Back to Journals » Journal of Blood Medicine » Volume 14
Hematological Abnormalities Among Malaria Infected Adult Patients in Association with ABO Blood Groups at Jinella Health Center, Harar, Eastern Ethiopia
Authors Asmerom H , Gemechu K , Sileshi B, Arkew M
Received 4 May 2023
Accepted for publication 11 August 2023
Published 21 August 2023 Volume 2023:14 Pages 463—476
DOI https://doi.org/10.2147/JBM.S419815
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Haftu Asmerom, Kabtamu Gemechu, Beza Sileshi, Mesay Arkew
School of Medical Laboratory Sciences, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
Correspondence: Haftu Asmerom, School of Medical Laboratory Sciences, College of Health and Medical Sciences, Haramaya University, P.O. Box: 235, Harar, Ethiopia, Tel +251910089878, Email [email protected]
Background: Hematological abnormalities are a common complication of malaria infection. However, there is a paucity of evidence regarding it among malaria-infected adult patients in association with the ABO blood group in Ethiopia, particularly in the Harari Region. Therefore, this study aimed to assess the hematological abnormalities among malaria-infected adult patients in association with ABO blood groups at Jinella Health Center, Harar, Eastern Ethiopia.
Methods: An institutional-based cross-sectional study was conducted from July 10, 2022, to January 10, 2023. Four milliliters of venous blood were collected from each study participant. Drops of blood were used for blood film preparation. ABO blood group was determined by agglutination test using monoclonal anti-sera (Agape Diagnostics Ltd., India). A complete blood count was done using the DxH 800 (Beckman Coulter, Inc, Miami, FL) hematology analyzer. The data were analyzed using SPSS version 26. Bivariable and multivariable logistic regression models were fitted. The level of significance was declared at a p-value of < 0.05.
Results: The study revealed that 47.2% (95% CI: 41.0 53.6) of the participants were anemic. Being female (AOR = 3.18, 95% CI = 1.67, 6.04), having the A blood group (AOR = 2.75, CI = 1.20, 6.31), and being infected with P. falciparum (AOR = 2.64, CI = 1.26, 5.53) were all significantly associated with malaria anemia. The overall prevalence of thrombocytopenia was also 67.7% (95% CI: 61.7– 73.4%). It was significantly associated with P. falciparum infection (AOR = 8.03, CI = 3.53, 18.25) and high parasitemia levels (AOR = 4.40, CI = 1.57, 12.32).
Conclusion: Patients with malaria who belonged to the “A” blood group in the study area had anemia as a serious health problem. Hence, frequently checking for anemia in patients with malaria who have blood group “A” can help with early detection and better management of anemia.
Keywords: malaria, hematological abnormalities, blood groups, Harar, Ethiopia
Background
The two Plasmodium species, Plasmodium falciparum (P. falciparum) and Plasmodium vivax (P. vivax) are the principal causes of malaria and pose the greatest risks to the world’s population.1 The most frequent side effects of malaria infection are hematological abnormalities, which affect red blood cells (RBC), white blood cells (WBC), and platelets (PLT).2,3
Hematological abnormalities are caused by P. falciparum-encoded polypeptides (RIFINs), sub-telomeric variants (STEVOR), and P. falciparum erythrocyte membrane protein 1 (PfEMP1) to ensure antigenic variation and evasion of host immunity and mediate the binding of parasite-infected RBC to the vascular endothelium (cytoadherence), to RBC (rosetting), to WBC, and sequestration of cells.4–6 The expression of STEVOR on the RBC leads to PfEMP1-independent binding of infected RBCs to uninfected RBCs (rosette formation), while antibodies targeting STEVOR in the merozoite can effectively inhibit invasion.7
The A, B, and H carbohydrate antigens on the surface of RBC make up the ABO blood group system, which is the most significant one in clinical practice when it comes to the relationship between malaria infection.8 It is one of the hereditary factors that may affect a person’s vulnerability to the clinical effects of malaria.5,8–10 Anemia, thrombocytopenia, leukopenia, and leukocytosis are hematological abnormalities that have been associated with malaria infection and are thought to be its distinguishing feature.11
Cytoadherence and rosetting contribute to the pathophysiology of malaria by obstructing blood flow, resulting in a shortage of oxygen in tissues, excessive lactate formation, and a decline in the pH of blood and tissues, which can result in severe anemia.5 It is characterized by hemoglobin (Hgb) levels that are below 12 g/dl for women and 13 g/dl for males.12
Another very common malarial consequence is thrombocytopenia, which is characterized by low PLT levels (<150 × 103 cells/μL).13 Leukopenia and leukocytosis are also seen throughout the world, notably in Africa.14,15 A study carried out in Ethiopia also revealed leucopenia and leukocytosis among malaria-infected patients.16 The ABO blood group is one of the genetic factors linked to human susceptibility to severe malaria infection.5 Some researchers have looked at the molecular nature of the interaction between the ABO blood group and P. falciparum via the binding of parasitized RBC to RBC.5,17 Yet, and this is crucial, the ABO blood group antigens are equally expressed on the vascular endothelium and serum proteins, indicating that they may be responsible for the development of severe malaria.18
According to several studies, people with blood groups A and B are more likely to develop severe malaria than people with blood group O19,20 and have a higher frequency of anemia.21 On the other hand, a few studies revealed that people with the O blood group had the highest proportion of malaria infections, followed by those with the A blood group.19,22 Hematological abnormalities have been observed in malaria patients. Nevertheless, there is not much information available regarding the prevalence of anemia, thrombocytopenia, leukopenia, and leukocytosis in association with the ABO blood groups. Therefore, this study aimed to assess the hematological abnormalities among malaria-infected adult patients in association with ABO blood groups at Jinella Health Center, Harar, Eastern Ethiopia.
Materials and Methods
Study Design, Area, and Period
A cross-sectional study was conducted in the Harar region, which is one of the ten regional states of the Federal Democratic Republic of Ethiopia, with Harar as the capital city. This region is found 526 kilometers east of Addis Ababa, the capital city of Ethiopia. According to the Harari Regional Health Bureau’s 2018 annual report, the region consists of four hospitals (two of which are public and private hospitals), and ten health centers. Of those ten health centers, the study was carried out at Jinella Health Center from July 10, 2022, to January 10, 2023.
Study Participants
The study population consisted of all adult patients (aged between 18 and 65 years old) who had been confirmed to have malaria and gave their consent to take part in the study. Contrarily, individuals who were positive for infectious diseases (Human Immunodeficiency Virus (HIV), Hepatitis B Surface Antigen (HBsAg), and Hepatitis C Virus (HCV)), patients who were pregnant, patients who had intestinal parasites, and patients with history of chronic disease (hypertension, heart disease, and diabetes mellitus) obtained through clinical records or patient cards were excluded from the study.
Sample Size Determination and Sampling Technique
The sample size for the prevalence of thrombocytopenia among malaria-infected patients was determined using single population proportion formula considering the following assumptions: 95% confidence level, 5% margin of error, and proportion of thrombocytopenia among malaria-infected patients conducted in Ethiopia (84%).23 This provided us with a sample size of 206. The sample size for the hematology abnormality in association with ABO blood groups was calculated in Open Epi Info 7.2.5 statistical software assuming the following assumptions: 95% confidence level, 80% power, equal unexposed to the exposed ratio (1:1), the proportion of anemia among malaria patients of A blood group (41.6%) and B blood group (22.9%).21 This provided a sample size of 231. The sample size calculated for the proportion of malaria anemia among blood groups (231) was used for this study as it was greater than the calculated sample size for thrombocytopenia among malaria-infected patients. After the addition of 10% of non-responders, a total of 254 study participants were recruited using a convenience sampling technique.
Data Collection Procedure
After being written in English, the questionnaire was translated into two regional dialects (Amharic and Afan Oromo), and then back into English. Before data collection, collectors selected patients who qualified for the inclusion criteria and obtained informed consent. Socio-demographic information, clinical records, and medical history of patients were collected by administering the questionnaire under the supervision of the principal investigator.
Laboratory Sample Collection and Malaria Diagnosis
Four mL of venous blood was drawn from each patient and put into ethylene diamine tetraacetic acid (EDTA) tubes using a syringe and needle. For microscopic analysis, 1 drop (about 30 µL) and 2 drops (about 60 µL) of blood from each EDTA test tube were used to prepare thin and thick blood films on labeled slides, respectively. Methanol was used to fix the thin film for 10 seconds. Later, both films were stained with 10% Giemsa for 10 minutes, then carefully washed and air-dried. The slides were examined under a light microscope with an oil immersion (x100) objective.24 The parasitemia level was calculated on a thick blood film using the following formula; 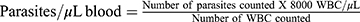 25 and it was defined as high parasitemia (>10 parasites per field), moderate parasitemia (1–10 parasites per field), and low parasitemia (1–100 parasites per 100 fields).12
25 and it was defined as high parasitemia (>10 parasites per field), moderate parasitemia (1–10 parasites per field), and low parasitemia (1–100 parasites per 100 fields).12
Blood Group Determination
ABO blood groups were typed using commercially available monoclonal antisera (Agape Diagnostics Ltd., India). Two drops of whole blood from an EDTA test tube were placed in each of three grease-free, clean glass slides on which the same proportion (two drops) of antisera for blood groups A, B, and Rh was applied. The blood and the antisera were mixed with an applicator stick. The slide was then tilted to detect agglutination, and the result was recorded accordingly as blood groups A+, B+, AB+, and O+, or A-, B-, AB-, and O-.26
Determination of Hgb Concentration, Total WBC, and PLT Counts
The remaining blood sample in the EDTA test tube was used to determine Hgb concentration, RBC, total WBC, and PLT counts using an automated hematology analyzer UniCel DxH 800 Coulter (Beckman Coulter, Inc, Miami, FL), following the protocol of the manufacturer. Major blood cells such as Hgb, RBC, total WBC count, and PLT were recorded. To define anemia in the patients, hemoglobin values of <12 g/dl for women and <13g/dl for men were used. Anemia was further classified as mild (Hgb 10.0–10.9 g/dl), moderate (Hgb 7–9.9 g/dl), and severe (Hgb <7g/dl).27,28 A WBC count of >10,000 cells/μL was classified as leukocytosis, while leucopenia was defined as a WBC count <4000 cells/μL.29 A PLT count <150, 000 cells/µL was used to define thrombocytopenia. It was further classified as mild (PLT between 100,000 and 150,000 cells/µL), moderate (PLT between 50,000 and 100,000 cells/µL), severe (PLT <50, 000 cells/µL).30,31
Statistical Analysis of the Data
Following data collection, the data were edited and cleaned, and each questionnaire’s completion and coding were verified. Data were entered into Epi-Data version 4.4.2.1, and all data were edited, organized, coded, exported, and analyzed using Statistical Package for Social Sciences (SPSS) version 26 statistical software. Tables, percentages, and frequencies were used to describe categorical variables. The normal distribution of continuous data was examined using the Kolmogorov–Smirnov test. Bivariable logistic regression analysis was used, and a Crude Odds Ratio (COR) with a 95% confidence interval (CI) was computed to assess the association between each independent variable and the outcome variables. Variables with a p-value of < 0.25 were considered candidates for multivariable logistic regression analysis. Model goodness-of-fit was checked by the Hosmer and Lemeshow test, and the final model was well-fitted with the included independent variables (P value = 0.82). The final model was performed to control the confounding variables and identify the associated factors by estimating the Adjusted Odds Ratio (AOR) with a 95% CI. Statistical significance was declared at a p-value of < 0.05.
Data Quality Control
The questionnaire was translated into the local dialects (Afan Oromo and Amharic) for data collection and then translated back into English. A week before the actual data collection, 5% of the questionnaires were pre-tested among adult patients having malaria at the Babile Health Center, which is about 24.9 kilometers from the study area. The completeness of each questionnaire was checked by the investigators daily. All laboratory activities were performed with strict adherence to standard operating procedures (SOP). The quality and expiration dates of the methanol used for thin blood fixation and Giemsa stain were checked. Giemsa was used to stain positive malaria slides to ensure the product’s quality. Before deeming the slide negative, up to 100 high-power fields were viewed. An expert laboratory technologist reviewed any discrepancies in the blood film results between the two laboratory professionals. Strict adherence to the automated hematology instructions was observed. Quality checks were carried out daily using low, normal, and high control levels to track an instrument’s performance over time before beginning sample analysis.
Ethical Consideration
The ethical approval was received from the Institutional Health Research Ethics Review Committee (IHRERC), College of Health and Medical Sciences, Haramaya University. Informed, voluntary, written, and signed consent was obtained before the initiation of the study from each participant and the health center head. Both the measurements and the interviews took place in private settings in different rooms. All possible identifiers were excluded from the questionnaires to protect participant confidentiality. The study was carried out in accordance with the Declaration of Helsinki.
Results
Socio-Demographic Characteristics
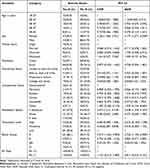
Of the total of 254 malaria-infected patients who took part in the study, 139 (54.7%) were male. The highest 86 (33.9%) number of participants was found to be within the age range of 18–27 years, while the least number was found to be within the age range of 58–65 years. One hundred thirty-six (53.5%) of the respondents were married, and 7 (2.8%) were widowed. The majority, 144 (56.7%) were living in rural areas, and 93 (36.6%) were able to write and read. Regarding occupational status, the highest 70 (27.5%) number of the study participants were farmers (Table 1).
 |
Table 1 Socio-Demographic Characteristics of the Study Participants at Jinella Health Center, Harar, Eastern Ethiopia from July 10, 2022-January 10, 2023 (N = 254) |
Association of ABO Blood Group with Malaria Infection
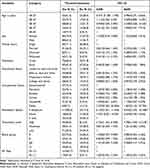
The ABO blood group of all malaria patients was also examined. Accordingly, the highest prevalence of malaria infection was observed among subjects with blood groups A and B, with a prevalence of 91 (35.8%) and 77 (30.3%), respectively. While individuals with blood group AB (34 (13.4%)) were the least affected. Predominantly, 64 (70.3%) A blood group, 21 (61.8%) AB blood group, and 25 (48.1%) O blood group patients were infected with P. falciparum whereas the highest 36 (46.8%) of B blood group malaria patients were infected with P. vivax. Eleven (12.1%) A blood group patients and 5 (14.7%) AB blood group patients were infected with mixed infections. Overall, there was a statistically significant association between ABO blood groups and malaria severity (χ2 = 19.575, p = 0.003). Regarding the Rh factor, the majority of 226 (89.0%) malaria patients were Rh-positive. One hundred twenty-seven (88.2%), 71 (88.8%), and 28 (93.3%) malaria-infected with P. falciparum, P. vivax, and mixed infection were Rh-positive, respectively. But there was no statistically significant association between the Rh factor and malaria severity (χ2 = 0.675, p = 0.71). (p = 0.71) (Table 2).
 |
Table 2 Cross-Tabulation of Chi-Square Analysis of ABO Blood Groups Among Malaria-Infected Adult Patients at Jinella Health Center, Harar, Eastern Ethiopia from July 10, 2022-January 10, 2023 (N=254) |
Association of ABO Blood Groups with Anemia
The mean hemoglobin level of the study participants was 12.1g/dl ± 2.8 and ranged from 5.7 to 17.4 g/dl. The overall prevalence of anemia among malaria-infected adult patients was 47.2% (95% CI: 41.0–53.6). Out of this prevalence, 53 (20.9%), 60 (23.6%), and 7 (2.8%) had mild, moderate, and severe anemia, respectively. Blood group A individuals (51.7%) had the highest prevalence of anemia, followed by blood group B individuals (22.5%). The lowest prevalence of anemia was 7.5% among blood group AB individuals. The most severe anemia was detected in individuals with blood group A (5 (71.4%)). Twenty-eight (46.7%) and 16 (26.7%) of the A and B blood groups, respectively, showed the highest levels of moderate anemia. The highest mild anemia appeared among 29 (54.7%) of blood group A individuals, and the lowest was among 3 (5.7%) of blood group AB individuals. There was a statistically significant association between the ABO blood group and the anemia status of the study participants (p < 0.001). The majority of mild, moderate, and severe anemias were Rh (+), with proportions of 47 (88.7%), 54 (90.0%), and 5 (71.4%), respectively (Table 3).
 |
Table 3 Cross-Tabulation of Chi-Square Analysis of ABO Blood Groups with Anemia Among Study Participants at Jinella Health Center, Harar, Eastern Ethiopia from July 10, 2022-January 10, 2023 (N =120) |
Association of ABO Blood Groups with Thrombocytopenia
The mean platelet value of the study participants was 140×103 cells/μL± 84.3 and ranged from 39 to 405×103 cells/μL. The overall prevalence of thrombocytopenia was 67.7% (95% CI: 61.6–73.4). The highest prevalence of thrombocytopenia was detected among individuals with blood group A (67 (39.0%)), followed by blood group B (47 (27.3%)). The lowest prevalence of thrombocytopenia was 24 (14.0%) in blood group AB individuals. Out of the total participants, 84 (33.1%), 60 (23.6%), and 28 (11.0%) had mild, moderate, and severe thrombocytopenia, respectively. The most severe thrombocytopenia was detected in individuals with blood group B (11 (39.6%)). However, the highest prevalence of moderate and mild thrombocytopenia was found among blood group A participants, with a prevalence of 22 (36.7%) and 36 (42.9%), respectively. There was no statistically significant association between the ABO blood group and thrombocytopenia in the study participants (p = 0.319). The majority of mild, moderate, and severe thrombocytopenia were Rh (+), with proportions of 71 (84.5%), 55 (91.7%), and 27 (96.4%), respectively (Table 4).
Association of ABO Blood Group with WBC Alteration
The mean WBC value of the study participants was 6.1×103 cells/μL± 3.1 and ranged from 2.2 to 14.1×103 cells/μL. Of the total number of study participants, 81 (31.9%) of them had leukopenia, and 33 (13.0%) of them also had leukocytosis. The highest number of leukopenia was observed among participants with blood group A (32 (39.5%)), followed by blood group O (21 (25.9%)). However, the lowest prevalence of leukopenia was 11 (13.6%) among blood group AB individuals. Seventy-two (88.9%) who had leukopenia were RH (+). Regarding leukocytosis, the highest number of leukocytosis was detected in blood group B (11 (33.3%)). However, the least leukocytosis appeared among blood group AB (4 (12.1%)) participants. Twenty-nine (87.9%) study participants who had leukocytosis were RH (+). There was no statistically significant association between the ABO blood group, Rh type, and WBC alterations (P = 0.243) (Table 5).
Factors Associated with Anemia
Bivariable and multivariable binary logistic regression analyses were performed to determine parameters associated with the anemia status of adult malaria patients. In the bi-variable binary logistic regression analysis; sex, marital status, educational status, parasitemia level, Plasmodium species, and blood group were significant at a p-value of <0.25 and considered candidates for multivariable logistic regression analysis. In multivariable logistic regression, sex, Plasmodium species, and blood group were found to have a significant association with anemia at a p-value of <0.05. The odds of being anemic among female malaria-infected patients were 3.2 times more likely (AOR = 3.18, 95% CI = 1.67, 6.04) than their male counterparts. Malaria patients infected with P. falciparum were 2.64 times more likely (AOR = 2.64, CI = 1.259, 5.53) to develop anemia than those infected with P. vivax. Blood group A malaria-infected patients were 2.75 times more likely (AOR = 2.75, CI = 1.20, 6.31) to develop anemia than blood group O patients (Table 6).
Factors Associated with Thrombocytopenia
Both bi-variable and multivariable binary logistic regression analyses were conducted to identify factors associated with thrombocytopenia in malaria-infected adult patients. In the bi-variable binary logistic regression analysis; sex, educational status, occupational status, parasitemia level, and Plasmodium species were significantly associated at a p-value of <0.25 and considered candidates for multivariable logistic regression analysis. In multivariable logistic regression, Plasmodium species and parasitemia level were found to have a significant association with thrombocytopenia at a p-value of <0.05. Malaria patients infected with P. falciparum were 8.03 times more likely (AOR = 8.03, CI = 3.53, 18.25) to develop thrombocytopenia than those infected with P. vivax. Malaria-infected patients with a high parasitemia level were 4.40 times more likely (AOR = 4.40, CI = 1.57, 12.32) to develop thrombocytopenia than malaria-infected patients with a low parasitemia level (Table 7).
Discussion
Hematological abnormalities are one of the most common complications of malaria infection, along with malarial anemia, thrombocytopenia, and leukocytosis or leucopenia.12 The findings of this study assessed the hematological abnormalities among malaria-infected adult patients in association with ABO blood groups at Jinella Health Center, Harar, Eastern Ethiopia. Accordingly, the hemoglobin test result showed that 47.2% of study participants were anemic. Being female, having an “A” blood group, and being infected with P. falciparum were identified as associated factors of anemia among malaria-infected patients in association with ABO blood groups. On the other hand, A PLT count <150, 000 cells/µL was used to define thrombocytopenia, and 67.7% of study participants had thrombocytopenia. Parasitemia level and P. falciparum were identified as associated factors of thrombocytopenia among malaria-infected patients in association with ABO blood groups.
The present study showed significantly varied degrees of malaria infection among the ABO blood groups (P = 0.003). The highest (35.8%) malaria infection was observed among individuals with A (35.8%), followed by B (30.3%), and O (20.5%) blood groups, while individuals with AB (13.4%) blood groups were the least affected. This result is in agreement with the findings done in Sri Lanka,32 Sudan,33 and across different corners of Ethiopia.19,22,34 However, this study was contrary to the findings conducted in China,35 Burkina Faso,36 Ghana37 and Nigeria38 which reported malaria infection was generally higher in O blood groups than other blood groups. This discrepancy in results could due to mutations in the genetic mechanisms (A, B, and H carbohydrate antigens), which can regulate protein activities during infection and antibodies against the malaria parasite.39,40
A higher proportion of malaria was found to be Rh-positive (89.0%) individuals than Rh-negative individuals, even though no statistically significant association was seen as similar studies reported in India,41 Senegal,42 and Nigeria.43,44 It is uncertain how the Rh- blood group could work as a protective or risk factor for malaria. However, the Rh-blood group changes the parasites’ adhesion properties to the RBC membrane and sequesters itself in less suitable areas for malaria infection.45
In this study, the overall anemia prevalence was 47.2%. Blood group A malaria-infected patients were 2.75 times more likely to develop anemia than blood group O patients. This finding was supported by some studies conducted in India,46 Nigeria,47 and Ethiopia.21 The high risk of anemia observed in malaria patients with blood group A might be attributed to the presence of specific receptors (band 3 and glycophorin A) and glycosylated adhesive molecules, which assist in the fast invasion of RBCs by P. falciparum.48
Whereas the current study disagreed with the results of the study conducted in Colombia49 that reported a more frequent occurrence of severe anemia among individuals with blood group O. Additionally, blood group AB subjects had the least anemia, in contrast to a study conducted in Cameroon,50 which reported the most anemic blood group. This inconsistency might be attributed to the presence of A and B antigens on the surface of RBC; cytoadherence, hence rosetting and sequestration, is increased in individuals with blood groups A and B.51 Another possible explanation may be due to the presence of sialic acid and galactose in the carbohydrate structure of glycophorin A or B, which are the major requisites for the entrance of P. falciparum merozoites into human RBC.52
The odds of being anemic among female malaria-infected patients were 3.2 times higher than those of their male counterparts. This finding was supported by a study done in India,53 and Cameroon.54 The possible explanation might be due to chronic blood loss and abnormal menstrual function among female participants.55 Malaria patients infected with P. falciparum were 2.64 times more likely to develop anemia than those infected with P. vivax. This result was in agreement with studies conducted in Colombia49 and Ethiopia.21 But in contrast with a study conducted in Australia.56 This discordant result of anemia in plasmodium species might be multifactorial and incompletely understood. But in P. falciparum, RBC sequestration reduces the proportion of parasitized RBC that traverse the spleen, while the increased deformability of infected RBC in vivax malaria may limit the proportion of RBC that are removed during passage through the splenic microcirculation.57,58
In this study, the overall prevalence of thrombocytopenia was 67.7%. The highest prevalence of thrombocytopenia was detected among study participants belonging to blood group A (39.0%), followed by blood group B (27.3%), while its distribution was lower in subjects with AB (14.0%) blood groups. The most severe thrombocytopenia was detected in individuals with blood group B (39.6%). However, the highest prevalence of moderate and mild thrombocytopenia was found among blood group A participants, with a prevalence of 22 (36.7%) and 36 (42.9%), respectively. According to the current study, no significant association was found (p = 0.319) between the blood group and thrombocytopenia, and all ABO blood groups showed similar falls in platelet levels, suggesting that thrombocytopenia was not affected by the ABO blood groups. Studies from India,59 and Pakistan60 showed similar findings.
Patients with P. falciparum malaria were 8.03 times more likely to develop thrombocytopenia than those with P. vivax malaria. This result was supported by studies conducted in India,61 Indonesia,62 Pakistan,63 and Sudan.64 The pathogenic mechanisms by which platelets mediate disease severity in patients with P. falciparum malaria remain to be delineated. However, a study has shown that the platelets of P. falciparum patients express Toll-like receptors (TLRs), which release prepared inflammatory mediators.65 Another reason for thrombocytopenia in malaria infections, particularly those brought on by P. falciparum, could be the immune-mediated degradation of circulating platelets.66
Malaria-infected patients with a high parasitemia level were 4.40 times more likely to develop thrombocytopenia than malaria-infected patients with a low parasitemia level. This result was supported by studies done in Thailand,67 Senegal,68 and Sudan.64 Evidence suggests that malaria thrombocytopenia at high parasitemia was found to significantly lower the PLT count.69 As malaria parasitemia increases, platelets play a critical role in enabling the cytoadherence of malaria-infected erythrocytes (IEs) to ECs, and Von Willebrand factor (VWF) then promotes this platelet-mediated IE cytoadherence during the malaria infection.70 Finally, platelets enhance IE clumping and sequestration, which further contributes to its decrease during the infection.71
Changes in WBC count are less dramatic than in other blood cell series and have been controversial. Of the total number of study participants, 81 (31.9%) had leukopenia. This study was in accordance with the studies conducted in Colombia72 and Pakistan.73 This could be a result of the sequestration of leukocytes, accelerated destruction, or decreased production.74 The highest number of leukopenia was observed among blood group A (39.5%), followed by blood group O study participants (25.9%), in contrast with the studies conducted in India59 and Pakistan60 which were higher in B and AB blood groups, respectively. This discrepancy in results across different studies might be due to the methods they used for the determination of WBC counts and statistical tests that assume Gaussian distributions of data.75
Regarding leukocytosis, 33 (13.0%) of the study participants had leukocytosis. The same results were reported from Burkina Faso76 and Thailand.67 The highest number of leukocytosis was detected in blood group B (33.3%), similar to a study conducted in Pakistan.60 This might be attributed to the increased production of leukocytes at the onset of an infection to engulf the invading parasite.77 The ABO blood group and WBC alterations among malaria-infected patients did not have a statistically significant correlation.
Strengths and Limitations of the Study
The study’s strength is that all hematological abnormalities were detected. However, due to its cross-sectional nature, the study is unable to demonstrate the temporal relationship between the hematological abnormalities and the identified related factors. Additionally, the study did not apply any reliable assays, like PCR, that can distinguish distinct malaria parasites with comparable characteristics.
Conclusion
Of the examined malaria-infected adult patients, almost half, more than half, one-third, and almost one-eighth of the patients had anemia, thrombocytopenia, leukopenia, and leukocytosis, respectively. There was a significant association between the ABO blood groups and susceptibility to malaria-related anemia. Being female, having an “A” blood group, and being infected with P. falciparum were identified as associated factors of anemia among malaria-infected patients about ABO blood groups. On the other hand, parasitemia level and P. falciparum were identified as associated factors of thrombocytopenia among malaria-infected patients in relation to ABO blood groups. There was no significant association between blood groups and susceptibility to malaria-developing thrombocytopenia, leukopenia, or leukocytosis. Therefore, there should be routine screening for anemia, thrombocytopenia, and WBC alterations for all malaria patients.
Abbreviations
BF, Blood Film; EDTA, Ethylene Diamine Tetra Acetic Acid; ECs, endothelial cells; Hgb, Hemoglobin; ICAM-1, Intravascular cell adhesion molecule-1; IEs, Infected erythrocytes; RBC, Red Blood Cell; P. Falciparum, Plasmodium Falciparum; PLT, Platelet; P. vivax, Plasmodium Vivax; VWF, Von Willebrand factor; WBC, White Blood Cell.
Acknowledgments
The authors are grateful to all Jinella Health Center staff members, the data collectors, supervisors, study participants, and questionnaire translators for their cooperation.
Author Contributions
All authors significantly contributed to the work that was published by participating in the ideation, study design, execution, data collection, analysis, and interpretation processes as well as in the writing, editing, and review of the article. All authors have agreed on the approval of the final manuscript to be published in the current journal and to be accountable for all aspects of the work.
Funding
This study received no specific funding from public, commercial, or not-for-profit funding agencies.
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
1. World Health Organization. Eliminating Malaria. World Health Organization; 2016.
2. Mohandas N, An X. Malaria and human red blood cells. Med Microbiol Immunol. 2012;201(4):593–598. doi:10.1007/s00430-012-0272-z
3. Cox D, McConkey S. The role of platelets in the pathogenesis of cerebral malaria. Cell Mol Life Sci. 2010;67(4):557–568. doi:10.1007/s00018-009-0211-3
4. Plewes K, Turner G, Dondorp A. Pathophysiology, clinical presentation, and treatment of coma and acute kidney injury complicating falciparum malaria. Curr Opin Infect Dis. 2018;31(1):69. doi:10.1097/QCO.0000000000000419
5. Wahlgren M, Goel S, Akhouri RR. Variant surface antigens of Plasmodium falciparum and their roles in severe malaria. Nat Rev Microbiol. 2017;15(8):479–491. doi:10.1038/nrmicro.2017.47
6. Yam XY, Niang M, Madnani KG, Preiser PR. Three is a crowd–new insights into rosetting in Plasmodium falciparum. Trends Parasitol. 2017;33(4):309–320. doi:10.1016/j.pt.2016.12.012
7. Niang M, Bei AK, Madnani KG, et al. STEVOR is a Plasmodium falciparum erythrocyte binding protein that mediates merozoite invasion and rosetting. Cell Host Microbe. 2014;16(1):81–93. doi:10.1016/j.chom.2014.06.004
8. Cserti M, Dzik H. The ABO blood group system and Plasmodium falciparum malaria. Blood. 2007;110(7):2250–2258.
9. Loscertales M, Owens S, O’Donnell J, Bunn J, Bosch‐Capblanch X, Brabin B. ABO blood group phenotypes and Plasmodium falciparum malaria: unlocking a pivotal mechanism. Adv Parasitol. 2007;65:1–50.
10. Driss A, Hibbert JM, Wilson NO, Iqbal SA, Adamkiewicz TV, Stiles JK. Genetic polymorphisms linked to susceptibility to malaria. Malar J. 2011;10(1):1–10. doi:10.1186/1475-2875-10-271
11. Das B, Ganguly R, Khuntia H, Bal M, Ranjit M. Hematological changes in severe P. falciparum malaria. Int J Curr Microbiol Appl Sci. 2017;6(6):1733–1739. doi:10.20546/ijcmas.2017.606.201
12. Al-Salahy M, Shnawa B, Abed G, Mandour A, Al-Ezzi A. Parasitaemia and its relation to hematological parameters and liver function among patients malaria in Abs, Hajjah, Northwest Yemen. Interdiscip Perspect Infect Dis. 2016;2016:1–5. doi:10.1155/2016/5954394
13. Bayleyegn B, Asrie F, Yalew A, Woldu B, Gonzalez Salazar F. Role of platelet indices as a potential marker for malaria severity. J Parasitol Res. 2021;2021:1–8. doi:10.1155/2021/5531091
14. D’souza JJ, Jayaprakash C, D’souza P, Abraham S, Suresh S, Shrinath M. Comparative hematological changes in malarial infection by P. vivax and P. falciparum: observations from the endemic region of Mangalore, India. Int J Appl Res. 2017;3(6):179–183.
15. Okafor UE, Tsoka-Gwegweni JM, Bibirigea A, Irimie A, Tomuleasa C. Parasitaemia and haematological changes in malaria-infected refugees in South Africa. S Afr Med J. 2016;106(4):413–416. doi:10.7196/SAMJ.2016.v106i4.9758
16. Sirak S, Fola A, Worku L, Biadgo B. Malaria parasitemia and its association with lipid and hematological parameters among malaria-infected patients attending at Metema Hospital, Northwest Ethiopia. Pathol Lab Med Int. 2016;8:43–50. doi:10.2147/PLMI.S118946
17. Goel S, Palmkvist M, Moll K, et al. RIFINs are adhesins implicated in severe Plasmodium falciparum malaria. Nat Med. 2015;21(4):314–317. doi:10.1038/nm.3812
18. Needs M. Human blood groups (3rd edn). Br J Biomed Sci. 2013;70(3):131.
19. Tadesse H, Tadesse K. Assessing the association of severe malaria infection and ABO blood groups in northwestern Ethiopia. J Vector Borne Dis. 2013;50(4):292.
20. Gayathri B, Harendra K, Gomathi N, Jeevan S, Reethesh R. Relationship between ABO blood groups and malaria with clinical outcome in rural area of South India. Glob J Med Public Health. 2013;2(2):1–7.
21. Tazebew B, Munshea A, Nibret E. Prevalence and association of malaria with ABO blood group and hemoglobin level in individuals visiting Mekaneeyesus Primary Hospital, Estie District, northwest Ethiopia: a cross-sectional study. Parasitol Res. 2021;120(5):1821–1835. doi:10.1007/s00436-021-07093-z
22. Zerihun T, Degarege A, Erko B. Association of ABO blood group and Plasmodium falciparum malaria in Dore Bafeno Area, Southern Ethiopia. Asian Pac J Trop Biomed. 2011;1(4):289–294. doi:10.1016/S2221-1691(11)60045-2
23. Awoke N, Arota A. Profiles of hematological parameters in Plasmodium falciparum and Plasmodium vivax malaria patients attending Tercha General Hospital, Dawuro Zone, South Ethiopia. Infect Drug Resist. 2019;12:521. doi:10.2147/IDR.S184489
24. World Health Organization, &UNICEF. Microscopy for the Detection, Identification and Quantification of Malaria Parasites on Stained Thick and Thin Blood Films in Research Settings: Procedure: Methods Manual. World Health Organization; 2015.
25. World Health Organization. Malaria Parasite Counting; Malaria Microscopy Standard Operating Procedures. World Health Organization; 2016. Available from: WHO/HTM/GMP/MM/SOP/2016.09.
26. Otajevwo F. Prevalence of malaria parasitaemia and its association with ABO blood grouping among students of Igbinedion University Okada, Nigeria. Br J Med Med Res. 2013;3(4):1164–1177. doi:10.9734/BJMMR/2013/1745
27. Lanier J, Park J, Callahan R. Anemia in older adults. Am Fam Physician. 2018;98(7):437–442.
28. Murphy J. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011.
29. Ngum NH, Fakeh NB, Lem AE, Mahamat O. Prevalence of malaria and associated clinical manifestations and myeloperoxidase amongst populations living in different altitudes of Mezam division, North West Region, Cameroon. Malar J. 2023;22(1):1–14. doi:10.1186/s12936-022-04438-6
30. Izak M, Bussel JB. Management of thrombocytopenia. F1000Prime Rep. 2014;6. doi:10.12703/P6-6
31. Mangrio GM, Memon MA, Shaikh MA, Memon HNA, Arwani S, Shah SZA. Vivax malaria: frequency, severity of thrombocytopenia and variation in Red Cell Distribution Width (RDW). Prof MedJ. 2015;22(05):559–564. doi:10.29309/TPMJ/2015.22.05.1267
32. Pathirana S, Alles H, Bandara S, et al. ABO-blood-group types and protection against severe, Plasmodium falciparum malaria. Ann Trop Med Parasitol. 2005;99(2):119–124. doi:10.1179/136485905X19946
33. Satti SMA. Association of ABO and Rh Blood Groups with Malaria Infection in 24 Algorashi Area in Aljazeera State-Sudan. Sudan University of Science & Technology; 2017.
34. Tekeste Z, Petros B. The ABO blood group and Plasmodium falciparum malaria in Awash, Metehara and Ziway areas, Ethiopia. Malar J. 2010;9(1):1–4. doi:10.1186/1475-2875-9-280
35. Zhang X, Yang M, Zhao H, Hu J, Li L. Relationship between malaria and ABO blood types in East China. Biomed Res Int. 2017;2017:1–3. doi:10.1155/2017/8163762
36. Bougouma EC, Ouedraogo A, Sirima SB. The ABO Blood Group System and Plasmodium Falciparum (Pf) Infection in Three Ethnic Groups Living in the Stable and Seasonal Malaria Transmission Areas of Burkina Faso (BF). IntechOpen; 2022.
37. Osisiogu EU, Agyapong GA, Mahmoud FC, Waqas FB, Appiah C, Nikoi CN. Prevalence of malaria among ABO blood groups in Ghana: a Case Study of Adentan Municipality. Int J Pathog Res. 2023;12(1):21–29. doi:10.9734/ijpr/2023/v12i1217
38. Tela I, Modibbo M, Adamu L, Taura M. Prevalence of malaria infection among ABO blood groups in Jama’are, Nigeria. RA J ApplRes. 2015;1(7):255–262.
39. Dolo A, Modiano D, Maiga B, et al. Difference in susceptibility to malaria between two sympatric ethnic groups in Mali. Am J Trop Med Hyg. 2005;72(3):243–248. doi:10.4269/ajtmh.2005.72.243
40. Seyoum S, Dagne K. ABO and rhesus blood-type frequencies in data from hospitals and the Red Cross in Ethiopia. Ethiop Med J. 1985;23(1):1–6.
41. Singh G, Urhekar A, Singh R. A study on correlation of malaria infection with A, B, O, RH blood group system. J Parasitolo Vector Biol. 2015;7(4):67–73.
42. Wotodjo AN, Richard V, Boyer S, et al. The implication of long-lasting insecticide-treated net use in the resurgence of malaria morbidity in a Senegal malaria endemic village in 2010–2011. Parasit Vectors. 2015;8(1):1–11. doi:10.1186/s13071-015-0871-9
43. Abah A, Grey A, Onoja H. Plasmodium malaria and ABO blood group among blood donors in Yenegoa, Bayelsa State, Nigeria. J Prim Health Care. 2016;6(4):1–4.
44. Ito E, Egwunyenga A, Ake J. Prevalence of malaria and human blood factors among patients in Ethiope East, Delta State, Nigeria. Int J Med Biomed Res. 2014;3(3):191–201. doi:10.14194/ijmbr.3.3.7
45. Rattanapan Y, Duangchan T, Wangdi K, Mahittikorn A, Kotepui M. Association between rhesus blood groups and malaria infection: a systematic review and meta-analysis. Trop Med Infect Dis. 2023;8(4):190. doi:10.3390/tropicalmed8040190
46. Ramalingam L, Raghavan GV. Association between blood groups and blood hemoglobin levels in rural population of Kanchipuram district of Tamil Nadu. Natl J Physiol Pharm Pharmacol. 2020;10(6):495.
47. Mbah JO, Njoku O, Nnachi AU, Nnachi IA, Nwinyimagu AJ. Incidence of antenatal malaria parasitaemia and the effect on the haemoglobin profile of pregnant women in Enugu East Local Government Area, Enugu, Nigeria. Am J Epidemiol Infect Dis. 2015;3(5):88–94.
48. Degarege A, Medhin G, Animut A, Legess M, Erko B. Association of ABO blood group and P. falciparum malaria related outcomes: a cross-sectional study in Ethiopia. Acta Trop. 2012;123(3):164–169. doi:10.1016/j.actatropica.2012.04.012
49. Herrera AM, Montoya LP, Arboleda M, Ortiz LF. Association of severe malaria with ABO-blood group types in an endemic zone of Colombia. CES Med. 2009;23(2):7–14.
50. Kwenti TE, Kwenti TDB. Anaemia and its association with month and blood phenotype in blood donors in Fako division, Cameroon. BMC Hhematol. 2016;16:1–7. doi:10.1186/s12878-016-0070-8
51. Afoakwah R, Aubyn E, Prah J, Nwaefuna EK, Boampong JN. Relative susceptibilities of ABO blood groups to Plasmodium falciparum malaria in Ghana. Adv Hematol. 2016;2016:1–4. doi:10.1155/2016/5368793
52. Abegaz SB, Erg n S. Human ABO blood groups and their associations with different diseases. Biomed Res Int. 2021;2021:1–9. doi:10.1155/2021/6629060
53. Shankar H, Singh MP, Hussain SSA, Phookan S, Singh K, Mishra N. Epidemiology of malaria and anemia in high and low malaria-endemic north-eastern districts of India. Front Public Health. 2022; 2022:10.
54. Sumbele IUN, Sama SO, Kimbi HK, Taiwe GS. Malaria, moderate to severe anaemia, and malarial anaemia in children at presentation to hospital in the Mount Cameroon area: a cross-sectional study. Anemia. 2016;2016:1–12. doi:10.1155/2016/5725634
55. Kaur IP, Kaur S. A comparison of nutritional profile and prevalence of anemia among rural girls and boys. J Exerc Sci Physiother. 2011;7(1):11–18. doi:10.18376//2011/v7i1/67618
56. Douglas NM, Anstey NM, Buffet PA, et al. The anaemia of Plasmodium vivax malaria. Malar J. 2012;11(1):1–14. doi:10.1186/1475-2875-11-135
57. Dondorp AM, Angus B, Chotivanich K, et al. Red blood cell deformability as a predictor of anemia in severe falciparum malaria. Am J Trop Med Hyg. 1999;60(5):733–737. doi:10.4269/ajtmh.1999.60.733
58. Suwanarusk R, Cooke BM, Dondorp AM, et al. The deformability of red blood cells parasitized by Plasmodium falciparum and P. vivax. J Infect Dis. 2004;189(2):190–194. doi:10.1086/380468
59. Deepa AV, Rameshkumar K, Ross C. ABO blood groups and malaria related clinical outcome. J Vector Borne Dis. 2011;48(1):7–11.
60. Burhan H, Hasan AS, Mansur-ul-Haque S, Zaidi G, Shaikh T, Zia A. Association between blood group and susceptibility to malaria and its effects on platelets, TLC, and Hb. J Infect Dev Ctries. 2016;10(10):1124–1128. doi:10.3855/jidc.6828
61. Gill MK, Makkar M, Bhat S, Kaur T, Jain K, Dhir G. Thrombocytopenia in malaria and its correlation with different types of malaria. Ann Trop Med Public Health. 2013;6(2). doi:10.4103/1755-6783.116521
62. Dini S, Douglas NM, Poespoprodjo JR, et al. The risk of morbidity and mortality following recurrent malaria in Papua, Indonesia: a retrospective cohort study. BMC Med. 2020;18:1–12. doi:10.1186/s12916-020-1497-0
63. Khan SJ, Abbass Y, Marwat MA. Thrombocytopenia as an indicator of malaria in adult population. Malar Res Treat. 2012;2012:1–4. doi:10.1155/2012/405981
64. Elnaim EG, Amer S, Abdalmanan M, et al. Association of thrombocytopenia, urine malaria antigens, and blood groups with malaria parasite density among Sudanese malaria patients at Sharg Al-Nile District in Khartoum State. Int J Adv Res Biol Sci. 2020;7(3):17–22.
65. Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123(18):2759–2767.
66. Pain A, Ferguson DJ, Kai O, et al. Platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes is a common adhesive phenotype and is associated with severe malaria. Proc Natl Acad Sci. 2001;98(4):1805–1810. doi:10.1073/pnas.98.4.1805
67. Kotepui M, Piwkham D, PhunPhuech B, Phiwklam N, Chupeerach C, Duangmano S. Effects of malaria parasite density on blood cell parameters. PLoS One. 2015;10(3):e0121057. doi:10.1371/journal.pone.0121057
68. Sylla K, Tine R, Sow D, et al. Anemia, thrombocytopenia, and changes in biochemical parameters occurring in patients with uncomplicated Plasmodium falciparum malaria: data analysis from antimalarial efficacy-randomized trials in Dakar and Kaolack Regions, Senegal. J Parasitol Res. 2022;2022. doi:10.1155/2022/1635791
69. Kochar D, Kochar S, Agrawal R, et al. The changing spectrum of severe falciparum malaria: a clinical study from Bikaner (northwest India). J Vector Borne Dis. 2006;43(3):104.
70. O’Sullivan JM, Preston RJ, O’Regan N, O’Donnell JS. Emerging roles for hemostatic dysfunction in malaria pathogenesis. Blood. 2016;127(19):2281–2288.
71. McMorran BJ, Marshall VM, de Graaf C, et al. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science. 2009;323(5915):797–800. doi:10.1126/science.1166296
72. Tobón-Castaño A, Mesa-Echeverry E, Miranda-Arboleda AF. Leukogram profile and clinical status in vivax and falciparum malaria patients from Colombia. J Trop Med. 2015;2015:796182. doi:10.1155/2015/796182
73. Ullah I, Ali MU, Ali S, Rafiq A. Sattar Z, Hussain S. Hematological Profile of Patients Having Malaria-positive Peripheral Blood Smears: A Cross-sectional Study at a Diagnostic Research Center in Khyber Pakhtunkhwa, Pakistan. Cureus. 2018;10(9):1–12. doi:10.7759/cureus.3376
74. Okeke OP, Imakwu CA, Eyo JE, Okafor FC. Effects of childhood malaria on the biochemical and haematological profiles of infected children in Anambra State, Nigeria. Int J Trop Dis. 2016;19(3):1–14.
75. McKenzie FE, Prudhomme WA, Magill AJ, et al. White blood cell counts and malaria. J Infect Dis. 2005;192(2):323–330. doi:10.1086/431152
76. Modiano D, Sirima BS, Konaté A, Sanou I, Sawadogo A. Leucocytosis in severe malaria. Trans R Soc Trop Med Hyg. 2001;95(2):175–176. doi:10.1016/S0035-9203(01)90152-X
77. Kayode O, Kayode A, Awonuga O. Status of selected hematological and biochemical parameters in malaria and malaria-typhoid co-infection. J Biol Sci. 2011;11(5):367–373. doi:10.3923/jbs.2011.367.373
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.