Back to Journals » Clinical Interventions in Aging » Volume 18
High ASA Physical Status and Low Serum Uric Acid to Creatinine Ratio are Independent Risk Factors for Postoperative Delirium Among Older Adults Undergoing Urinary Calculi Surgery
Authors Liu J, Li J , Gao D, Wang J, Liu M, Yu D
Received 4 November 2022
Accepted for publication 10 January 2023
Published 19 January 2023 Volume 2023:18 Pages 81—92
DOI https://doi.org/10.2147/CIA.S395893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Maddalena Illario
Jing Liu,1,2 Jianli Li,1 Dongyan Gao,1 Jing Wang,1 Meinv Liu,1 Dongdong Yu1
1Department of Anesthesiology, Hebei General Hospital, Shijiazhuang City, People’s Republic of China; 2Graduate Faculty, Hebei North University, Zhangjiakou City, People’s Republic of China
Correspondence: Jianli Li, Department of Anesthesiology, Hebei General Hospital, Shijiazhuang city, Hebei Province, 050051, People’s Republic of China, Tel +81 85988447, Email [email protected]
Purpose: This study was to investigate the incidence and potential predictive factors for postoperative delirium (POD) in older people following urinary calculi surgery, and to establish the corresponding risk stratification score by the significant factors to predict the risk of POD.
Patients and Methods: We retrospectively analyzed the perioperative data of 195 patients aged 65 or older who underwent elective urinary calculi surgery between September 2020 and September 2022. POD was defined by chart-based method, and the serum uric acid to creatinine (SUA/Cr) ratio as well as neutrophil-to-lymphocyte ratio (NLR) were calculated, respectively. Identification of the risk factors for POD was performed by univariate and multivariate logistic regression analysis. Moreover, the risk stratification score was developed based on the regression coefficients of the associated variables.
Results: In 195 eligible patients following urinary calculi surgery, the median age was 69 (66– 72) and 19 patients ultimately developed POD (9.7%). The results by univariate analysis showed that patients with advanced age, high American Society of Anesthesiologists (ASA) physical status (≥ 3) and low SUA/Cr ratio (≤ 3.3) were more likely to develop POD, but dexmedetomidine can significantly decrease the risk of the occurrence of POD. The multivariate analysis further indicated that high ASA physical status (≥ 3) and low SUA/Cr ratio (≤ 3.3) were independently associated with POD, and the POD incidence could obviously be elevated with the increase of risk stratification score. Moreover, patients with delirium had longer hospital stays.
Conclusion: POD is frequent in geriatric patients following urinary calculi surgery (9.7%). The high ASA physical status (≥ 3) and low SUA/Cr ratio (≤ 3.3) were effective predictors of POD. The corresponding risk stratification based on these factors could be beneficial to determining patients who are susceptible to POD, and thus better preventing and reducing the occurrence of POD. However, large prospective studies are needed to confirm this finding.
Keywords: postoperative delirium, serum uric acid to creatinine ratio, ASA physical status, risk stratification score, urinary calculi surgery, older adults
Graphical Abstract:
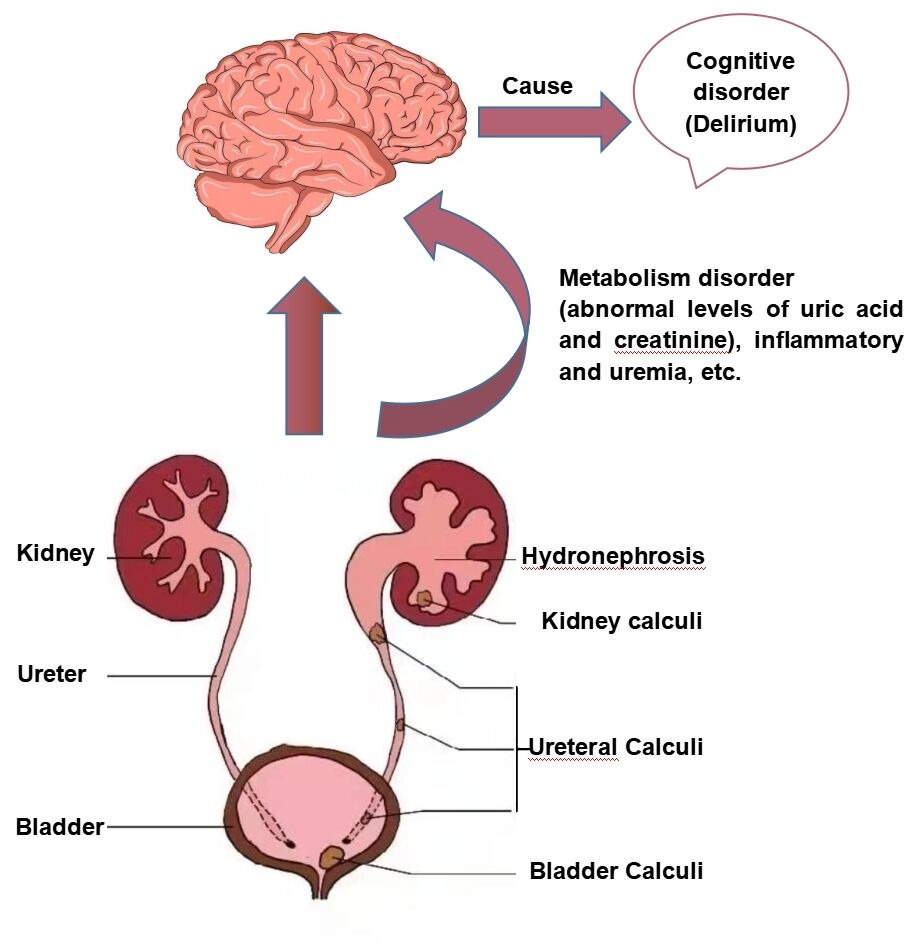
Introduction
Postoperative delirium (POD) is a usually neurological complication in older patients following urological surgery, mainly presented as a transient disorder in consciousness level, disorientation, and inattention after surgery in accordance with the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V).1 The prevalence of POD is different, depending on the type of urological surgery and assessment method. It was reported that the POD incidence was 29% in radical cystectomy, 21% in transurethral resection of the prostate (TURP), and 10% in transurethral resection of bladder tumor (TURBT).2 In addition, POD can result in various adverse consequences, such as prolonged hospital stays, higher risk of dementia, as well as increased mortality.3,4 Therefore, it is imperative to explore effective strategies for preventing and treating POD. POD is triggered by the interaction between multiple predisposing and precipitating factors.5 A better understanding of risk factors for delirium may aid the clinical decision-making process and contribute to reducing morbidity and mortality in the future. Currently, several common risk factors associated with POD have been ascertained in various urological surgeries, such as advanced age, cerebrovascular disease, preoperative cognitive impairment, and longer surgical time, etc.6–8
Urolithiasis, as a common urological disease that appears in any part of the kidney, bladder, and urethra, has been prevalent in geriatric patients worldwide over the past few decades due to environmental and metabolic factors.9 According to reports, the prevalence of urinary stones in China ranges from 4.11% to 6.4%.10,11 To date, the validated treatment methods for urolithiasis include drug therapy, minimally invasive surgery, and open surgery.12 Since minimally invasive surgery, such as percutaneous nephrolithotomy and ureteroscopic holmium laser lithotrips, etc, can result in less trauma and faster postoperative recovery than open surgery, it is widely considered as a more safe and effective surgical procedure for urinary calculi in the elderly.13,14 Unfortunately, senile patients undergoing urinary calculi surgery are not only often accompanied by some comorbidities (hypertension, diabetes and chronic kidney disease, etc), anxiety or depression, electrolyte disturbance, infection and pain preoperatively, but also suffer from some postoperative complications, such as bleeding, pulmonary embolism, even septic shock, etc.15–18 It is noteworthy that numerous studies verified that the above poor conditions and related complications would increase the certain risk of the episode of POD in other surgical types, such as orthopedic, vascular, and thoracic surgery, etc.19–22 Moreover, it is well known that inflammation might be the potential pathophysiological mechanism involved in the development of POD.23 Surprisingly, according to the literature, the proportion of infectious stones accounts for 10–15% of all urinary calculi.24 Additionally, it has been shown that the prevalence of common infectious complications in patients following urinary stone surgery, including fever and systemic inflammatory response syndrome (SIRI), could be as high as 27.4%.25 Given the above clinical characteristics of urinary stone, we deem older patients undergoing urinary calculi surgery might be at high risk for POD, and it is crucial to identify its associated risk factors for preventing POD. Nevertheless, to our knowledge, the POD incidence and potential risk factors among older adults after urinary calculi surgery have not been investigated so far.
Given the context, this study aimed to determine the POD incidence and to identify the related risk factors in older patients following urinary calculi surgery. Furthermore, the risk stratification was established according to related risk factors in order to serve as better guidance for postoperative clinical management of patients.
Materials and Methods
Ethics Approval
This retrospective cohort study was performed at Hebei General Hospital after approval of the Medical Ethics Committee of Hebei General Hospital (2,022,146) and complied with the Declaration of Helsinki. The requirement for informed consent was waived due to the nature of the retrospective study, and the study conformed to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Study Setting and Population
This study took place in Hebei General Hospital from September 2020 to September 2022, and recruited all patients aged ≥65 years old who were scheduled to undergo selective urinary calculi surgery. Patients with delirium, coma, gout, and severe renal insufficiency or renal failure preoperatively and hospitalization for only 1 day postoperatively, were excluded. Moreover, those with intake of the drugs affecting the level of uric acid and incomplete data were also excluded from this study.
Data Collection
Based on the review of the available information in our database, perioperative variables were abstracted from the electronic medical records and were classified into three stages: preoperative, intraoperative, and postoperative. Preoperative data included age, sex, American Society of Anesthesiologists (ASA) physical status, Body Mass Index (BMI), smoking and drinking history, preoperative pain scores, preoperative comorbidities (eg, hypertension, diabetes, coronary heart disease (CHD), etc) and medical history (eg, β-blockers, steroids, benzodiazepines, etc). In addition, the potential variables during the procedure consisted of surgical types and positions, anesthesia method, anesthetics usage (eg, midazolam, dexmedetomidine, sufentanil, etc), duration of surgery, intraoperative lactic acid level, etc. Postoperative factors containing total times of postoperative analgesics, the length of hospital stay, Intensive care unit (ICU) admission, and some common postoperative complications (eg, fever, septic shock and pneumonia), were also collected in the medical chart. Preoperative pain was evaluated using the Visual Analogue Scale (VAS).26 Besides, we also recorded some preoperative laboratory indicators, including white blood cell count, hemoglobin, serum albumin, calcium, aspartate transaminase/alanine (AST/ALT), blood urea nitrogen (BUN), creatinine, serum uric acid (SUA), blood glucose, as well as D-dimer, etc. The neutrophil-to-lymphocyte ratio (NLR) was calculated as neutrophil count divided by lymphocyte count. The serum uric acid-to-creatinine (SUA/Cr) ratio was calculated as serum uric acid divided by creatinine. Based on the median age or SUA/Cr ratio in delirium patients, the cut-off values for age and SUA/Cr ratio were set at 70 and 3.3, respectively.27
Bias
We took a series of measures to minimize the bias caused by this retrospective study. First, all potential risk factors were extracted by a doctor, and the accuracy was checked by another doctor. Any dispute was decided by a third doctor. Second, in order to ensure the authenticity and reliability of statistics, we only put patients with complete data into the final statistical analysis and adopted multivariate regression analysis to adjust the confounding factors.
Delirium Assessment
POD was diagnosed using a chart-based method during any postoperative stage.28 Meanwhile, we carefully examined all medical and nursing records to evaluate delirium. Referring to the study by Xu et al,29 patients were included in the POD group according to the following conditions: (1) if the case noted delirium was diagnosed by a psychiatrist; (2) if the doctor’ advice revealed the use of antipsychotics, specifically olanzapine, haloperidol, and quetiapine; (3) if two or more experienced anesthesiologists agreed on the diagnosis of POD by the review of electronic medical records (delirium was screened regularly by trained nurses two times one day). Supplementary Table 1 shows the abstracted symptoms related to DSM-V criteria.
Sample Size
First, we reviewed the patient data from September 2020 to December 2020, and revealed that two of 23 patients developed POD (8.7%). Meanwhile, the reported POD incidence ranged from 7% to 35% in urological surgery according to a previous literature review.2 Therefore, we assumed that the prevalence of POD (π) following urinary calculi surgery was 10%, with a relative error ε of 20%, an allowable error E of 5%, and a confidence interval (1–α) of 95%. The sample size was calculated as approximately 166.
Statistical Analysis
All data were analyzed using IBM SPSS statistics software version 25.0 (SPSS Inc., Chicago, IL). Quantitative data conformed to non-normal distribution by Shapiro–Wilk (SW) test and were presented as median, with quartiles 1 and 3 [M (Q1–Q3)]. Mann–Whitney U-test was performed for group comparisons. Qualitative data were described as number (n) or rate (%), and group differences were compared by chi-square test or Fisher test. The multicollinearity between related variables was analyzed using tolerance (Tol) or variance inflation factor (VIF). All variables with P<0.05 were firstly executed univariate logistic regression analysis, followed by stepwise forward multivariate logistic regression analysis, and thus adjusting confounding factors. The effects of related factors were expressed as odds ratio (OR) with 95% confidence interval (CI), and the Hosmer and Lemeshow goodness-of-fit test was conducted to reflect the model fitness for the logistic regression. Besides, the risk stratification score was calculated in line with the regression coefficients (β) of all independent predictors. The accuracy of risk stratification score in predicting POD was determined by area under curve (AUC) from the receiver operator characteristic (ROC) curve. P<0.05 demonstrated statistical significance.
Results
Study Population and Delirium Rate
The study initially included 229 patients, 34 patients of whom were eliminated complying with the exclusion criteria, and the remaining 195 patients were ultimately analyzed (Figure 1). Nineteen of 195 patients were diagnosed as POD (9.7%). The onset of delirium commonly occurred within the postoperative 2 days (Figure 2A). The POD incidence was highest in ureteroscopic lithotripsy (47.4%), followed by percutaneous nephrolithotomy (36.8%) and transurethral holmium laser cystolithotripsy (15.8%; Figure 2B).
 |
Figure 1 Flow chart of study population. |
The results in Table 1 show the median age was 68 (range 66–72) years, and the number of male patients was 135 (69.2%). There was statistical significance in age (P=0.047), ASA physical status (P=0.011), and β-blockers usage (P=0.045) between the two groups. Besides, dexmedetomidine could significantly decrease the risk of the occurrence of POD (P=0.024). In terms of laboratory data, the SUA/Cr ratio in the POD group was lower than that in the non-POD group (3.3 (2.8–4.9) vs 4.2 (3.6–5.1), P=0.025), and the SUA/Cr ratio ≤3.3 dramatically increased the incidence of POD (P<0.001). Conversely, patients who developed delirium had a higher level of intraoperative lactic acid, compared to those without delirium (1.6(1.2–2.0) vs 1.3 (1–1.6), P=0.019).
 |
Table 1 Characteristics of Patients with and without Delirium |
Multicollinearity Diagnosis Among Potential Risk Factors
As presented in Supplementary Table 2, our results showed no multicollinearity among potential risk factors (all Tol >0.1 or VIF <10).
Risk Factors for Delirium
In univariate regression analysis, the unadjusted results indicated that age, ASA physical status, β-blockers and dexmedetomidine usage, the SUA/Cr ratio, as well as intraoperative lactic acid level were identified as the potential risk factors for POD. Ultimately, the multivariate logistic regression analysis suggested that the independent risk factors associated with POD were high ASA physical status (≥3) (OR=4.050; 95% CI=1.368–11.991; P=0.012) and low SUA/Cr ratio (≤3.3) (OR=8.772; 95% CI=3.019–25.483; P<0.001). Moreover, the Hosmer and Lemeshow goodness-of-fit test manifested the regression model fitted very well with a χ2 value of 1.164 and P-value of 0.884, as demonstrated in Table 2.
 |
Table 2 Analyses of Risk Factors for Postoperative Delirium |
The Establishment and Predictive Value of Risk Stratification Score
The risk stratification score was developed using the regression coefficients of independent variables from the multivariate regression model (Table 2). The calculation formula was as follows: 1.399×(ASA grade ≥3)+2.172×(the SUA/Cr ratio ≤3.3). Given the simplification for clinical application, we assigned a weight score of 1 and 2, for corresponding to β of 1–2 and >2–3, respectively. The maximum risk score was 3, and the POD incidence in older patients undergoing urinary calculi surgery was 2% in score 0, 10.5% in score 1, 22.7% in score 2, and 42.9% in score 3 (Figure 3A). This risk classification was highly correlated with the development of POD (P<0.001). Moreover, Figure 3B notes the high predictive accuracy of risk score by the ROC curve, with the AUC of 0.802 (P<0.001, 95% CI=0.698–0.907).
Clinical Outcomes of Patients
As indicated in Table 3, the length of hospital stay was longer in patients with delirium (P<0.05). Nevertheless, other clinical outcomes had no statistic differences between both groups (P>0.05).
 |
Table 3 Comparison of Clinical Outcomes in Both Groups |
Discussion
POD, a common and serious trouble in the clinical environment, occurs in 7–35% of older adults after urological surgery, which often results in various negative outcomes and seriously hinders the postoperative recovery of patients.2,3 However, POD can be prevented, with its potential risk factors identification being the first step. Several previous studies investigated the incidence of POD and related risk factors in radical cystectomy and TURB in recent years.7,8 Nonetheless, as far as we know, this retrospective cohort study was for the first time to analyze the POD incidence and to determine the potential risk variables in older patients following urinary calculi surgery. Our results showed the independent risk factors for POD included high ASA physical status (≥3) and low SUA/Cr ratio (≤3.3). Furthermore, we developed the risk score of POD to stratify patients into four categories according to these independently significant factors (scores 0–3). The patients with a higher risk score were correlated with the increased risk of POD (P<0.001), and the AUC of 0.802 demonstrated the high predictive accuracy of this risk stratification score.
The POD incidence was approximately 9.7% in the current study. Unlike our results, other studies found a lower incidence of POD in general urological surgery, such as 3.4% and 4.7%.6,30 However, the POD rate was relatively higher in radical cystectomy (29%) and TURB (21%) than this study.7,8 These findings suggested the differences in POD rate might be a result of surgical type, diagnostic criteria, including age range and sample size. Also, we observed that the episode of delirium in older patients with urolithiasis generally occurred within the first 48 hours after surgery, which was consistent with a study by Matsuki et al.6 So, clinicians should evaluate delirium in the early stages after surgery to better prevent and reduce complications related to POD.
As previously mentioned, ASA physical status was identified as an independent risk factor for POD.31,32 In line with their results, our result demonstrated that high ASA physical status (≥3) can increase the likelihood of developing POD. ASA physical status is a preliminary assessment of the patient’s tolerance to anesthesia, mainly based on the damaged general physical condition and multiple comorbidities.33 Although some common comorbidities, such as diabetes and cerebrovascular diseases, etc, had no statistic differences between the two groups in this study, we deemed that it might be that the combined effect of multiple comorbidities increased the baseline susceptibility in older patients, together with surgical stress, which ultimately led to the onset of POD. Generally speaking, clinicians should be alert to the older adults undergoing urinary calculi surgery with high ASA classification to reduce the occurrence of POD.
To date, a large body of evidence illuminated the role of UA in the nervous system. To be specific, UA could trigger the expression of proinflammatory factors by activating the NF-κB pathway in the hypothalamus or directly cross the blood–brain barrier (BBB) to induce inflammation, which finally resulted in cognitive disorders.34,35 In contrast, other authors believed UA might exert a protective effect on cognitive function after neurological diseases, such as Alzheimer’s disease and stroke due to its antioxidant properties with the capacity to neutralize and scavenge prooxidant molecules.36,37 A recent study verified that low SUA level was associated with the increased risk of POD in older patients with hip fracture surgery.29 Yet surprisingly, no significant difference in the UA level was observed between two groups in our study, however, our multivariate analysis revealed that the SUA/Cr ratio was an independent risk factor for POD in elderly patients undergoing urinary calculi surgery after adjusting confounding factors. The SUA/Cr ratio, an indicator of renal function-standardized UA, has been verified to be relevant to adverse outcomes in multiple diseases, such as metabolic and cardiovascular diseases (CVD), etc.38,39 Interestingly, our study firstly focused on the predictive value of the SUA/Cr ratio on POD and the results showed that a low SUA/Cr ratio (≤3.3) could lead to an obvious increase in POD incidence (OR=0.250; 95% CI=0.117–0.532; P<0.001). We deemed that the SUA/Cr ratio would be more sensitive in predicting POD in older patients following urinary calculi surgery, which might be a result of eliminating the effect of renal function on UA.40 Similar to our research, prior studies demonstrated the SUA/Cr level in patients with Parkinson’s disease (PD) was lower and was negatively associated with the stages of PD.41,42 Nevertheless, other studies indicated the high SUA/Cr ratio could result in poorer functions outcomes and the increased risk of stroke recurrence after ischemic stroke, which might be explained by the controversial role of UA and its physiological metabolism.43,44 Thus, our conclusion should be interpreted with caution and more prospective researchis needed to support this hypothesis.
Besides, there was an interesting phenomenon that elderly patients undergoing upper urinary tract stone surgery had a higher likelihood of developing POD in our finding (Figure 3B). Patients with an upper urinary tract stone often developed acute kidney injury (AKI) after surgery, which might be related to the increased risk of POD.45,46 Unfortunately, we did not measure and compare the postoperative renal function index among various surgeries, including estimated glomerular filtration rate (eGFR), creatinine, and BUN. Therefore, more prospective studies with large samples should be needed to further explore the role of urinary calculi surgery at different sites in the occurrence of POD.
Several limitations need to be addressed. First, we performed this retrospective study in a single-center institution, and multi-center prospective studies are needed to verify our results. Second, this study excluded some patients with incomplete data, which may bias POD incidence. Finally, some other factors associated with POD, such as cognitive impairment, frailty, body temperature, etc, were not included in this study.
Conclusion
In conclusion, the prevalence of POD was 9.7% in older adults following urinary calculi surgery in this study. Additionally, the high ASA physical status (≥3) and low SUA/Cr ratio (≤3.3) were independently associated with POD. The risk stratification score according to these determined risk factors would contribute to early identifying older patients at high-risk of POD to provide optimal clinical care for geriatric patients after urinary calculi surgery. Even so, more large prospective studies are required to confirm whether a preoperative low SUA/Cr ratio could serve as a risk marker for POD, which could enable proactive interventions.
Data Sharing Statement
All raw data can be provided from the corresponding author (Jianli Li) without reservation to support the conclusions of this article.
Ethics Approval
This study has been approved of the the Medical Ethics Committee of Hebei General Hospital (2022146) and registered at the Chinese Clinical Trial Registry (ChiCTR2200065243). This cohort study complied with the Declaration of Helsinki, and the requirement for informed consent was waived due to the nature of the retrospective study. All information of the patients was confidential.
Acknowledgment
All authors appreciated support from Hebei General Hospital.
Author Contributions
All authors made a significant contribution to the reported work, whether in terms of the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
The study was supported by the Key Research and Development Program of Hebei Province (Grant No. 19277714D).
Disclosure
All authors declare no conflicts of interest in this work.
References
1. Sanyaolu L, Scholz AFM, Mayo I, et al. Risk factors for incident delirium among urological patients: a systematic review and meta-analysis with GRADE summary of findings. BMC Urol. 2020;20(1):169. doi:10.1186/s12894-020-00743-x
2. Leotsakos I, Katafigiotis I, Gofrit ON, Duvdevani M, Mitropoulos D. Postoperative Delirium after Urological Surgery: a Literature Review. Curr Urol. 2019;13(3):133–140. doi:10.1159/000499280
3. Kirfel A, Guttenthaler V, Mayr A, Coburn M, Menzenbach J, Wittmann M. Postoperative delirium is an independent factor influencing the length of stay of elderly patients in the intensive care unit and in hospital. J Anesth. 2022;36(3):341–348. doi:10.1007/s00540-022-03049-4
4. Mohanty S, Gillio A, Lindroth H, et al. Major surgery and long term cognitive outcomes: the effect of postoperative delirium on dementia in the year following discharge. J Surg Res. 2022;270:327–334. doi:10.1016/j.jss.2021.08.043
5. Oh ST, Park JY. Postoperative delirium. Korean J Anesthesiol. 2019;72(1):4–12. doi:10.4097/kja.d.18.00073.1
6. Matsuki M, Tanaka T, Takahashi A, et al. Incidence and risk factors of postoperative delirium in elderly patients undergoing urological surgery: a multi-institutional prospective study. Int J Urol. 2020;27(3):219–225. doi:10.1111/iju.14172
7. Large MC, Reichard C, Williams JTB, et al. Incidence, risk factors, and complications of postoperative delirium in elderly patients undergoing radical cystectomy. Urology. 2013;81(1):123–128. doi:10.1016/j.urology.2012.07.086
8. Tai S, Xu L, Zhang L, Fan S, Liang C. Preoperative risk factors of postoperative delirium after transurethral prostatectomy for benign prostatic hyperplasia. Int J Clin Exp Med. 2015;8(3):4569–4574.
9. McCarthy JP, Skinner TA, Norman RW. Urolithiasis in the elderly. Can J Urol. 2011;18(3):5717–5720.
10. Zhang X, Ma J, Wang N, Lin C. Urinary stone composition analysis of 3684 patients in the eastern Shandong region of China. J Int Med Res. 2020;48(3):300060519887266. doi:10.1177/0300060519887266
11. Ye Z, Zeng G, Yang H, et al. The status and characteristics of urinary stone composition in China. BJU Int. 2020;125(6):801–809. doi:10.1111/bju.14765
12. Raheem OA, Khandwala YS, Sur RL, Ghani KR, Denstedt JD. Burden of urolithiasis: trends in prevalence, treatments, and costs. Eur Urol Focus. 2017;3(1):18–26. doi:10.1016/j.euf.2017.04.001
13. Türk C, Petřík A, Sarica K, et al. EAU Guidelines on Interventional Treatment for Urolithiasis. Eur Urol. 2016;69(3):475–482. doi:10.1016/j.eururo.2015.07.041
14. Liu Q, Guo X, Li J. Holmium laser lithotripsy reduces complications and relieves postoperative pain in elderly patients with urinary calculi. Am J Transl Res. 2022;14(8):5614–5621.
15. Karoli R, Fatima J, Karoli Y, et al. Study of association of metabolic syndrome and risk factors of nephrolithiasis. J Assoc Physicians India. 2021;69(1):32–35.
16. Ní Néill E, Richards HL, Hennessey D, Ryan EM, Fortune DG. Psychological distress in patients with urolithiasis: a systematic review and meta-analysis. J Urol. 2022;209:58–70. doi:10.1097/JU.0000000000003032
17. Amri M, Naouar S, Ben Khalifa B, Hmidi N, Braiek S, ElKamel R. Predictive factors of bleeding and fever after percutaneous nephrolithotomy. Tunis Med. 2019;97(5):667–674.
18. Tao W, Ming X, Zang Y, et al. The clinical outcomes of flexible ureteroscopy and laser lithotripsy (FURSL) for treatment of the upper urinary tract calculi. J Xray Sci Technol. 2022;30(1):123–133. doi:10.3233/XST-210992
19. Yang Q, Wang J, Chen Y, Lian Q, Shi Z, Zhang Y. Incidence and risk factors of postoperative delirium following total knee arthroplasty: a retrospective Nationwide Inpatient Sample database study. Knee. 2022;35:61–70. doi:10.1016/j.knee.2022.02.006
20. Zhang HJ, Ma XH, Ye JB, Liu CZ, Zhou ZY. Systematic review and meta-analysis of risk factor for postoperative delirium following spinal surgery. J Orthop Surg Res. 2020;15(1):509. doi:10.1186/s13018-020-02035-4
21. Visser L, Prent A, Banning LBD, van Leeuwen BL, Zeebregts CJ, Pol RA. Risk Factors for Delirium after Vascular Surgery: a Systematic Review and Meta-Analysis. Ann Vasc Surg. 2021;76:500–513.
22. Liu J, Li J, He J, Zhang H, Liu M, Rong J. The Age-adjusted Charlson Comorbidity Index predicts post-operative delirium in the elderly following thoracic and abdominal surgery: a prospective observational cohort study. Front Aging Neurosci. 2022;14:979119. doi:10.3389/fnagi.2022.979119
23. Simone MJ, Tan ZS. The role of inflammation in the pathogenesis of delirium and dementia in older adults: a review. CNS Neurosci Ther. 2011;17(5):506–513. doi:10.1111/j.1755-5949.2010.00173.x
24. Flannigan R, Choy WH, Chew B, Lange D. Renal struvite stones--pathogenesis, microbiology, and management strategies. Nat Rev Urol. 2014;11(6):333–341. doi:10.1038/nrurol.2014.99
25. Streltsova OS, Vlasov VV, Grebenkin EV, et al. Controlled fragmentation of urinary stones as a method of preventing inflammatory infections in the treatment of urolithiasis (experience in successful clinical use). Sovrem Tekhnologii Med. 2021;13(3):55–61. doi:10.17691/stm2021.13.3.07
26. da Costa BR, Saadat P, Basciani R, Agarwal A, Johnston BC, Jüni P. Visual Analogue Scale has higher assay sensitivity than WOMAC pain in detecting between-group differences in treatment effects: a meta-epidemiological study. Osteoarthritis Cartilage. 2021;29(3):304–312. doi:10.1016/j.joca.2020.10.004
27. Janssen TL, Steyerberg EW, Faes MC, et al. Risk factors for postoperative delirium after elective major abdominal surgery in elderly patients: a cohort study. Int J Surg. 2019;71:29–35. doi:10.1016/j.ijsu.2019.09.011
28. Kuhn E, Du X, McGrath K, et al. Validation of a consensus method for identifying delirium from hospital records. PLoS One. 2014;9(11):e111823. doi:10.1371/journal.pone.0111823
29. Xu L, Lyu W, Wei P, et al. Lower preoperative serum uric acid level may be a risk factor for postoperative delirium in older patients undergoing Hip fracture surgery: a matched retrospective case-control study. BMC Anesthesiol. 2022;22(1):282. doi:10.1186/s12871-022-01824-0
30. Sato T, Hatakeyama S, Okamoto T, et al. Slow gait speed and rapid renal function decline are risk factors for postoperative delirium after urological surgery. PLoS One. 2016;11(5):e0153961. doi:10.1371/journal.pone.0153961
31. Gold C, Ray E, Christianson D, et al. Risk factors for delirium in elderly patients after lumbar spinal fusion. Clin Neurol Neurosurg. 2022;219:107318. doi:10.1016/j.clineuro.2022.107318
32. Kim EM, Li G, Kim M. Development of a risk score to predict postoperative delirium in patients with hip fracture. Anesth Analg. 2020;130(1):79–86. doi:10.1213/ANE.0000000000004386
33. Li G, Walco JP, Mueller DA, Wanderer JP, Freundlich RE. Reliability of the ASA physical status classification system in predicting surgical morbidity: a retrospective analysis. J Med Syst. 2021;45(9):83. doi:10.1007/s10916-021-01758-z
34. Shao X, Lu W, Gao F, et al. Uric acid induces cognitive dysfunction through hippocampal inflammation in rodents and humans. J Neurosci. 2016;36(43):10990–11005. doi:10.1523/JNEUROSCI.1480-16.2016
35. Lu W, Xu Y, Shao X, et al. Uric acid produces an inflammatory response through activation of NF-κB in the hypothalamus: implications for the pathogenesis of metabolic disorders. Sci Rep. 2015;5:12144. doi:10.1038/srep12144
36. Boccardi V, Carino S, Marinelli E, et al. Uric acid and late-onset Alzheimer’s disease: results from the ReGAl 2.0 project. Aging Clin Exp Res. 2021;33(2):361–366. doi:10.1007/s40520-020-01541-z
37. Sun Z, Feng J, He M, et al. Higher uric acid is associated with better discharge recovery and short-term outcome in stroke patients treated with thrombolysis. Neurol Sci. 2021;42(8):3225–3231. doi:10.1007/s10072-020-04919-z
38. Han AL, Lee HK. Association of the metabolic dysfunction-associated fatty liver disease with serum uric acid-to-creatinine ratio. Metab Syndr Relat Disord. 2022;20(7):370–376. doi:10.1089/met.2022.0013
39. Wang A, Tian X, Wu S, et al. Metabolic factors mediate the association between serum uric acid to serum creatinine ratio and cardiovascular disease. J Am Heart Assoc. 2021;10(23):e023054. doi:10.1161/JAHA.121.023054
40. Arévalo-Lorido JC, Carretero-Gómez J, Robles NR. Serum uric acid levels and outcome during admission in acute ischaemic stroke, depending on renal function. Int J Neurosci. 2018;128(10):906–912. doi:10.1080/00207454.2018.1441150
41. Songsomboon C, Tanprawate S, Soontornpun A, Wantaneeyawong C, Louthrenoo W. Serum uric acid, serum uric acid to serum creatinine ratio and serum bilirubin in patients with parkinson’s disease: a case-control study. J Clin Med Res. 2020;12(3):172–179. doi:10.14740/jocmr4079
42. Zhong LL, Song YQ, Tian XY, Cao H, Ju KJ. Level of uric acid and uric acid/creatinine ratios in correlation with stage of Parkinson disease. Medicine. 2018;97(26):e10967. doi:10.1097/MD.0000000000010967
43. Gong Y, Tian X, Zhou Y, et al. Association between serum uric acid to serum creatinine ratio and poor functional outcomes in patients with acute ischemic stroke. Eur J Neurol. 2022;29(11):3307–3316. doi:10.1111/ene.15521
44. Sun X, Lv J, Wu Z, Shi J, Huang H. Serum uric acid to serum creatinine ratio and risk of stroke recurrence in young adults with ischemic stroke. Neuropsychiatr Dis Treat. 2022;18:2031–2039. doi:10.2147/NDT.S378576
45. Fulla J, Prasanchaimontri P, Wright HC, et al. Acute kidney injury and percutaneous nephrolithotomy: incidence and predictive factors. World J Urol. 2022;40(2):563–567. doi:10.1007/s00345-021-03874-4
46. Wan R, McKenzie CA, Taylor D, Camporota L, Ostermann M. Acute kidney injury as a risk factor of hyperactive delirium: a case control study. J Crit Care. 2020;55:194–197. doi:10.1016/j.jcrc.2019.10.013
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



