Back to Journals » Open Access Emergency Medicine » Volume 16
Iatrogenic Vascular Injuries in Resource-Limited Setting: A 4-Year Experience Monocentric Retrospective Study
Authors Almadwahi NY, Alkadri AM , Fadhel A, Alshujaa M, Ahmed F , Badheeb M
Received 17 November 2023
Accepted for publication 17 April 2024
Published 18 April 2024 Volume 2024:16 Pages 57—64
DOI https://doi.org/10.2147/OAEM.S450213
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hans-Christoph Pape
Nabeel Yahya Almadwahi,1 Ali Mohahmmed Alkadri,2 Ali Fadhel,3 Mohamed Alshujaa,1 Faisal Ahmed,4 Mohamed Badheeb5
1Department of Vascular Surgery, School of Medicine, Sana’a University, Sana’a, Yemen; 2Department of Vascular Surgery, School of Medicine, Ibb University, Ibb, Yemen; 3Department of Cardio-Pediatric Surgery, School of Medicine, Sana’a University, Sana’a, Yemen; 4Department of Urology, School of Medicine, Ibb University, Ibb, Yemen; 5Department of Internal Medicine, Yale New-Haven Health/Bridgeport Hospital, Bridgeport, CT, USA
Correspondence: Ali Mohahmmed Alkadri, Department of Vascular Surgery, School of Medicine, Ibb University, Ibb, Yemen, Tel +967776089579, Email [email protected] Faisal Ahmed, Department of Urology, School of Medicine, Ibb University, Ibb, Yemen, Tel/Fax +967 4428950, Email [email protected]
Background: Iatrogenic vascular injuries (IVIs) due to diagnostic and therapeutic interventions are known but rare or probably under-reported. We present our four-year findings on patients with IVIs after catheterization or surgery who underwent vascular surgical repairs in a resource-limited setting.
Methods: A retrospective case series study between Jun 2018 and Sep 2022 of 35 patients diagnosed with IVIs and treated surgically at our hospital was included. The data on IVIs including patient characteristics, causes and type of injury, treatment, and outcomes were collected and analyzed.
Results: The mean age was 37.12± 17.0 years, and most patients (65.7%) were male. Of the 35 IVIs, 21 were caused by percutaneous procedures, while 14 occurred intraoperatively and affected various arteries and veins. The main injured vessels were the femoral artery (20%) and direct blood vessel puncture made by non-qualified specialists (42.9%) during dialysis cannulation was the main cause. The intraoperative IVI affected the inferior vena cava in three patients, the aorta in two patients, the external iliac artery in four, the tibial and popliteal arteries in four, and the internal carotid artery in one. The following types of repairs were recorded: direct suture of the vessel with or without endarterectomy (71.4%), synthetic patch placement (25.7%), ligation (8.6%), bypass or interposition graft (14.3%), and thromboembolectomy (5.7%). Vascular repair was successful in 32 (91.4%) patients while three patients (8.6%) were expired. Complications occurred in 7 (20%) patients, of which superficial wound infections were the common complication (11.6%) and were treated with proper antibiotic therapy.
Conclusion: Prompt identification of IVIs, as well as proper triage for future treatment, can enhance patient outcomes. Our data showed that non-qualified specialists seem to be responsible for the majority of IVIs. For that, we emphasize the importance of performing vascular procedures by a qualified specialist with adequate training.
Keywords: iatrogenic, vascular injury, adverse event, vascular surgical procedure
Introduction
The expansion of endovascular interventions, along with the increasing complexity of patients and procedures, and the broadening scope of various medical specialties, has coincided with a heightened utilization of these interventions for purposes such as hemodynamic monitoring and nutrition delivery. This trend may have contributed to a rise in the incidence of Iatrogenic Vascular Injury (IVI).1,2 Although IVIs are reported to have a relatively low incidence, their deleterious effects pose a significant threat to patient survival. They are associated with risks such as limb loss, prolonged hospitalization, and increased overall healthcare costs.1,3 Furthermore, national studies have indicated that IVIs account for up to 50% of all vascular injuries in developed countries.4 However, data on the prevalence, causes, surgical techniques, and prognosis of IVIs in middle and low-income countries are scant, partly due to limited record-keeping and a scarcity of vascular surgery specialists. Additionally, most existing reports on vascular injuries either cover a broad range of injury types or focus on selected cases under unique circumstances.3,5
While IVI is considered an adverse event associated with any vascular procedure, its occurrence and severity are influenced by multiple factors, including the type of procedure, the surgeon’s expertise, and overall health service quality.6
Many studies have described the IVIs’ etiology, their treatments, and the role of vascular surgeons.3–5 However, there are few reports in our country regarding this issue,7 due to a scarcity of vascular surgery specialists and many traumatic vascular injuries might not reach or not receive the optimal care due to logistics, financial, and time constraints. This study aims to describe our 4- year experience with patients who underwent surgical repair of IVIs after catheterization or major surgical operation in a resource-limited setting where inadequate healthcare funding, a lack of skilled personnel, and the absence of an academic curriculum focused on this sophisticated surgical procedure are still the main challenge. It also seeks to emphasize the crucial role of vascular surgeons in providing consultations and support to other surgical services and their patients.
Materials and Methods
Study Design
A retrospective case series study between Jun 2018 and Sep 2022 of all patients diagnosed with IVIs and treated at our teaching hospital in the vascular surgery department (Al-Thora General Hospital, Sana’a, Yemen) during the study period was included. The study was approved by the Ethics Research Committees of Sana’a University, which was carried out in accordance with the Declaration of Helsinki.
Inclusion Criteria
Patients referred to the vascular surgery department for vascular injury or foreign body retention after interventions/surgeries, including those resulting in IVIs, and supported a written informed consent were included.
Exclusion Criteria
All patients with isolated injury to the superficial venous system, vascular injuries caused by trauma, those treated by a general surgeon, and patients treated in other centers were excluded from the study.
Data Collections and Study Outcomes
The data on iatrogenic vascular injury patients included demographic characteristics such as age and gender, symptoms, time to diagnosis, mechanism, and location of the injury, specialty involved, repair techniques, limb salvage rate, complication rates, and mortality. Initial control of the intraoperative IVIs was achieved by manually compressing the bleeding site while dissecting the surrounding area of the injury to establish definitive vascular control using vascular clamps. We made sure to avoid blind clamping and took special care in the application and handling of the clamps to prevent further IVI. Before compression or clamping, all patients received intravenous heparin (75 to 100 IU/kg IV bolus over 10 minutes). Any injuries sustained during surgery were immediately repaired. The injuries related to the cannulation or catheter were diagnosed based on a combination of signs, symptoms, and Color Flow Duplex Imaging (CFDI) findings. CFDI accurately determined the exact location of the vascular lesion in these patients. The criteria for surgical intervention included increased pressure on the skin, rapid expansion, low blood pressure, accompanying distal ischemia, and a large hematoma (Figure 1). The main outcomes were surgical success, patient survival, and organ salvage. To define salvageability, the limb should be considered vascularly viable, indicated by warmth, intact pulses, and preserved motor and sensory functions.1
 |
Figure 1 Arterial aneurysms caused by repeated central line access (A) Right subclavian artery aneurysm. (B) left subclavian to axillary artery aneurysm. (C) left brachial artery pseudoaneurysm). |
Statistical Analysis
Quantitative data were described using mean and /or median and standard deviation, while qualitative data were expressed in frequency and percentage. The normality of data was tested using Kolmogorov–Smirnov and Shapiro–Wilk test; when the data were non-normally distributed, a nonparametric test was used. The chi-square test was performed to assess the association between nominal and categorical variables regarding the number of causal factors for IVIs. A p-value < 0.05 was considered statistically significant. Statistical analyses in this study were conducted using IBM SPSS version 22 software (IBM Corp., Armonk, New York).
Result
During the study period, thirty-five cases of IVI were identified and met the inclusion criteria. The mean age of patients was 37.12 ± 17.0 years, ranging from 5 months to 62 years, with 7 (20%) being older than 50 years. A majority of the patients, 65.7%, were male. The time from injury to presentation varied widely, with the shortest being 10 minutes and the longest being 3 months, median averaging 2.7 days. Patient characteristics and causes of IVI are detailed in Table 1. The distribution of IVIs by medical specialty was as follows: Cardiology 6 (17.1%), Orthopedic Surgery 4 (11.4%), Gynecology 4 (11.4%), General Surgery 3 (8.6%), and Urology 3 (8.6%). Notably, about 15 (42.9%) of the IVIs were caused by non-physician (healthcare) practitioners and were statistically significant (p< 0.05). The most frequently injured vessel was the femoral artery, involved in 7 cases (20%), followed by the brachial artery in 6 cases (17.1%).
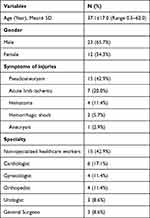 |
Table 1 Patient Characteristics and Causes of Iatrogenic Vascular Injuries |
Of the 35 IVIs, the primary cause of IVI was direct blood vessel puncture during dialysis cannulation by non-qualified specialists for end-stage kidney disease patients, accounting for 42.9% (15 cases). Other causes included injuries to the femoral artery (6 cases) during percutaneous coronary intervention (PCI) procedures, the thoracic aorta (1 case) due to a pseudoaneurysm following patent ductus arteriosus closure, and the abdominal aorta (1 case) inadvertently ligated during a tumor resection by a gynecologist, leading to intraoperative death (Figure 2). Inferior vena cava (IVC) injuries occurred in 3 cases: two during biliary tract surgery and one during tumor resection, with two resulting in intraoperative deaths. Iliac artery injuries occurred in 4 cases during pelvic tumor resections and gynecologic operations. Additionally, the internal carotid artery in one during thyroidectomy, and tibial and popliteal artery injuries were noted in orthopedic procedures in 4 cases (Table 2). All patients underwent open surgical repair. The average operative time was 104.4 ± 14.4 minutes, ranging from 67 to 130 minutes. Vascular repairs included direct suturing of the vessel with or without endarterectomy in 25 (71.4%) cases, synthetic patch placement in 9 (25.7%) cases, ligation in 3 (8.6%) cases, bypass or interposition grafting in 5 (14.3%) cases, and thrombo-embolectomy in 2 (5.7%) cases, as detailed in (Table 3). The repair was successful in 32 (91.4%) cases, while 3 patients (8.6%) died during surgery. In dead cases, open vascular repair was tried. However, due to the severity of injured vessels and severe shock, the patients expired intraoperatively. The average follow-up period was 1.5 ± 0.6 months. Complications occurred in 7 (20%) cases, including superficial wound infections in 4 (11.6%) cases treated with proper antibiotics. Graft thrombosis occurred in one patient, one case required amputation, and one case needed reoperation due to failed surgical repair.
 |
Table 2 The Anatomical Distribution of Vascular Injuries |
 |
Table 3 Operative and Postoperative Characteristics of IVIs |
Discussion
The epidemiological analysis of IVI is hampered by the absence or limitations inherent within national databases and registries, compounded by the heterogeneity of patient populations and a selection bias in injury studies. This limitation is particularly pronounced in middle- and low-income nations.8 In this study, we analyze the patterns and outcomes of IVI in a singular, resource-constrained setting over four years.
IVIs, which account for 10% of all cases in most published papers, are on the rise as endovascular therapies become more prevalent. The study revealed a mean age of 37.12 ± 17.0 years, with 20% above 50 years. Civilian trauma is more frequent in youngsters, but it can develop at any age due to a variety of circumstances.9
Previous literature has consistently demonstrated a disproportionate prevalence of IVI amongst male subjects, a trend observable irrespective of socioeconomic status and income levels.10,11 However, the pattern of these injuries remains inconsistent. For instance, a comprehensive report from the United States indicated a higher incidence rate amongst female subjects, albeit lacking statistical significance.6 Further, single-center reports have documented inconsistent gender distributions.12,13 In contrast, our research denotes a predominance of male subjects.
Consistent with antecedent reports, IVI predominantly involves the arterial system.2 The pattern and severity of IVI are contingent upon various factors, including the etiology, nature, and anatomical location of the injury. These can range from asymptomatic pseudoaneurysms, identified upon clinical examination, to more severe manifestations such as acute limb ischemia and active hemorrhage necessitating urgent surgical intervention.14 The patterns of IVI injuries were seen as pseudoaneurysm (42.9%), followed by acute limb ischemia (20.0%), then hematoma (11.4%), hemorrhagic shock (5.7%), and true aneurysm (2.9%), similar findings were reported by Dabas et al15 and all cases required open repair because of large size, failed USG guided compression, or due to compromised viability of the skin.
In this study, a true aneurysm (involvement of the entire wall of the vessel) was seen in 2.9%. We hypothesize that trauma-induced compression of the arterial wall causes a contusion of the arterial media, which leads to wall weakness and fusiform dilation. This process differs from pseudoaneurysms, which occur when fibrous tissue surrounds a posttraumatic hematoma with continuous arterial flow. However, it is difficult to point out that these aneurysms existed before the trauma. Similar reports were mentioned by Jedynak and associations and Goncu et al.16,17
This study observed a higher trend of IVIs when performed by non-specialized healthcare practitioners, accounting for 42.9% of cases within our cohort, with a significant proportion related to direct blood vessel puncture during dialysis cannulation. While the collaboration within medical teams is fundamental; however, we posit that vascular access procedures necessitate comprehensive initial training and rigorous supervision before implementation. Such a protocol has been associated with comparable outcomes among physicians.18 Additionally, this approach should be also implemented for physicians in training.19
The second IVI cause was due to cardiac catheterizations, with a notably higher rate of complications observed through femoral access. Although multiple studies have corroborated an elevated incidence of complications via femoral access,20 the prevalence in our investigation does not necessarily imply increased utilization of this method. Instead, it may reflect the complexity and advanced nature of the cases handled. Further research is required to elucidate the patterns of vascular injury in cardiac interventions. Additionally, our study was unable to ascertain whether vascular access in the included cases was established using ultrasound guidance or if closure devices were employed, which have been associated with enhanced success rates and reduced complication rates.21 Overall, the necessity for surgical interventions in the context of complex endovascular procedures was found to be relatively minimal, less than 4%.22
Major iatrogenic injuries to the abdominal vasculature are relatively rare but can be catastrophic, with reported mortality rates reaching as high as 60%-90%, if unrecognized promptly.23 Despite their rarity, advancements in surgical techniques have significantly influenced the incidence and management of such IVIs. Our study included cases of ligation injury to the abdominal aorta and IVC injuries during pelvic tumor resections, some of which led to intra-operative fatalities. Indeed, IVI presents a formidable challenge during gynecological tumor resections, primarily due to the limited specialized vascular surgical training among gynecologists. Notably, the highest risk of IVI was observed during tumor resection, necessitating heightened vigilance during this stage.24 The involvement of a multidisciplinary team in pre-operative planning, including the potential intra-operative participation of a vascular surgeon, is imperative for achieving optimal outcomes in managing these high-risk tumor resections.25 It is important to note that surgical interventions for aortic injuries also present considerable risks, including a mortality rate of 16% and the potential for paraplegia, which can occur in up to 25% of cases.26 Recently, endovascular repair has gained popularity, largely due to its comparatively lower complication rates, as it obviates the need for clamping or thoracotomy.26 In the context of our study, one patient presented with aortic trauma during tumor resection. In another case, thoracic aortic trauma was presented as a pseudoaneurysm two years following patent ductus arteriosus closure. These patients, characterized by unstable hemodynamic parameters, were treated with synthetic graft interpositions, with no subsequent occurrences of mortality, paraplegia, or other complications. Pseudoaneurysms after endovascular PDA repair are rare and can be caused by infection, rupture in the suture line, or stenotic ductus. There are three types: decreased curvature of the aortic arch due to higher arterial pressure. In other types, it can originate from the ductus arteriosus or pulmonary artery, often due to reperfusion or damage to the arterial wall.7 In our case, it arose from the aortic arch in the descending part of the aorta, resulting from reperfusion caused by a ligature damaging the arterial wall. The pseudoaneurysms were large in diameter (8 × 9 cm), which needed urgent repair.
Overall, the management of iatrogenic vascular injuries is inherently dependent on the nature of the injury and the affected blood vessels. In our cohort, primary repair was the most commonly implemented approach, a decision likely influenced by the complex nature of IVIs. Indeed, such an approach requires exceptional precision to avert complications like tension or stenosis, particularly when end-to-end anastomosis is considered.27 Corroborating our findings, a similar study reported that primary repair was the most frequently utilized surgical procedure in 65% of patients, with end-to-end anastomosis and saphenous vein graft interposition applied in 44% and 38% of cases, respectively.28 Additionally, a previous study recommended saphenous vein graft interposition as the most effective surgical intervention for injuries to the superficial femoral artery.29 On the other hand, non-surgical interventions should be initially considered for pseudoaneurysms. These encompass ultrasound-guided compression, biodegradable collagen injection, the use of coated stents, coil embolization, and various vascular closure devices.8
The exact criteria for IVI definitions remain a subject of controversy. There is a lack of a single all-encompassing definition of iatrogenic vascular injuries which led to heterogeneous reporting of IVIs under various categories and less than a true reflection of the incidence of IVIs.15 In this study, we include only patients referred to the vascular surgery department and treated by vascular surgeons.
Study Limitations
This study possesses several limitations, most notably as a single-center experience, it predominantly represents more advanced cases of IVI that necessitated surgical intervention by a vascular surgeon, and it does not encompass cases that were managed intra-operatively by general surgeon or through conservative management. Additionally, the relatively small sample size and the retrospective design of the study render it vulnerable to selection and misclassification biases. Furthermore, the study was unable to determine whether ultrasound-guided vascular access was employed, nor could it ascertain the usage of closure devices in endovascular interventions. Our result needs to be validated in a large cohort study with strict criteria for IVIs, including multicenter with different levels of facilities.
Conclusion
Prompt recognition of iatrogenic injury and appropriate triage for further treatment can improve patient outcomes. Our data showed that non-qualified specialists seem to be responsible for the majority of IVIs. For that, we emphasize the importance of performing vascular procedures by a qualified specialist with adequate training.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The Sana’a University’s ethics committee approved the study protocol and all patients provided informed consent before data capturing.
Consent for Publication
All authors reviewed the manuscript and approved its submission.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Khan FH, Yousuf KM, Bagwani AR. Vascular injuries of the extremities are a major challenge in a third world country. J Trauma Manag Outcomes. 2015;9(1):5. doi:10.1186/s13032-015-0027-0
2. Giswold ME, Landry GJ, Taylor LM, Moneta GL. Iatrogenic arterial injury is an increasingly important cause of arterial trauma. Am J Surg. 2004;187(5):
3. Shanmugavelayutham C, Ilayakumar P, Elancheralathan K, Velladurachi B, Amalorpavanathan J, Rajkumar M. Iatrogenic vascular injuries: an institutional experience. Indian J Vasc Endovasc Surg. 2019;6(1):19–22. doi:10.4103/ijves.ijves_46_18
4. Fingerhut A, Leppäniemi AK, Androulakis GA, et al. The European experience with vascular injuries. Surg Clin North Am. 2002;82(1):175–188. doi:10.1016/S0039-6109(03)00147-6
5. Yoo TK, Min SK, Ahn S, et al. Major vascular injury during nonvascular surgeries. Ann Vasc Surg. 2012;26(6):825–832. doi:10.1016/j.avsg.2012.01.010
6. Miranda J, Dongarwar D, Salihu HM, et al. Gender, Racial and Ethnic Disparities in Iatrogenic Vascular Injuries among the Ten Most Frequent Surgical Procedures in the United States. Ann Vasc Surg. 2022;80:18–28. doi:10.1016/j.avsg.2021.09.044
7. Almadwahi N, Fadhel A, Alkadri A, Ahmed F, Badheeb M, Alshujaa M. Iatrogenic pseudoaneurysm of patent ductus arteriosus following prior PDA closure in a teenager. J Pediatr Surg Case Rep. 2022;85:102441. doi:10.1016/j.epsc.2022.102441
8. Baghi I, Herfatkar MR, Shokrgozar L, Poor-Rasuli Z, Aghajani F. Assessment of Vascular Injuries and Reconstruction. Trauma Mon. 2015;20:e30469.
9. Wani ML, Ahangar AG, Ganie FA, Wani SN, Wani NU. Vascular injuries: trends in management. Trauma Mon. 2012;17(2):266–269. doi:10.5812/traumamon.6238
10. Usman R, Jamil M, Anwer MF. Evaluation, surgical management and outcome of traumatic extremity vascular injuries: a 5-year level-1 trauma centres experience. Ann Vasc Dis. 2018;11(3):312–317. doi:10.3400/avd.oa.18-00068
11. Van Waes OJ, Van Lieshout EM, Hogendoorn W, Halm JA, Vermeulen J. Treatment of penetrating trauma of the extremities: ten years’ experience at a Dutch level 1 trauma center. Scand J Trauma Resusc Emerg Med. 2013;21(1):2. doi:10.1186/1757-7241-21-2
12. Guraya SY. Extremity vascular trauma in Pakistan. Saudi Med J. 2004;25(4):498–501.
13. De’Ath HD, Galland RB. Iatrogenic and non-iatrogenic vascular trauma in a district general hospital: a 21-year review. World J Surg. 2010;34(10):2363–2367. doi:10.1007/s00268-010-0647-5
14. Rudström H, Bergqvist D, Björck M. Iatrogenic vascular injuries with lethal outcome. World J Surg. 2013;37(8):1981–1987. doi:10.1007/s00268-013-2061-2
15. Dabas A, Katiyar A, Srivastava S, et al. A single-center 5-year experience of iatrogenic vascular injuries and their outcomes. Indian j Vasc Endovasc Surg. 2022;9(3):229–235. doi:10.4103/ijves.ijves_20_22
16. Jedynak J, Frydman G. Idiopathic true aneurysm of the radial artery: a rare entity. EJVES Extra. 2012;24(4):e21–e22. doi:10.1016/j.ejvsextra.2012.08.001
17. Goncu T, Toktas F, Tiryakioglu O, Yumun G, Demirtas S, Yavuz S. Posttraumatic true aneurysm of the axillary artery following blunt trauma. Case Rep Med. 2010;2010:2. doi:10.1155/2010/210391
18. Sakai H, Hirosue M, Iwata M, Terasawa T. The effect of introducing a nurse-practitioner-led peripherally inserted central venous catheter placement program on the utilization of central venous access device: a retrospective study in Japan. J Vasc Access. 2023;11297298231173160. doi:10.1177/11297298231173160
19. Kreeftenberg HG, Aarts JT, Bindels A, van der Meer NJM, van der Voort PHJ. Procedures performed by advanced practice providers compared with medical residents in the ICU: a prospective observational study. Crit Care Explor. 2020;2(4):e0101. doi:10.1097/CCE.0000000000000101
20. Chiarito M, Cao D, Nicolas J, et al. Radial versus femoral access for coronary interventions: an updated systematic review and meta-analysis of randomized trials. Catheter Cardiovasc Interv. 2021;97(7):1387–1396. doi:10.1002/ccd.29486
21. Pang N, Gao J, Zhang B, et al. Vascular closure devices versus manual compression in cardiac interventional procedures: systematic review and meta-analysis. Cardiovasc Ther. 2022;2022:8569188. doi:10.1155/2022/8569188
22. Bolia A. Subintimal angioplasty in lower limb ischaemia. J Cardiovasc Surg. 2005;46:385–394.
23. Kobayashi LM, Costantini TW, Hamel MG, Dierksheide JE, Coimbra R. Abdominal vascular trauma. Trauma Surg Acute Care Open. 2016;1(1):e000015. doi:10.1136/tsaco-2016-000015
24. Buras AL, Chern JY, Chon HS, Shahzad MM, Wenham RM, Hoffman MS. Major vascular injury during gynecologic cancer surgery. Gynecol Oncol Rep. 2021;37:100815. doi:10.1016/j.gore.2021.100815
25. Bergentz S-E, Bergqvist D. Vascular injuries in orthopedic surgery. In: Iatrogenic Vascular Injuries. Berlin, Heidelberg: Springer; 1989:87–94.
26. Temizkan V. The effect of early endovascular intervention on the outcome of traumatic vascular injuries. Turk J Thorac Cardiovasc Surg. 2013;21(1):63–68. doi:10.5606/tgkdc.dergisi.2013.5765
27. Pereira BM, Chiara O, Ramponi F, et al. WSES position paper on vascular emergency surgery. World J Emerg Surg. 2015;10(1):49. doi:10.1186/s13017-015-0037-2
28. Yazıcı S. Analysis of peripheral vascular injuries: a social catastrophe. Dicle Med J. 2014;41(3):441–445. doi:10.5798/diclemedj.0921.2014.03.0451
29. Wahlgren CM, Riddez L. Penetrating Vascular Trauma of the Upper and Lower Limbs. Curr Trauma Rep. 2016;2(1):11–20. doi:10.1007/s40719-016-0035-1
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.