Back to Journals » Journal of Blood Medicine » Volume 14
Hyperviscosity Syndrome Induced Bilateral Visual and Auditory Impairment in Therapy Resistant Waldenström Macroglobulinemia with MYD88 and CXCR4 Mutations
Authors Plante MM, Kimbrough EO , Agarwal AK, Jiang L , Bourgeois K, Stamper GC, Stewart MW, Tun HW
Received 2 June 2023
Accepted for publication 21 November 2023
Published 15 December 2023 Volume 2023:14 Pages 639—648
DOI https://doi.org/10.2147/JBM.S424072
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Marie M Plante,1 ErinMarie O Kimbrough,2 Amit K Agarwal,3 Liuyan Jiang,4 Kirk Bourgeois,4 Greta C Stamper,5 Michael W Stewart,6 Han W Tun2
1Department of Internal Medicine, Mayo Clinic, Jacksonville, FL, USA; 2Division of Hematology and Oncology, Mayo Clinic, Jacksonville, FL, USA; 3Department of Radiology, Mayo Clinic, Jacksonville, FL, USA; 4Department of Pathology, Mayo Clinic, Jacksonville, FL, USA; 5Department of Otolaryngology and Audiology, Mayo Clinic, Jacksonville, FL, USA; 6Department of Ophthalmology, Mayo Clinic, Jacksonville, FL, USA
Correspondence: Han W Tun, Division of Hematology and Oncology, Mayo Clinic, 4500 San Pablo Road S, Jacksonville, FL, USA, Tel +1 904 953 7290, Fax +1 904 953 2315, Email [email protected]
Abstract: Hyperviscosity syndrome (HVS) is an emergent complication of Waldenström macroglobulinemia (WM) characterized by visual, neurologic, and rarely auditory impairment. We report a 69-year-old female with MYD88 and CXCR4-mutant WM who developed HVS resulting in bilateral blindness and deafness associated with neurologic manifestations including confusion, severe generalized weakness, and imbalance. Ophthalmologic evaluation revealed bilateral central retinal vein occlusion (CRVO), diffuse retinal hemorrhages, macular edema, and serous macular detachments (SMD). Magnetic resonance imaging of the brain showed bleeding in the inner ears. Management was challenging as her WM was resistant to systemic therapies including bendamustine + rituximab (BR) and rituximab + bortezomib + dexamethasone (RVD). Bruton’s tyrosine kinase inhibitors could not be used initially due to ongoing lower gastrointestinal bleeding. She required five total sessions of plasma exchange and was finally initiated on zanubrutinib, achieving a partial response. She also received intravitreal bevacizumab with rapid resolution of the retinal hemorrhages but with little improvement of the SMD. She had partial restoration of her hearing in the right ear and only slight improvement in her bilateral visual deficits. The management of HVS in frail, elderly patients with therapy-resistant WM can be challenging. In these cases, plasma exchange is required until an effective systemic therapy can be safely instituted. Genomic profiling is important in the management of WM as it can predict treatment resistance and guide therapeutic decisions.
Keywords: hyperviscosity syndrome, Waldenström macroglobulinemia, central retinal vein occlusion, exudative maculopathy, serous macular detachment, cochlear hemorrhage, plasma exchange, genomic sequencing, CXCR4
Introduction
Waldenström macroglobulinemia (WM) is a lymphoplasmacytic lymphoma, predominantly in the bone marrow, associated with an immunoglobulin M (IgM) monoclonal protein of any size.1 It is a B-cell non-Hodgkin lymphoma with an estimated incidence of 3–4 cases per million people per year. It is more common among white males with a mean age of 65–70 years at diagnosis.2–4 Structurally, IgM molecules are large and form pentamers.5 Consequently, the presence of an increased IgM monoclonal protein can give rise to hyperviscosity syndrome (HVS). HVS is a clinical diagnosis aided by laboratory and pathology findings, therefore serum viscosity measurement is not required to make the diagnosis.5 Although there is no strict immunoglobulin level or viscosity level threshold to define HVS, there are well-characterized associated ranges that may better clarify true HVS verses an alternative diagnosis.5 HVS can develop at different IgM and viscosity levels and vary from patient to patient, however it typically occurs at a viscosity level ≥ 4.0 centipoise (cpoise) and only develops ~15% of WM patients with an IgM level above 6000 mg/dL.5–8 HVS occurs in 10–30% of patients with WM, and WM accounts for nearly 85% of HVS cases.9,10 In a cohort of 825 newly diagnosed WM patients, 14% developed symptoms consistent with HVS.11 CXCR4 mutations, found in 30% of patients with WM, are associated with increased likelihood of developing HVS.12
The typical clinical manifestations of HVS include visual disturbances—most often blurred vision, mucosal hemorrhage, and neurologic abnormalities.6 Less commonly, patients can develop cardiac manifestations such as new onset high-output heart failure or pulmonary hypertension.5,6 Other frequent symptoms include bilateral epistaxis or gingival bleeding and visual changes with retinopathy which results from shearing forces that damage small, susceptible vessels.6 Exposed venules overlying mucosal surfaces are especially prone to bleeding because of poor underlying support, which occurs in the oropharynx, gingiva, gastrointestinal tract, retina, and on the surface of the brain.6 Central neurologic manifestations can range from mild headache and presyncope to a comatose state.6 Peripheral neuropathy can occur and is especially common when auto-IgM antibodies are present.5 Other symptoms such as hearing loss or vestibular symptoms can occur from stagnant blood within cochlear veins.6 Fundoscopic examination is important in the assessment of HVS. Retinopathy can be present even in an individual without visual complaints. Examination can reveal hemorrhages, exudates, microaneurysms, and papilledema.5 Central retinal vein occlusion (CRVO) is described as a “blood and thunder” retina with retinal venous engorgement or “sausaging.” Specific retinal findings such as optic disc swelling, dilated veins, as well as dot/blot and flame hemorrhages in all four quadrants should raise concern for CRVO which is an ophthalmologic emergency.13 If clinical suspicion is high for HVS and retinopathy is present, laboratory testing to measure serum viscosity should be collected to confirm the diagnosis along with immediate initiation of plasma exchange to prevent further damage followed by systemic therapy.
We report therapeutic challenges in a frail, elderly patient who developed bilateral blindness and deafness related to HVS in the setting of therapy-resistant WM with CXCR4 mutations.
Case Presentation
Six months before coming to our institution, a 69-year-old white female presented to an outside facility with severe anemia. She was diagnosed with a B-cell lymphoma, likely follicular lymphoma, based on a bone marrow biopsy. She initially received two cycles of bendamustine + rituximab (BR) and developed severe pancytopenia and sepsis. She was then treated with five weekly infusions of single agent rituximab (R). She had a rapid decline in her vision followed by hyperacute onset bilateral deafness associated with vertigo, tinnitus, and imbalance. Magnetic resonance imaging (MRI) of the brain and lumbar puncture were unrevealing. Clinical laboratory studies revealed a serum IgM of 4470 mg/dL (37–286 mg/dL) and serum protein electrophoresis with immunofixation (SPEP with IFE) demonstrated an IgM lambda monoclonal protein with an M-spike of 4.9 g/dL. She continued to decline further, developing bilateral pneumonia and was ultimately discharged to hospice. While enrolled in hospice, she had a rapid improvement in her respiratory status as well as strength and decided to pursue additional evaluation.
She presented to our institution. She was quite ill with bilateral visual and auditory impairment, severe lethargy, and confusion requiring immediate hospitalization for further workup.
Her admission labs were significant for anemia with a hemoglobin of 6.2 g/dL (11.6–15.0 g/dL) and thrombocytopenia with a platelet count of 98 x 109/L (157–450 x 109/L). The white blood cell count was normal. Her beta-2-microglobulin (B2M) was elevated at 4.02 mcg/mL (1.21–2.70 mcg/mL), C-reactive protein was normal, and LDH was normal. Quantitative immunoglobulins revealed an elevated IgM of 4770 mg/dL (37–286 mg/dL) with decreased IgA of 43 mg/dL (61–356 mg/dL) and a decreased IgG of 78 mg/dL (767–1590 mg/dL). The lambda free light chains (FLC) were elevated at 25.9 mg/dL (0.57–2.63 mg/dL), kappa FLC were low at 0.18 mg/dL (0.33–1.94 mg/dL), and the kappa/lambda FLC ratio was abnormal at 0.0071 (0.26–1.65). SPEP with IFE confirmed a monoclonal IgM lambda with an M-spike of 3.6 g/dL. Serum viscosity was markedly elevated and > 6.6 cpoise (1.4–1.8 centipoises).
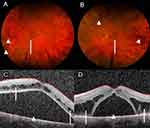
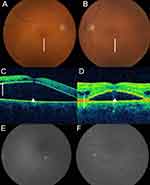
MRI brain revealed bilateral small volume internal ear hemorrhage, Figure 1A–C. Computed tomography of the chest, abdomen, and pelvis did not show any lymphadenopathy or organomegaly. Ophthalmologic examination was significant for decreased visual acuity of 20/150 on the right (OD) and 20/100 on the left (OS). Fundoscopic examination showed diffuse intraretinal hemorrhages, mild venous dilation and tortuosity, and serous macular detachments (SMD), Figure 2A and B. Initial optical coherence tomography (OCT) showed significant subretinal fluid bilateral and intraretinal fluid OS, Figure 2C and D. These findings were consistent with bilateral CRVO with macular edema and exudative detachments.
Repeat bone marrow biopsy revealed a diffuse and interstitial proliferation of small to medium lymphocytes and plasma cells, Figure 3A. Immunohistochemistry (IHC) studies revealed the lymphoplasmacytic infiltrate was strongly and diffusely positive for CD20, CD79a, and CD138, Figure 3B–F. In addition, light chain stains demonstrated lambda-restriction within the lymphocytes and plasma cells. Flow cytometry identified a monoclonal lambda restricted B-cell population that was negative for CD5, CD10, and CD103. Fluorescence in situ hybridization (FISH) studies were negative for MYC, BCL2, and BCL6 gene rearrangements. Molecular studies by polymerase chain reaction (PCR) on the aspiration specimen revealed MYD88 L265P and CXCR4 gene mutations. Next generation sequencing performed on bone marrow aspirate confirmed the MYD88 and CXCR4 mutations, and additional pathogenic mutations involving ETV6, ASXL1, BCORL1, and ATR were detected. Table 1 provides a summary of the diagnostic evaluation including IHC and genomic testing. Clinical, laboratory, bone marrow pathology, and imaging findings were consistent with HVS secondary to WM. She was immediately started on plasma exchange.
 |
Table 1 Summary of Pathology Results and Genomic Testing |
The patient underwent a total of 3 plasma exchange sessions with serial improvement in the IgM levels as follows: 4770 mg/dL, 3550 mg/dL, 2040 mg/dL to 1010 mg/dL on the day of discharge. Unfortunately, her hospitalization was complicated by an episode of significant hematochezia which was attributed to benign rectal and perianal ulcers found on endoscopic evaluation. Due to the GI bleed, retinal hemorrhages, and inner ear hemorrhages, rituximab + bortezomib + dexamethasone (RVD) was initiated in lieu of a Bruton’s tyrosine kinase inhibitor (BTKi) to avoid increasing her risk of bleeding. She also received monthly bilateral intravitreal bevacizumab 1.25 mg injections. Her hearing was markedly improved on her right side on the day of discharge. She also had modest improvement in her vision. Her visual acuity improved from 20/100 to 20/50 OS, but there was no improvement OD.
Unfortunately, she had a relapse of the HVS after 2 cycles of RVD. She developed mental status changes and profound weakness in association with a critical rise in IgM to 6680 mg/dL. She was stabilized with two sessions of plasma exchange with IgM level improving to 1850 mg/dL. She was then started on BTKi with zanubrutinib 160 mg twice daily. She responded well to zanubrutinib achieving a partial response with resolution of the anemia and a reduction in the IgM level to 761 mg/dL. A graph demonstrating pertinent IgM levels throughout her clinical course is shown in Figure 4.
Serial fundoscopic examinations are shown with eventual resolution of the retinal hemorrhages (Figure 5A and B). Repeat OCT revealed minimal improvement in the bilateral subretinal fluid collections and intraretinal fluid OS despite intravitreal injections and systemic therapy (Figure 5C and D). Fluorescein angiography (FA) revealed no evidence of retinal vascular or pigment epithelial leakage to explain the etiology of the intra- and subretinal fluid (Figure 5E and F). These findings suggest that her visual symptoms were likely due to WM-related SMD, a type of exudative retinopathy, rather than bilateral CRVO.
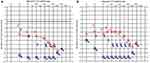
In terms of her auditory function, the initial testing revealed significant hearing loss in the right and profound deafness in the left ear (Figure 6A). Repeat audiology testing two months later revealed partial recovery in the right ear but persistent profound deafness in the left ear (Figure 6B). The modest improvement allowed her to obtain amplification hearing aids for use in the right ear to maximize her remaining auditory function.
At the time of writing, she remains well and has not required any further plasma exchange. She enjoys a decent quality of life and functional status and is able communicate well. She remains on continuous therapy with zanubrutinib and has not developed any major toxicities.
Discussion
Our case clearly identifies therapeutic challenges in managing frail, elderly patients with therapy-resistant WM-associated HVS. Our patient was likely predisposed to symptomatic HVS based on the CXCR4 mutations as demonstrated in prior studies.12 Additionally, CXCR4 mutations have been associated with therapy resistance.12 She was resistant to BR and RVD and required multiple sessions of plasma exchange until she responded to zanubrutinib. It is also possible that the development and relapse of HVS was partly due to IgM flare related to rituximab-based therapy together with intrinsic therapy resistance associated with the CXCR4 mutations. Our case represents one of only a few reported cases with concurrent auditory and visual deficits secondary to WM-associated HVS. Furthermore, our patient had SMD, a rare ophthalmologic manifestation of WM with only a few published cases to date.14,15 Table 2 summarizes other reported cases of combined auditory and visual deficits in patients with HVS-related WM.
 |
Table 2 Summary of Reported WM-Related HVS with Combined Ophthalmologic and Auditory Manifestations |
The visual and auditory manifestations in our patient are quite interesting. The improvement in her hearing was sub-optimal, and she had no significant improvement in her vision. Her auditory impairment was secondary to bleeding within the inner ears and hyperviscosity-induced cochlear damage. She had a partial restoration of her hearing in the right ear with plasma exchange. Her visual impairment did not respond to plasma exchange and intravitreal bevacizumab. Although the hyperviscosity-induced vasculopathy with retinal hemorrhages improved and eventually cleared with therapy, the WM-associated SMD did not improve as evidenced by the persistent intra- and subretinal fluid. The pathophysiology of WM-related SMD has not been fully characterized. Some authors speculate IgM leaks into the subretinal space through damaged external limiting membrane resulting in an osmotic gradient which traps fluid.14,15 Others argue the hyperglobulinemia disturbs the retinal pigment epithelium pump and results in an accumulation of subretinal fluid.14,15 Based on the findings in our case, we suggest that WM-associated SMD does not respond well to currently available treatments including plasma exchange and intravitreal injection of bevacizumab. Further research is necessary to determine the optimal management of WM-associated SMD.
HVS is an emergent complication of WM and warrants rapid initiation of plasma exchange.8 Plasma exchange has been used successfully in the management of HVS since the late 1950s with brisk reversal of retinopathy and other manifestations. It is especially effective in WM as IgM is 80% intravascular. Each session reduces viscosity by 20–30% with a reduction in IgM levels by 30–50%.5,8 In a small study of 9 patients with HVS secondary to WM, retinal venous diameter decreased in each patient by an average of 15.3% with a 46.5% average reduction in IgM levels and a 44.7% average reduction in serum viscosity.19 Plasma exchange requires central line access. A single session lasts approximately 90 minutes with an average of 1–1.5 plasma volumes exchanged per session.5,6 It can be repeated on successive days while monitoring serial serum viscosity levels. It is usually not necessary to achieve normal viscosity levels for symptomatic improvement. Plasma exchange should be continued to maintain the serum viscosity below each individual’s symptomatic threshold and until systemic pharmacologic therapies take effect.5 Our patient required two additional rounds of plasma exchange before she responded to zanubrutinib therapy.
Plasma exchange does not treat the underlying malignant process responsible for HVS; therefore, WM-directed systemic therapy should also be initiated quickly. Under the current NCCN guidelines, WM can be treated with chemoimmunotherapy such as BR, proteasome inhibitor-based therapy such as RVD, and targeted therapy with BTK inhibitors like ibrutinib with or without rituximab.20 As demonstrated in our case, rituximab monotherapy or rituximab-based regimens should be used with caution in the setting of HVS. Rituximab can cause a transient spike in IgM levels in 30–70% of patients. Close monitoring is warranted after initiation of R-based therapies.5 Therapy resistance associated with CXCR4 mutations can complicate the clinical course of WM-associated HVS. Plasma exchange should be repeated for symptomatic patients until significant response to systemic therapy has been achieved.
Genomic profiling of WM can help with predict therapeutic response and guide selection of appropriate systemic therapy.21 The most common somatic mutations in WM are MYD88 (95–97%), CXCR4 (30–40%), ARID1A (17%), and CD79B (8–15%).21 Our patient had MYD88 and CXCR4 mutations with additional pathogenic mutations involving ETV6, ASXL1, BCORL1, and ATR. The identification and characterization of MYD88 and CXCR4 mutations have encouraged targeted drug development such as BTKi and CXCR4 inhibitors.21 ETV6 mutations are present in 3.64% while ATR is altered in 1.54% of non-hodgkin lymphoma patients.22 CXCR4 mutations have been associated with resistance to ibrutinib and varying levels of resistance to bendamustine and bortezomib.7 With regards to our patient, she did not respond to bendamustine and bortezomib, and responded well to zanubrutinib. She stopped requiring plasma exchange following the initiation of zanubrutinib.
Conclusion
Therapeutic management of HVS is quite challenging in frail, elderly patients with therapy-resistant WM related to CXCR4 mutations. HVS is an emergent complication of WM which warrants rapid initiation of plasma exchange. Emergent plasma exchange may need to be repeated until an effective systemic therapy has been identified. R-based regimens should be used with caution in the presence of HVS due to IgM flare. WM-associated SMD does not appear to respond to the currently available therapies and warrants further research. Genomic profiling is important in management of WM as it predicts therapy resistance and guides therapeutic decisions.
Abbreviations
B2M, beta-2-microglobulin; BR, bendamustine + rituximab; BTKi, Bruton’s tyrosine kinase inhibitor; CRVO, central retinal vein occlusion; cpoise, centipoise; FA, Fluorescein angiography; FISH, fluorescence in situ hybridization; FLC, free light chains; HVS, hyperviscosity syndrome; IF, immunofixation; IgM, immunoglobulin M; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; OCT, optical coherence tomography; OD, right eye; OS, left eye; PCR, polymerase chain reaction; R, rituximab; RVD, rituximab, bortezomib, dexamethasone; SMD, serous macular detachment; SPEP, serum protein electrophoresis; WM, Waldenström Macroglobulinemia.
Consent for Publication
The study participant has given written informed consent to participate as well as consent to publish her data and images. IRB approval was not required to publish the case details.
Acknowledgment
The abstract of this paper was presented virtually at the International Summit on Hematology and Blood Disorders as a poster presentation with interim findings. The poster’s abstract is available online at: https://magnusconferences.com/hematology-blood/program/scientific-program/2023/hyperviscosit-syndrome-induced-bilateral-visual-and-auditory-impairment-in-cxcr4-mutant-waldenstrom-macroglobulinemia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Waldenström JG. Macroglobulinemia--a review. Haematologica. 1986;71(6):437–440.
2. Groves FD, Travis LB, Devesa SS, et al. Waldenström’s macroglobulinemia: incidence patterns in the United States, 1988–1994. Cancer. 1998;82(6):1078–1081. doi:10.1002/(SICI)1097-0142(19980315)82:6<1078::AID-CNCR10>3.0.CO;2-3
3. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-643569
4. Varettoni M, Ferrari A, Frustaci AM, et al. Younger patients with Waldenström Macroglobulinemia exhibit low risk profile and excellent outcomes in the era of immunotherapy and targeted therapies. Am J Hematol. 2020;95(12):1473–1478. doi:10.1002/ajh.25961
5. Stone MJ, Bogen SA. Role of plasmapheresis in Waldenström’s macroglobulinemia. Clin Lymphoma Myeloma Leuk. 2013;13(2):238–240. doi:10.1016/j.clml.2013.02.013
6. Gertz MA. Acute hyperviscosity: syndromes and management. Blood. 2018;132(13):1379–1385. doi:10.1182/blood-2018-06-846816
7. Gertz MA. Waldenstrom Macroglobulinemia: tailoring therapy for the individual. J Clin Oncol. 2022;40(23):2600–2608. doi:10.1200/JCO.22.00495
8. Schwartz J, Padmanabhan A, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the Seventh Special Issue. J Clin Apher. 2016;31(3):149–162. doi:10.1002/jca.21470
9. Stone MJ, Bogen SA. Evidence-based focused review of management of hyperviscosity syndrome. Blood. 2012;119(10):2205–2208. doi:10.1182/blood-2011-04-347690
10. Shrestha S, Poddar E, Bashyal B, et al. Bilateral central retinal vein occlusion as an initial presentation of Waldenström macroglobulinemia: a case report. J Med Case Rep. 2023;17(1):59. doi:10.1186/s13256-023-03778-4
11. Gustine JN, Meid K, Dubeau T, et al. Serum IgM level as predictor of symptomatic hyperviscosity in patients with Waldenström macroglobulinemia. Br J Haematol. 2017;177(5):717–725. doi:10.1111/bjh.14743
12. Castillo JJ, Moreno DF, Arbelaez MI, et al. CXCR4 mutations affect presentation and outcomes in patients with Waldenström macroglobulinemia: a systematic review. Expert Rev Hematol. 2019;12(10):873–881. doi:10.1080/17474086.2019.1649132
13. Kiew S, Ting D. Diagnosis and management of central retinal vein occlusion. EyeNet Magazine; 2018.
14. Baker PS, Garg SJ, Fineman MS, et al. Serous macular detachment in Waldenström macroglobulinemia: a report of four cases. Am J Ophthalmol. 2013;155(3):448–455. doi:10.1016/j.ajo.2012.09.018
15. Leskov I, Knezevic A, Gill MK. Serous macular detachment associated with Waldenstrom macroglobulinemia managed with ibrutinib: a case report and new insights into pathogenesis. Retin Cases Brief Rep. 2021;15(4):490–494. doi:10.1097/ICB.0000000000000837
16. Watson JA, Olson DJ, Zhang AY. Hyperviscosity retinopathy due to waldenström macroglobulinemia: a case report and literature review. J Vitreoretin Dis. 2021;5(6):520–524. doi:10.1177/2474126420987142
17. Ershler W. Waldenström macroglobulinemia with hyperviscosity: illustrative case series. Relias Media; 2012.
18. Sedhom R. View of Waldenstrom’s macroglobulinemia presenting as syncope. Global J Med Res. 2016;16(F5):17–18.
19. Menke MN, Feke GT, McMeel JW, Treon SP. Effect of plasmapheresis on hyperviscosity-related retinopathy and retinal hemodynamics in patients with Waldenstrom’s macroglobulinemia. Invest Ophthalmol Vis Sci. 2008;49(3):1157–1160. doi:10.1167/iovs.07-1254
20. Anderson KC, Alsina M, Bensinger W, et al. Waldenström Macroglobulinemia/Lymphoplasmacytic Lymphoma, version 1.2023. Natl Compr Canc Netw. 2022;10(10):1211–1219.
21. Treon SP, Xu L, Guerrera ML, et al. Genomic landscape of Waldenström macroglobulinemia and its impact on treatment strategies. J Clin Oncol. 2020;38(11):1198–1208. doi:10.1200/JCO.19.02314
22. André F, Arnedos M, Baras AS; AACR Project GENIE Consortium. AACR project GENIE: powering precision medicine through an International Consortium. Cancer Discov. 2017;7(8):818–831. doi:10.1158/2159-8290.CD-17-0151
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.