People’s Hospital.
Consent for Publication
No information or images that could lead to identification of a study participant were mentioned in our study.
Author Contributions
All authors made significant contributions to conception, study design, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from Jiangsu Association for Science and Technology (TJ2021019), Science Foundation of Kangda College of Nanjing Medical University (KD2020KYJJZD021), Doctoral Science Foundation of Taixing People’s Hospital Foundation Project (TRYBS2022001) and Instructive Project of Jiangsu Provincial Health Commission (Z2023052).
Disclosure
The authors declare no conflict of interest.
References
1. Feigin VL, Stark BA, Johnson CO., GBD. Stroke collaborators. global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021; 20(10): 795–820. doi:10.1016/S1474-4422(21)00252-0
2. Ding C, Wu Y, Chen X, et al. Global, regional, and national burden and attributable risk factors of neurological disorders: the global burden of disease study 1990–2019. Front Public Health. 2022;10:952161. doi:10.3389/fpubh.2022.952161
3. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi:10.1016/S0140-6736(16)00163-X
4. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke Association. Stroke. 2019;50(12): e344–e418. doi:10.1161/STR.0000000000000211
5. Hao Y, Yang D, Wang H, et al. Predictors for symptomatic intracranial hemorrhage after endovascular treatment of acute ischemic stroke. Stroke. 2017;48(5):1203–1209. doi:10.1161/STROKEAHA.116.016368
6. Tian B, Tian X, Shi Z, et al. Clinical and imaging indicators of hemorrhagic transformation in acute ischemic stroke after endovascular thrombectomy. Stroke. 2022;53(5):1674–1681. doi:10.1161/STROKEAHA.121.035425
7. van der Steen W, van der Ende NAM, Luijten SPR, et al. Type of intracranial hemorrhage after endovascular stroke treatment: association with functional outcome. J Neurointerv Surg. 2023;15(10):971–976. doi:10.1136/jnis-2022-019474
8. Kang Z, Wu L, Sun D, et al. Proximal hyperdense middle cerebral artery sign is associated with increased risk of asymptomatic hemorrhagic transformation after endovascular thrombectomy: a multicenter retrospective study. J Neurol. 2023;270(3):1587–1599. doi:10.1007/s00415-022-11500-5
9. Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD. Global brain inflammation in stroke. Lancet Neurol. 2019;18(11):1058–1066. doi:10.1016/s1474-4422(19)30078-x
10. van Bodegraven EJ, van Asperen JV, Robe PAJ, Hol EM. Importance of GFAP isoform-specific analyses in astrocytoma. Glia. 2019;67(8):1417–1433. doi:10.1002/glia.23594
11. Abdelhak A, Foschi M, Abu-Rumeileh S, et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurol. 2022;18(3):158–172. doi:10.1038/s41582-021-00616-3
12. Shen XN, Huang SY, Cui M, et al. Plasma glial fibrillary acidic protein in the Alzheimer disease continuum: relationship to other biomarkers, differential diagnosis, and prediction of clinical progression. Clin Chem. 2023;69(4):411–421. doi:10.1093/clinchem/hvad018
13. Ayrignac X, Le Bars E, Duflos C, et al. Serum GFAP in multiple sclerosis: correlation with disease type and MRI markers of disease severity. Sci Rep. 2020;10(1):10923. doi:10.1038/s41598-020-67934-2
14. Benussi A, Ashton NJ, Karikari TK, et al. Serum Glial Fibrillary Acidic Protein (GFAP) is a marker of disease severity in frontotemporal lobar degeneration. J Alzheimers Dis. 2020;77(3):1129–1141. doi:10.3233/jad-200608
15. Foerch C, Niessner M, Back T, et al. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin Chem. 2012;58(1):237–245. doi:10.1373/clinchem.2011.172676
16. Katsanos AH, Makris K, Stefani D, et al. Plasma glial fibrillary acidic protein in the differential diagnosis of intracerebral hemorrhage. Stroke. 2017;48(9):2586–2588. doi:10.1161/STROKEAHA.117.018409
17. Pujol-Calderon F, Zetterberg H, Portelius E, et al. Prediction of outcome after endovascular embolectomy in anterior circulation stroke using biomarkers. Transl Stroke Res. 2022;13(1):65–76. doi:10.1007/s12975-021-00905-5
18. Chen CH, Chu HJ, Hwang YT, et al. Plasma neurofilament light chain level predicts outcomes in stroke patients receiving endovascular thrombectomy. J Neuroinflammation. 2021;18(1):195. doi:10.1186/s12974-021-02254-4
19. Shi G, Li M, Zhou R, et al. Procalcitonin related to stroke-associated pneumonia and clinical outcomes of acute ischemic stroke after IV rt-PA treatment. Cell Mol Neurobiol. 2022;42(5):1419–1427. doi:10.1007/s10571-020-01031-w
20. Shi G, Ke D, Gong P, et al. Serum YKL-40 levels and white matter hyperintensities in patients with acute ischemic stroke. J Inflamm Res. 2023;16:311–319. doi:10.2147/JIR.S398701
21. Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. trial of org 10172 in acute stroke treatment. Stroke. 1993;24(1):35–41. doi:10.1161/01.str.24.1.35
22. Higashida RT, Furlan AJ. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34(8): e109–37. doi:10.1161/01.STR.0000082721.62796.09
23. Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650–2663. doi:10.1161/STROKEAHA.113.001972
24. von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981–2986. doi:10.1161/STROKEAHA.115.010049
25. Zhang X, Xie Y, Wang H, et al. Symptomatic intracranial hemorrhage after mechanical thrombectomy in Chinese ischemic stroke patients: the ASIAN score. Stroke. 2020;51(9):2690–2696. doi:10.1161/STROKEAHA.120.030173
26. Pencina MJ, D’Agostino RB, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi:10.1002/sim.2929
27. Alba AC, Agoritsas T, Walsh M, et al. Discrimination and calibration of clinical prediction models: users’ guides to the medical literature. JAMA. 2017;318(14):1377–1384. doi:10.1001/jama.2017.12126
28. Ospel JM, Qiu W, Menon BK, et al. Radiologic patterns of intracranial hemorrhage and clinical outcome after endovascular treatment in acute ischemic stroke: results from the ESCAPE-NA1 trial. Radiology. 2021;300(2):402–409. doi:10.1148/radiol.2021204560
29. Mohamed A, Shuaib A, Saqqur M, Fatima N. The impact of leptomeningeal collaterals in acute ischemic stroke: a systematic review and meta-analysis. Neurol Sci. 2023;44(2):471–489. doi:10.1007/s10072-022-06437-6
30. van der Steen W, van der Ende NAM, van Kranendonk KR, et al. Determinants of symptomatic intracranial hemorrhage after endovascular stroke treatment: a retrospective cohort study. Stroke. 2022;53(9):2818–2827. doi:10.1161/STROKEAHA.121.036195
31. Venditti L, Chassin O, Ancelet C, et al. Pre-procedural predictive factors of symptomatic intracranial hemorrhage after thrombectomy in stroke. J Neurol. 2021;268(5):1867–1875. doi:10.1007/s00415-020-10364-x
32. Heimfarth L, Passos FRS, Monteiro BS, Araujo AAS, Quintans Junior LJ, Quintans JSS. Serum glial fibrillary acidic protein is a body fluid biomarker: a valuable prognostic for neurological disease - A systematic review. Int Immunopharmacol. 2022;107:108624. doi:10.1016/j.intimp.2022.108624
33. Chen Y, Zhou S, Yang S, et al. Developing and predicting of early mortality after endovascular thrombectomy in patients with acute ischemic stroke. Front Neurosci. 2022;16:1034472. doi:10.3389/fnins.2022.1034472
34. Kam W, Holmes DN, Hernandez AF, et al. Association of recent use of non-vitamin k antagonist oral anticoagulants with intracranial hemorrhage among patients with acute ischemic stroke treated with alteplase. JAMA. 2022;327(8):760–771. doi:10.1001/jama.2022.0948
35. Yi T, Zhang Y, Chen WH, et al. Impact of leukoaraiosis in patients with acute ischemic stroke treated with thrombectomy: a post hoc analysis of the DIRECT-MT trial. J Neurointerv Surg. 2023;15(2):139–145. doi:10.1136/neurintsurg-2021-018293
36. Choi KH, Kim JH, Kang KW, et al. Impact of microbleeds on outcome following recanalization in patients with acute ischemic stroke. Stroke. 2019;50(1):127–134. doi:10.1161/STROKEAHA.118.023084
37. Liu D, Nie X, Pan Y, et al. Adverse outcomes associated with higher mean blood pressure and greater blood pressure variability immediately after successful embolectomy in those with acute ischemic stroke, and the influence of pretreatment collateral circulation status. J Am Heart Assoc. 2021;10(5): e019350. doi:10.1161/JAHA.120.019350
38. Chen Y, Nguyen TN, Mofatteh M, et al. Association of early increase in body temperature with symptomatic intracranial hemorrhage and unfavorable outcome following endovascular therapy in patients with large vessel occlusion stroke. J Integr Neurosci. 2022;21(6):156. doi:10.31083/j.jin2106156
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

