Back to Journals » Journal of Blood Medicine » Volume 15
Hematological Abnormalities and Associated Factors Among Patients with Hypothyroidism at the University of Gondar Comprehensive Specialized Hospital
Authors Berta DM , Gelaw Y , Shiferaw E , Melkamu A , Legese GL , Adane T , Mandefro B
Received 18 December 2023
Accepted for publication 16 March 2024
Published 23 March 2024 Volume 2024:15 Pages 157—169
DOI https://doi.org/10.2147/JBM.S453015
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Dereje Mengesha Berta,1 Yemataw Gelaw,1 Elias Shiferaw,1 Abateneh Melkamu,2 Gebrehiwot Lema Legese,3 Tiruneh Adane,1 Befikad Mandefro4
1Department of Hematology and Immunohematology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Medical Laboratory Science, College of Medicine and Health Sciences, Debre Markos University, Debre Markos, Ethiopia; 3Department of Internal Medicine, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 4Department of Medical Laboratory Science, College of Medicine and Health Sciences, Arba Minch University, Arba Minch, Ethiopia
Correspondence: Dereje Mengesha Berta, Department of Hematology and Immunohematology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia, Tel +251910594497, Email [email protected]
Objective: Abnormalities in blood cells are frequently associated with thyroid hormone disorders as a result of their involvement in the proliferation and production of blood cells. This study aimed to determine the magnitude and associated factors of hematological abnormalities in patients with hypothyroidism.
Methods: A cross-sectional study was conducted from January 1 to June 30, 2023, at the University of Gondar Comprehensive Specialized Hospital. The present study included a total of 300 patients with hypothyroidism prospectively using the systematic random sampling technique. The hematological parameter data were collected using data extraction sheets, whereas the associated factor data were collected using both structured questionnaires and data extraction sheets. For complete blood cell counts, 4 mL of anticoagulated venous blood was collected and analyzed. The data were entered into Epi-data version 3.1 and analyzed with Stata version 14. Both bivariate and multivariate logistic regressions were performed to identify factors associated with hematological abnormalities. A P value < 0.05 was considered to indicate statistical significance.
Results: The median value of red blood cell, hemoglobin, mean cell volume, white blood cell, and platelet were 4.63 x1012/μL, 14 g/dL, 84.3fl, 5.3 x103/μL, and 228, respectively. The overall incidences of anemia, leucopoenia, and thrombocytopenia in patients with hypothyroidism were 26.3% (95% confidence interval (CI): 21– 32), 15.7% (95% CI: 14.2– 17.2), and 9% (95% CI: 7.5– 10.5), respectively. Lymphopenia was detected in 9% (95% CI: 8.6– 10.1) of the patients, and neutropenia was detected in 6% (95% CI: 4.4– 7.6) of the patients. Only three factors, female sex (adjusted odds ratio (AOR) =2.1, 95% CI=1.3– 3.1), alcohol consumption (AOR= 3.8, CI=1.7– 8.9), and febrile illness (AOR=2.7, 95% CI=1.3– 5.4), were found to be significantly associated factors for anemia.
Conclusion: The present study revealed heterogeneous hematological abnormalities in patients with hypothyroidism. Thus, early diagnosis and monitoring strategies are required to minimize complications in patients.
Keywords: hematological abnormalities, anemia, leucopenia, thrombocytopenia, thyroid dysfunction
Introduction
Thyroid hormones are produced by the thyroid gland and are found in front of the neck.1 It commonly results in dysfunction in individuals with endocrine disorders. It affects approximately 300 million people worldwide, with rates ranging from 4–10%, across different geographic locations.2 Dysfunction is more prevalent in women than in men.3 Due to thyroid hormone dysfunction, hypothyroidism occurs in 4.1% of 1000 women and 0.6% of 1000 males annually.4,5 Similarly, thyroid dysfunction is noted in approximately 18% of the elderly population, but more than half of the population knows little about this condition.6
The thyroid gland synthesizes hormones such as triiodothyronine (T3) and thyroxine (T4). These hormones play a crucial role in regulating metabolic processes,7 modulating metabolism, and hematopoiesis.8,9 During hematopoiesis, thyroid hormones regulate the cell cycle, differentiation, and proliferation of erythrocytes, leucocytes, and platelets.10
Thyroid hormones regulate erythropoiesis through various mechanisms. It involves stimulating the production of erythropoietin, erythroid colony-forming units, and erythroid burst-forming units that induce erythropoiesis.11,12 Besides, hormones regulate the signal transduction pathways involved in erythropoiesis. Furthermore, hormones are involved in balancing iron, vitamin B12, and 2.3-diphosphoglycerate levels during erythropoiesis.4 With regard to leucopoiesis, this hormone regulates leukopoiesis by increasing the synthesis of granulocyte-monocyte colony-forming units and interleukin 3.11,13 Evidence shows that thyroid hormone is not directly involved in thrombocytopoiesis but that increased concentrations of this hormone minimize the life span of thrombocytes.8
Dysfunction of thyroid hormones (hyperthyroidism and hypothyroidism) affects the production, differentiation, function, and survival of almost all blood cells.8 Hypothyroidism is a chronic disease in which the thyroid gland produces a lower amount of thyroid hormone than needed in the body.14 Hematological abnormalities such as anemia, leucopoenia, and thrombocytopenia are frequently observed in patients with hypothyroidism.15 However, the magnitude of hematological abnormalities and risk factors for hypothyroidism vary from place to place.16 Moreover, the associations between thyroid hormone levels and platelet count and mean platelet volume (MPV) are inconsistent.17,18 Despite the fact that hypothyroidism has a significant effect on hematological parameters, there is variability in its magnitude and risk factors from place to place. In Ethiopia, only a limited number of studies have been performed. Even the reported study did not reveal all the hematological abnormalities. Furthermore, the associated factors were not thoroughly examined. Thus, the main objective of this study was to assess hematological abnormalities and associated factors among patients with hypothyroidism for early diagnosis of these abnormalities to decrease morbidity and mortality at the University of Gondar Comprehensive Specialized Hospital, Northwest, Ethiopia.
Methods and Materials
Study Design and Period
A hospital-based cross-sectional study was conducted from January 1 to June 30, 2023.
Study Area
The study was performed at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. The hospital is located in the central Gondar zone, Gondar town, Amhara Regional State. The town has an elevation of 2133 meters above sea level. The hospital provides health care to more than seven million people, including residents of Gondar town and neighboring zones. According to the data, the hospital provides services for approximately 1700 thyroid dysfunction patients annually.19
Populations
All patients with hypothyroidism who attended the University of Gondar Comprehensive Specialized Hospital were used as source populations in this study. Meanwhile, the study used patients with hypothyroidism who attended the study area during the data collection period and who fulfilled the inclusion criteria as a study population.
Variables
The dependent variable of the study was hematological abnormalities, whereas the independent variables were sociodemographic characteristics (age, sex, marital status, residence, educational status, and occupation), behavioral characteristics (smoking status, alcoholism, meat consumption, vegetable consumption, and iodine salt, consumption), and clinical characteristics (parasitic infection and febrile illness).
Eligibility Criteria
The current study included a total of 300 patients who consecutively visited the hospital during the data collection period and were newly diagnosed with hypothyroid disease by a physician. Patients with known hematological disorders; who were taking nonsteroidal anti-inflammatory drugs, phenazopyridine, or penicillin, who were pregnant, who recently received blood transfusions, who had surgery within the last three months and who had active traumatic bleeding were excluded from the present study.
Sample Size Determination and Sampling Technique
The sample size was calculated based on a single population proportion formula: n= (Z α/2)2 p (1-p)/d2 using 95% CI, Zα/2=1.96, margin of error (d) 5%, and prevalence 26.5%, which was reported from a similar previous study. Consequently, the sample size of this study was calculated as (1.96)2 × 0.265 (1–0.265)/(0.05)2, and 300 participants were ultimately included. Study participants included in this study were recruited using a systematic random sampling technique. Participants were selected based on two regular intervals (K).
Data Collection and Laboratory Methods
During the data collection period, two trained nurse professionals collected sociodemographic and behavioral characteristic data from the participants via a structured questionnaire and face-to-face interview. Moreover, the professionals collected the clinical data of the participants via an observation using data extraction sheet. In addition, complete blood count (CBC) data, stool examination data, and blood film data were collected after analysis using data extraction sheets by two trained laboratory professionals.
For CBC analysis, four milliliters of venous blood were collected by two trained laboratory professionals aseptically using a 19-gauge syringe. After collection, the blood was transferred to dipotassium ethylene diamine-tetraacetic acid (K2EDTA) tubes, the tube was labeled with a unique identification number. To avoid blood clotting, the blood was mixed with EDTA anticoagulant gently. The collected whole blood was analyzed within 4 hours of being collected using a Beckman Coulter UniCel DxH 800 (Beckman Coulter, United States) automated hematology analyzer. The analyzer works based on the Coulter principle, VCS (volume, conductivity, and light scatter) technique, and spectrophotometry principle. During the analysis of the blood samples, the manufacturer’s instructions were followed strictly. After analysis, the outputs of the analyzer were recorded in the data extraction sheets.
Current study included all patients who confirmed as having hypothyroidism by physicians. As a result, the result of thyroid function test was collected from patients medical chart. In the study setting, for thyroid function test, over night fasting venous blood was collected by laboratory professionals. All blood samples used for thyroid function analysis were collected by using plain tubes and allowed for 30 minutes at room temperature for clot formation. Then the serum was separated by centrifugation for 15 min at 2500 rpm using a centrifuge. After separation of serum, thyroid hormone level was measured using chemiluminescence immunoassay analyzer. The analyzer works based on the principle of immunoreaction. The binding of antigen and antibody result’s immunoreaction. Subsequent addition of substrate in immune reaction results formation of light the intensity. The intensity of light formed is directly proportional to the amount of thyroid hormone level. Approximately 1 g of fresh stool sample from each respondent was collected by two trained laboratory professionals in a labeled, dry, clean, and leakproof container. After the collection of stool samples, the professionals prepared a wet mount and examined the parasites microscopically; the results were subsequently recorded on data extraction sheet. Similar professionals who examined stool samples prepared both thin and thick blood films for the detection of hemoparasite. The smears were prepared using a drop of anticoagulated venous blood and stained with a 10% Giemsa stain. Finally, the hemoparasite were examined and recorded on a data extraction sheet.
Data Quality Control
For data quality, the questionnaires were initially prepared in the English language, translated to the local language, and subsequently converted to the English language. These questionnaires were pretested at Tibebe Gihon Hospital Bahir Dar, Ethiopia, to assure consistency and validity. In addition, training was given to the data collectors to assure the quality of the data. Moreover, the data quality was assured by close inspection of the data collection process on site by the principal investigators and supervisor to ensure its clarity, accuracy, completeness, and consistency. Furthermore, standard operating procedures were followed strictly during CBC analysis, stool examinations, and blood film examinations to assure the quality of the laboratory results.
Data Analysis and Interpretation
After the data were manually checked for completeness, the data were coded, entered into Epidata version 3.1, and subsequently analyzed via Stata version 14. Descriptive statistics such as frequencies and percentages were employed to summarize the data. Tables and charts used to present descriptive data. The presence of statistical associations between variables was assessed by chi-square and Fisher’s exact tests. Moreover, bivariate and multivariate logistic regression models were used to evaluate the associations between hematological abnormalities and hypothyroidism in patients. The strength of associations was assessed by the crude odds ratio (COR) and AOR with 95% CI. A P value less than .05 was considered to indicate statistical significance. Assumptions such as the Shapiro‒Wilk test were used to test the normality of continuous variables, whereas the Hosmer–Lemeshow test was used to check the model’s fitness.
Operational Definitions
As defined by the World Health Organization (WHO), anemia is a condition in which the hemoglobin level is less than 12.0 g/dL for women and <13.0 g/dL for men after adjusting for altitude. In addition, according to the definition of the WHO, anemia can be classified as microcytic, normocytic, or macrocytic if the mean cell volume (MCV) is less than 80 fL, between 80 and 100 fL, or greater than 100 fL, respectively.20 On the other hand, leucopenia and thrombocytopenia were defined as a WBC count less than 3×103 cells/µL and a platelet count less than 90×103 cells/µL, respectively.21 Hypothyroidism was defined as a blood hormone level T4 or T3 less than 6.09 μg/dL, T3 less than 0.87 g/dL, or a TSH hormone level greater than 5.6 μIU/L.22
Ethical Consideration
After review, ethical approval was obtained from the Ethical Review Committee of the School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar (reference number SBMLS/194/15). We conducted the current study in accordance with the Helsinki Declaration’s principles. Before the data collected, all participant above the age of 18 years provided informed, voluntary, written, and signed consent. In addition, written informed consent was obtained from the child’s parent or legal guardian and oral assent was obtained from children under 18 years of age after explaining the risks and benefits of the study. All other ethical issues were assured during the data collection period and after the data were collected.
Results
Sociodemographic, Behavioral and Clinical Characteristics of the Study Participants
The current study included a total of 300 participants. Of the participants, 267 (89%) were female. Approximately half of the study participants (148/49.33%) were aged 46–75 years. The mean age of the participants was 46 ± 11.96 years. More than half of the participants were from rural areas (190/63.33%) and were unable to read or write (158/52.66%). Of the participants included in this study, 41 (13.67%) were iodine salt consumers. Stool and blood film examination revealed that 11 (3.67%) participants had intestinal parasite infections, and 4 (1.33%) of the patients had malaria (Table 1).
Hematological Profiles of Patients with Hypothyroidism
After adjusting for altitude, the median Hb concentration was 14 g/dL (interquartile range (IQR): 12, 16), with a range of 4.3–18.0 g/dL. In addition, the median WBC and platelet counts were 5.3 x103/µL (IQR: 4.3, 6.1) and 228 x103/µL (IQR: 224, 233), respectively. The WBC and platelet counts ranged from 1.9 to 12.2×103 cells/μL and from 20–601×103 cells/μL, respectively. Furthermore, the median (IQR) RBC indices, such as the MCV, MCH, and MCHC, were 87 fl (84.3, 89.2), 30.7 pg (28, 33.4), and 35 g/dL (33.8, 37.6), respectively (Table 2).
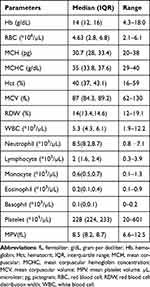 |
Table 2 Hematological Profile of Patients with Hypothyroidism at the University of Gondar Comprehensive Specialized Hospital, Ethiopia, from January to June 2023 (n=300) |
The Magnitude of Hematological Abnormalities in Patients with Hypothyroidism
The overall magnitude of anemia among patients with hypothyroidism was 81 (26.3%, 95% CI: 21–32%). Among the total anemia cases, 52 (64.2%, 95% CI: 62–66%), 25 (30.86%, 95% CI: 28.5–34.5%), and 4 (4.9%, 95% CI: 3.8–5.6%) were normocytic normochromic, microcytic hypochromic, and macrocytic hypochromic, respectively. In addition, the overall incidences of leucopenia and thrombocytopenia were 47 (15.7%, 95% CI (14.2–17.2)) and 28 (9%, 95% CI (7.5–10.5)), respectively. The majority of WBC abnormalities observed in the present study were lymphopenia (28; 9%, 95% CI (8.6–10.1)) and neutropenia (17; 6%, 95% CI (4.4–7.6)) (Figure 1). On the other hand, among patients with hypothyroidism the current founds erythrocytosis, leucocytosis and thrombocytosis with the frequency of 3(1%), 4(1.3%), 3(1%), respectively (Table 3).
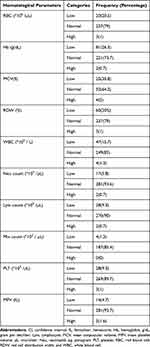 |
Table 3 Magnitude of Hematological Change in Patients with Hypothyroidism at the University of Gondar, Comprehensive Specialized Hospital, 2023 (N =300) |
 |
Figure 1 Magnitude of hematological abnormalities among patients with hypothyroidism at the University of Gondar Comprehensive Specialized Hospital, Ethiopia, from January to June 2023 (n=300). |
Factors Associated with Anemia
The association between anemia and hypothyroidism was assessed using both bivariate and multivariate logistic regressions. Being female increased the risk of anemia approximately twofold (AOR=2.1, 95% CI=1.1 −3.1) compared with being male. Similarly, anemia was approximately four (AOR= 3.8, CI= 1.7–8.9) times more likely to occur in patients who used alcohol than in nonusers. Patients who had febrile illness approximately three times (AOR =2.7295% CI: 1.3–5.4) more likely to develop anemia than did those without febrile disease (Table 4).
Discussion
Changes in hematological parameters such as RBC, WBC, and platelet counts are commonly observed in patients with hypothyroidism. As a result, it is important to assess the magnitude of hematological abnormalities and associated factors among patients with hypothyroidism to minimize associated complications.23–25 The current study also aimed to assess hematological abnormalities and associated factors among patients with hypothyroidism.
The most common hematological abnormality observed in this study was anemia (26.3%; 95% CI: 21–32%). Based on the WHO classification, the magnitude of anemia among patients with hypothyroidism in the study area was considered a moderate public health problem.26 The magnitude of anemia was greater than that in the general population.27 The high magnitude of anemia in patients with hypothyroidism could be related to the effect of thyroid hormones during erythropoiesis; hence, thyroid hormone regulates the production of RBCs, and the production of those hormones results in anemia.28 In addition, the greater magnitude of anemia might be due to the age of the patients included in this study; hence, the majority (49.3%) of patients were elderly. As age advances, the function of the bone marrow decreases, which can reduce the production of RBCs and may cause anemia.
The magnitude of anemia in the current study was in line with studies performed in Kenya (28.4%)9 and Iraq (31.3%).20 However, these figures were greater than those of studies conducted in New York (5.9%),29 Switzerland (5.9 and 6.54%),30,31 Italy (7.5 and 12%),32,33 Spain (18.6%),34 and the UK (4.2%).35 The reasons for these discrepancies may be related to socioeconomic status and the early treatment of anemia in those countries since most of those countries have developed standard nutrition, and early treatment of anemia in these regions may minimize the magnitude of anemia. On the other hand, the frequency was lower than that reported in studies performed in Saudi Arabia (36%, 37% and 60.27%),15,36,37 Turkey (41%),38 Tunisia (53.5%)39 and India (5 and 79.5%).40,41 The possible explanation for the variability could be attributed to variations in clinical characteristics, dietary habits, or factors that determine nutrition and health-being.42–45
With regard to the morphological classification of anemia, the current study revealed all types of anemia. Of these, the majority was normocytic normochromic (52 [64.2%, 95% CI: 62–66%]). These findings are supported by studies conducted in Poland, Turkey, and India.16,38,46 This type of anemia is expected in patients with hypothyroidism. A low concentration of thyroid hormone in patients with disease is linked to the suppression of blood cell production in the bone marrow because this hormone has an effect on erythropoietin.47,48
In the present study, it was discovered that being female, drinking alcohol and having a febrile illness are significant aggravating factors of anemia. The association between female sex and anemia may be related to biological variability, the prevalence of thyroid disease, and menstrual loss of blood in females compared with males. On the other hand, the association between alcohol consumption and anemia might be due to the effect of alcohol on iron absorption. Alcohol inhibits the function of enzymes, which induces the production of hemoglobin. This may depress the production of RBCs directly in the bone marrow.49–52 In addition, the association between febrile illness and anemia may be due to the immune activation of different cytokines and cells during infection, which particularly stimulate hepcidin production and capture circulating iron, respectively.53–55
The second most common abnormality found in this study was leucopenia (15.7%, 95% CI: 14.2–17.2), with a predominance of lymphopenia (9%, 95% CI: 8.6–10.1). Leucopenia in hypothyroidism is caused by the suppression of WBC production and differentiation because thyroid hormone is involved in this process. In addition, leucopenia may result from the destruction of WBCs by thyroid-stimulating antibodies.56 Furthermore, thyroid hormone deficiency induces hyperproduction of reactive oxygen species (ROS) and impairs the integrity of cell surface markers. This may enhance the apoptosis of WBCs and increase the frequency of leucopenia in patients with hypothyroidism.57,58 This figure was higher than that in a study in Kenya (12.2%).9 These variations might be due to the variability of behavioral characteristics, clinical characteristics, and inflammatory disease.
Thrombocytopenia was identified in 2.6% (95% CI: 1.3–5.1) of the study participants. These findings are in agreement with previous studies conducted in Kenya (4.7%),9 Japan (4.6%),59 Iraq (4%),20 and Poland (5%).60 The cause of the low PLT may be the distraction of platelets by thyroid stimulation antibodies61 and rapid consumption of platelets by hyperreactivity during thyroid dysfunction.62
The current study found erythrocytosis 3(1%), leucocytosis 4(1.3%), and thrombocytosis 3(1%) among patients with hypothyroidism. The finding supported by study conducted in Iraq.20 The possible explanation for occurrence of those abnormalities may be related with increasing of chronic inflammatory conditions in some thyroid patients, these leading to the production of a various of pro-inflammatory cytokines and chemokines including IL-6, thrombopoietin, erythropoietin, and other inflammatory factors.
Limitations of the Study
As a limitation, the study examined stool samples only from saline wet mounts, which may decrease the probability of detecting intestinal parasites. The differential diagnosis of anemia was not performed. As a result of the low patient sample size, the study included a small sample size, which may affect the representativeness of the findings.
Conclusion and Recommendations
In the present study, anemia, leucopenia and thrombocytopenia were the most common hematological abnormalities. Anemia is a moderate public health factor among patients with hypothyroidism. In addition, being female, alcohol consumption, and having febrile illness were found to be significant aggravating factors of anemia. Thus, early diagnosis and follow-up strategies are needed to reduce complications, mortality and morbidity in patients with hypothyroidism.
Abbreviations
CBC, Completed Blood Count; Hb, Hemoglobin; Hct, Hematocrit; MCH, Mean corpuscular hemoglobin; MCHC, Mean corpuscular hemoglobin concentration; MCV, Mean corpuscular volume; MPV, Mean platelet volume; PDW, Platelet distribution width; RBCs, Red blood cells; RDW, Red cell distribution width; SOP, Standard Operational Procedure; T3, Triiodothyronine; T4, Thyroxine; TSH, Thyroid stimulating hormone; WBC, White blood cells; WHO, World Health Organization.
Data Sharing Statement
All the data supporting these findings are contained within the manuscript.
Consent to Participate and Ethical Approval
We confirm that all the procedures were performed in accordance with the Helsinki Declaration’s principles. Ethical approval was obtained from the Ethical Review Committee of the School of Biomedical and Laboratory Sciences, College of Medicine and Health Science, the University of Gondar (SBLS/194/2015). The objective and purpose of the study were explained to the medical directors, and permission was obtained to collect the data. Written informed consent was obtained from each adult study participant to collect the data. Besides, written informed consent was obtained from the child’s parent or legal guardian and oral assent was obtained from children under 18 years of age after explaining the risks and benefits of the study. No unauthorized person had access to the collected data. The findings of this study are linked to the responsible bodies.
Acknowledgments
Special thanks to all the study participants for their willingness to participate and for providing the necessary information during the data collection. Finally, we thank University of Gondar for financial support.
Author Contributions
All authors made a significant contribution to the study reported whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation or all the areas: took part in drafting, revising, critically reviewing the article: gave final approval of the version to be published: have agreed on the journal to which the article to which the article has been submitted and agreed to accountable for all aspects of the work.
Funding
The authors declare that the University of Gondar provided financial support for this work with funding number of 10124. Funder has no other role in this study.
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this manuscript.
References
1. Khan YS. Histology, Thyroid Gland. StatPearls Publishing; 2022.
2. Garmendia Madariaga A, Santos Palacios S, Guillén-Grima F, Galofré JC. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab. 2014;99(3):923–931. doi:10.1210/jc.2013-2409
3. Asmelash D, Tesfa K, Biadgo B. Thyroid dysfunction and cytological patterns among patients requested for thyroid function test in an endemic goiter area of Gondar, North West Ethiopia. Int J Endocrinol. 2019;2019(10):910. doi:10.1155/2019/9106767
4. Kh SD. Some physiological and biochemical changes in women with hyperthyroidism. Tikrit J Pure Sci. 2016;21(2):37–40.
5. Altomare M, La Vignera S, Asero P, et al. High prevalence of thyroid dysfunction in pregnant women. J Endocrinol Invest. 2013;36(6):407–411. doi:10.3275/8658
6. Bashir H, Bhat MH, Farooq R, et al. Comparison of hematological parameters in untreated and treated subclinical hypothyroidism and primary hypothyroidism patients. Med J Islamic Republic Iran. 2012;26(4):172.
7. Golde D, Bersch N, Chopra I, Cline M. Thyroid hormones stimulate erythropoiesis in vitro. Br J Hematol. 1977;37(2):173–177. doi:10.1111/j.1365-2141.1977.tb06833.x
8. Kawa MP, Machaliński B. Hematopoiesis dysfunction associated with abnormal thyroid hormones production. Thyroid Disorder Focus Hyperthyr. 2014;3:181–205.
9. Iddah M, Macharia B, Ng’wena A, Keter A, Ofulla A. Thyroid hormones and hematological indices levels in thyroid disorders patients at moi teaching and referral hospital, Western Kenya. Int Scholarly Res Notices. 2013;2013:2.
10. Tata JR. The road to nuclear receptors of thyroid hormone. BBA. 2013;1830(7):3860–3866. doi:10.1016/j.bbagen.2012.02.017
11. Pushparaj T. Correlation of thyroid stimulating hormone levels and hematological parameters among euthyroids, hypothyroids and hyperthyroids. Univ J Pre Paraclin Sci. 2021;7(1):197–280.
12. Maggio M, De Vita F, Fisichella A, et al. The role of the multiple hormonal dysregulation in the onset of “anemia of aging”: focus on testosterone, IGF-1, and thyroid hormones. Int J Endocrinol. 2015;2015:1–22. doi:10.1155/2015/292574
13. Dorgalaleh A, Mahmoodi M, Varmaghani B, et al. Effect of thyroid dysfunctions on blood cell count and red blood cell indice. Iran J Pedia Hematol Oncol. 2013;3(2):73–77.
14. Chiovato L, Magri F, Carlé A. Hypothyroidism in context: where we’ve been and where we’re going. Adv Ther. 2019;36(S2):47–58. doi:10.1007/s12325-019-01080-8
15. Alqahtani SAM. Prevalence and Characteristics of Thyroid Abnormalities and Its Association with Anemia in ASIR Region of Saudi Arabia: a Cross-Sectional Study. Clin Pract. 2021;11(3):494–504. doi:10.3390/clinpract11030065
16. Das C, Sahana PK, Sengupta N, Giri D, Roy M, Mukhopadhyay P. Etiology of anemia in primary hypothyroid subjects in a tertiary care center in Eastern India. Indian J Endocrinol Metab. 2012;16(Suppl 2):361. doi:10.4103/2230-8210.104093
17. Erikci AA, Karagoz B, Ozturk A, et al. The effect of subclinical hypothyroidism on platelet parameters. Hematology. 2009;14(2):115–117. doi:10.1179/102453309X385124
18. Ford H, Toomath R, Carter J, Delahunt J, Fagerstrom J. Mean platelet volume is increased in hyperthyroidism. Am J Hematol. 1988;27(3):190–193. doi:10.1002/ajh.2830270308
19. Health informatics. Administrative Information From University of Gondar Comprehensive Specialized Hospital. Health informatics; 2022.
20. Ahmed SS, Mohammed AA. Effects of thyroid dysfunction on hematological parameters: case controlled study. Ann Med Surg. 2020;57(10):52–55. doi:10.1016/j.amsu.2020.07.008
21. Enawgaw B, Birhan W, Abebe M, et al. Hematological and immunological reference intervals for adult population in the state of Amhara, Ethiopia. Trop Med Int Health. 2018;23(7):765–773. doi:10.1111/tmi.13071
22. University of Gondar Clinical chemistry laboratory thyroid hormone reference range 2022 (Unpublished data).
23. Park S, Han CR, Park JW, et al. Defective erythropoiesis caused by mutations of the thyroid hormone receptor α gene. PLoS genetics. 2017;13(9):e1006991. doi:10.1371/journal.pgen.1006991
24. Kawa MP, Grymula K, Paczkowska E, et al. Clinical relevance of thyroid dysfunction in human hematopoiesis: biochemical and molecular studies. Euro J Endocrinol. 2010;162(2):295. doi:10.1530/EJE-09-0875
25. Davis FB, Cody V, Davis PJ, Borzynski L, Blas SD. Stimulation by thyroid hormone analogs of red blood cell Ca2+-ATPase activity in vitro. Correlations between hormone structure and biological activity in a human cell system. J Biol Chem. 1983;258(20):12373–12377. doi:10.1016/S0021-9258(17)44185-8
26. Chulilla JAM, Colás MSR, Martín MG. Classification of anemia for gastroenterologists. World J Gastroenterol. 2009;15(37):4627. doi:10.3748/wjg.15.4627
27. Alemu T, Umeta M. Prevalence and predictors of” small size” babies in Ethiopia: in-depth analysis of the Ethiopian demographic and health survey, 2011. Ethiopian J Health Sci. 2016;26(3):243–250. doi:10.4314/ejhs.v26i3.7
28. Lima CS, Wittmann DEZ, Castro V, et al. Pancytopenia in untreated patients with Graves’ disease. Thyroid. 2006;16(4):403–409. doi:10.1089/thy.2006.16.403
29. Fein HG, Rivlin RS. Anemia in thyroid diseases. Med Clin North Am. 1975;59(5):1133–1145. doi:10.1016/S0025-7125(16)31963-0
30. Floriani C, Feller M, Aubert CE, et al. Thyroid dysfunction and anemia: a prospective cohort study and a systematic review. Thyroid. 2018;28(5):575–582. doi:10.1089/thy.2017.0480
31. M’Rabet‐Bensalah K, Aubert CE, Coslovsky M, et al. Thyroid dysfunction and anemia in a large population‐based study. Clin Endocrinol. 2016;84(4):627–631. doi:10.1111/cen.12994
32. Jafarzadeh A, Poorgholami M, Izadi N, Nemati M, Rezayati M. Immunological and hematological changes in patients with hyperthyroidism or hypothyroidism. Clin Invest Med. 2010;33(5):271–279. doi:10.25011/cim.v33i5.14352
33. Sibilla R, Santaguida MG, Virili C, et al. Chronic unexplained anemia in isolated autoimmune thyroid disease or associated with autoimmune related disorders. Clin Endocrinol. 2008;68(4):640–645. doi:10.1111/j.1365-2265.2007.03091.x
34. Velarde-Mayol C, de la Hoz-García B, Del Cañizo-Fernández-Roldán C, Hernández-López AM, Loza-Candia I, Cardona-Hernández A. Pernicious anemia and autoimmune thyroid diseases in elderly people. Revista Espanola de Geriatria y Gerontol. 2015;50(3):126–128. doi:10.1016/j.regg.2014.10.004
35. van Vliet NA, Kamphuis AE, den Elzen WP, et al. Thyroid function and risk of anemia: a multivariable-adjusted and Mendelian randomization analysis in the UK biobank. J Clin Endocrinol Metab. 2022;107(2):e643–e52. doi:10.1210/clinem/dgab674
36. Refaat B. Prevalence and characteristics of anemia associated with thyroid disorders in non-pregnant Saudi women during the childbearing age: a cross-sectional study. Biomed J. 2015;38(4):307–316. doi:10.4103/2319-4170.151032
37. Suhail N, Alsel BTA, Batool S. Prevalence and association of thyroid dysfunction with anemia/body iron status among northern Border Saudi population. Int J Med Res Health Sci. 2020;9(3):1–7.
38. Mehmet E, Aybike K, Ganidagli S, Mustafa K. Characteristics of anemia in subclinical and overt hypothyroid patients. Endocr J. 2012;59(3):213–220. doi:10.1507/endocrj.EJ11-0096
39. Omar S, Kanoun F, Hammami M, et al. Erythrocyte abnormalities in thyroid dysfunction. Tunis Med. 2010;88(11):783–788.
40. Mishra AK, Anand R, Verma SP, Gupta KK. Study of impact of subclinical hypothyroidism on iron status and hematological profile. Int J Adv Med. 2018;5(2):446–451. doi:10.18203/2349-3933.ijam20181087
41. Gautam SK, Sharma A, Sharma K. Iron Deficiency Anemia And Its Association With Thyroid Dysfunction In School Going Adolescent Girls Of Scheduled Tribes In Udaipur. Int J Med Sci Educ. 2018;5(1):71–76.
42. Balarajan Y, Ramakrishnan U, Özaltin E, Shankar AH, Subramanian S. Anemia in low-income and middle-income countries. Lancet. 2011;378(9809):2123–2135. doi:10.1016/S0140-6736(10)62304-5
43. Shi Z, Hu X, Yuan B, Pan X, Dai Y, Holmboe-Ottesen G. Association between dietary patterns and anemia in adults from Jiangsu Province in Eastern China. Br J Nutr. 2006;96(5):906–912. doi:10.1017/BJN20061785
44. Hasan MM, Magalhaes RJS, Garnett SP, et al. Anemia in women of reproductive age in low-and middle-income countries: progress toward the 2025 global nutrition target. Bull World Health Organ. 2022;100(3):196. doi:10.2471/BLT.20.280180
45. Olsen A, Magnussen P, Ouma J, Andreassen J, Friis H. The contribution of hookworm and other parasitic infections to hemoglobin and iron status among children and adults in western Kenya. Trans Royal Soc Trop Med Hyg. 1998;92(6):643–649. doi:10.1016/S0035-9203(98)90795-7
46. Szczepanek-Parulska E, Hernik A, Ruchała M. Anemia in thyroid diseases. Pol Arch Intern Med. 2017;127(5):352–360. doi:10.20452/pamw.3985
47. Stadler J, Ade J, Ritzmann M, Hoelzle K, Hoelzle LE. Detection of a novel hemoplasma species in fattening pigs with skin alterations, fever and anemia. Vet Rec. 2020;187(2):66. doi:10.1136/vr.105721
48. Fenta DA, Nuru MM, Yemane T, Asres Y, Wube TB. Anemia and related factors among highly active antiretroviral therapy experienced children in Hawassa comprehensive specialized hospital, southern Ethiopia: emphasis on patient management. Drug Healthc Patient Safe. 2020;12:49. doi:10.2147/DHPS.S230935
49. Milman NT. A review of nutrients and compounds, which promote or inhibit intestinal iron absorption: making a platform for dietary measures that can reduce iron uptake in patients with genetic hemochromatosis. J Nutri Metabol. 2020;2020:1–15. doi:10.1155/2020/7373498
50. Ioannou GN, Dominitz JA, Weiss NS, Heagerty PJ, Kowdley KV. The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia. Gastroenterology. 2004;126(5):1293–1301. doi:10.1053/j.gastro.2004.01.020
51. Lewis G, Wise MP, Poynton C, Godkin A. A case of persistent anemia and alcohol abuse. Nat Clin Pract Gastroenterol Hepatol. 2007;4(9):521–526. doi:10.1038/ncpgasthep0922
52. Yokoyama A, Yokoyama T, Brooks PJ, et al. Macrocytosis, macrocytic anemia, and genetic polymorphisms of alcohol dehydrogenase‐1 B and aldehyde dehydrogenase‐2 in J apanese alcoholic men. Alcoholism. 2014;38(5):1237–1246. doi:10.1111/acer.12372
53. Geissler C, Singh M. Iron, meat and health. Nutrients. 2011;3(3):283–316. doi:10.3390/nu3030283
54. Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood J Am Soc Hematol. 2003;101(7):2461–2463.
55. Weiss G, Schett G. Anemia in inflammatory rheumatic diseases. Nat Rev Rheumatol. 2013;9(4):205–215. doi:10.1038/nrrheum.2012.183
56. Volpé R. The immunomodulatory effects of anti-thyroid drugs are mediated via actions on thyroid cells, affecting thyrocyte-immunocyte signaling: a review. Curr Pharm Des. 2001;7(6):451–460. doi:10.2174/1381612013397898
57. Nechyporuk V, Korda M, Pentiuk L, Kovalchuk O, Andriichuk V. Implementation of programmed cell death in circulating neutrophils and its special characteristics in experimentally induced hyperhomocysteinemia in a setting of thyroid dysfunction. Polski Merkuriusz Lekarski. 2020;48(288):437–442.
58. Łacka K, Maciejewski A. The role of apoptosis in the etiopathogenesis of autoimmune thyroiditis. Polski merkuriusz lekarski. 2012;32(188):87–92.
59. Ito S, Fujiwara S-I, Murahashi R, et al. Clinical association between thyroid disease and immune thrombocytopenia. Ann Hematol. 2021;100(2):345–352. doi:10.1007/s00277-020-04343-5
60. Szczepanek-Parulska E, Adamska M, Korda O, et al. Changes in complete blood count parameters influenced by endocrine disorders. Endokrynologia Polska. 2021;72(3):261–270. doi:10.5603/EP.a2021.0059
61. Marta GN, de Campos FP. Immune thrombocytopenia and autoimmune thyroid disease: a controversial overlap. Autopsy and Case Reports. 2015;5(2):45–48. doi:10.4322/acr.2015.002
62. Cheung E, Liebman HA. Thyroid disease in patients with immune thrombocytopenia. Hematology. 2009;23(6):1251–1260. doi:10.1016/j.hoc.2009.08.003
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


