Back to Journals » Clinical Interventions in Aging » Volume 19
Imaging of Sarcopenia in Type 2 Diabetes Mellitus
Authors Wang D, Zhang G, Yu Y, Zhang Z
Received 7 October 2023
Accepted for publication 17 January 2024
Published 26 January 2024 Volume 2024:19 Pages 141—151
DOI https://doi.org/10.2147/CIA.S443572
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Dingyue Wang, Gaosen Zhang, Yana Yu, Zhen Zhang
Department of Ultrasound, the First Affiliated Hospital China Medical University, Shenyang City, Liaoning Province, 110001, People’s Republic of China
Correspondence: Zhen Zhang, Email [email protected]
Abstract: Sarcopenia is an age-related condition characterized by the loss of skeletal muscle mass, muscular strength, and muscle function. In older adults, type 2 diabetes mellitus (T2DM) constitutes a significant health burden. Skeletal muscle damage and deterioration have emerged as novel chronic complications in patients with diabetes, often linked to their increased longevity. Diabetic sarcopenia has been associated with increased rates of hospitalization, cardiovascular events, and mortality. Nevertheless, effectively managing metabolic disorders in patients with T2DM through appropriate therapeutic interventions could potentially mitigate the risk of sarcopenia. Utilizing imaging technologies holds substantial clinical significance in the early detection of skeletal muscle mass alterations associated with sarcopenia. Such detection is pivotal for arresting disease progression and preserving patients’ quality of life. These imaging modalities offer reproducible and consistent patterns over time, as they all provide varying degrees of quantitative data. This review primarily delves into the application of dual-energy X-ray absorptiometry, computed tomography, magnetic resonance imaging, and ultrasound for both qualitative and quantitative assessments of muscle mass in patients with T2DM. It also juxtaposes the merits and limitations of these four techniques. By understanding the nuances of each method, clinicians can discern how best to apply them in diverse clinical scenarios.
Keywords: diagnostic imaging, older adults, sarcopenia, skeletal muscle mass, type 2 diabetes mellitus
Introduction
Sarcopenia, a syndrome characterized by the deterioration of muscle mass and function (including muscle strength and/or physical performance) as individuals age, has emerged as a pressing global concern. This is attributed to its association with escalated risks of mortality, frailty, and diminished mobility among the older adults.1,2 Type 2 diabetes mellitus (T2DM) is one of the most prevalent metabolic disorders, affecting almost a quarter of the population aged 65 years and above. This proportion is anticipated to escalate in the forthcoming decades.3 Older adults grappling with T2DM experience elevated incidences of functional disability, coexisting ailments, and various geriatric conditions, alongside microvascular and macrovascular complications.4 A novel chronic complication of T2DM is the damage and degeneration of the skeletal muscle, which is attributed to the extended survival of patients with T2DM.5,6 The vulnerability to sarcopenia is notably augmented in patients with T2DM. T2DM is associated with excessive loss of skeletal muscle and trunk fat mass in older adults. Older women withT2DM are at especially high risk for loss of skeletal muscle mass.7 While the rate of deterioration might vary contingent on the muscle cluster, it invariably leads to compromised muscle.8
The interplay between these two factors may potentiate adverse outcomes, notably functional regression and disability.9 The decline in muscle mass seems to be an inevitable part of the aging trajectory, and variations in the rate of degeneration among different populations imply that modifiable behavioral elements can influence the onset of sarcopenia.10 A particularly effective strategy in thwarting sarcopenia is resistance-based training.11 Moreover, alongside lifestyle modifications, due consideration must be accorded to several hypoglycemic drugs, which could yield varied effects on the underlying pathophysiological irregularities giving rise to sarcopenia. Our investigation delves into pertinent reviews on this subject, aimed at facilitating the early clinical identification of susceptibility to sarcopenia in patients with T2DM and the timely initiation of interventions to ameliorate muscle mass loss. This proactive approach seeks to minimize the incidence of falls and fractures in patients with T2DM, consequently enhancing their quality of life. Furthermore, it aids medical practitioners in selecting appropriate imaging modalities to track alterations in the muscle structure and mass among patients with T2DM during the course of diabetes. This monitoring process is vital for evaluating the efficacy of relevant treatments.
Definition and Diagnosis of Sarcopenia
Age-related loss of muscle mass and function is a prevalent phenomenon. Muscle strength undergoes a reduction of 20–40% during the seventh and eighth decades of life, and this decline exacerbates progressively over time.12 In 1988, Irwin Rosenberg first introduced the term “sarcopenia” to delineate the age-related deterioration of skeletal muscle mass and function.13 According to the European Working Group on Sarcopenia in Older People (EWGSOP), sarcopenia is currently defined as a “syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength”.14 This definition was revised in 2019 to describe sarcopenia as “a muscle disease originating from adverse muscle changes that accumulate over a lifetime”. EWGSOP proposes a screening and diagnostic approach for sarcopenia termed “Find-Assess-Confirm-Severity” (F-A-C-S; Figure 1).1 Similarly, the Asian Sarcopenia Working Group (AWGS) adopts a comparable strategy for sarcopenia, suggesting an adjusted cutoff value specifically suited for Asians, accounting for their anthropometric characteristics of the Asian population.15 Central to the diagnosis of sarcopenia is the measurement of muscle mass. Clinical practice and research employ diverse qualitative and quantitative methodologies to ascertain muscle mass. Imaging modalities, including dual-energy X-ray absorptiometry (DXA), computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound (US; Table 1), serve to evaluate muscle mass and quality, consequently facilitating sarcopenia diagnosis.
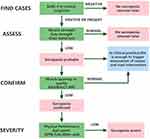 |
Figure 1 Screening and diagnostic approach for sarcopenia termed “Find-Assess-Confirm-Severity”. Adapted from Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T et al Sarcopenia: revised European consensus on definition and diagnosis. Age and ageing. 2019;48(1):16–31.1 |
 |
Table 1 Advantages and Disadvantages of the Primary Imaging Techniques for Body Composition and Mass/Fat Assessment |
Association Between T2DM and Sarcopenia
The skeletal muscle, which is the largest organ in the human body, also serves as a regulator of glucose homeostasis and is responsible for 80% of postprandial circulating glucose uptake.16 Muscle insulin resistance, which disrupts the amount and timing of glucose uptake, is the initial metabolic defect in T2DM.17 As individuals age, there is a gradual decline in mitochondrial function in human skeletal muscle along with a decrease in muscle mass, strength, and overall muscle function.18 In the case of insulin resistance and T2DM, there is delayed insulin action and glucose uptake by skeletal muscle resulting in decreased overall glucose uptake.19
In addition, anomalies in lipid metabolism and obesity frequently induce insulin resistance. Altered adipose tissue growth and function lead to fluctuations in the concentrations of adipokines and cytokines, causing chronic inflammation, mitochondrial impairment, disruption of the insulin signaling pathway in the skeletal muscle, and eventual muscular atrophy. Adipose tissue secretes proinflammatory cytokines such as interleukin 6 (IL-6), interleukin 1 (IL-1), and tumor necrosis factor alpha (TNF-α).20 These inflammatory molecules significantly affect key nodes within the pancreatic route, consequently influencing insulin sensitivity, fostering insulin resistance, and hastening the onset of sarcopenia.
These metabolic defects overlap, and insulin resistance occurs at the junction of aging, muscle atrophy/muscle loss, and obesity, forming a toxic feedback loop where each exacerbates the other.21
Muscle Mass and Behavioral Interaction
Although the decline in muscle mass is often considered an inevitable aspect of aging, there exists variation in the rate of muscle mass loss across different populations.10 This variability suggests that certain behavioral factors might influence the onset and progression of sarcopenia. Among the strategies for preserving muscle quantity and quality, resistance exercise training is a reliable approach.1 Notably, resistance-based training proves equally effective in enhancing muscle strength, size, and mass among older adults with T2DM.11 Investigation into resistance training at varying intensities revealed positive outcomes in terms of muscle number, assessed through mid-thigh muscle cross-sectional area measurements.22,23 However, to ensure the continuous and safe engagement of older adults in training, it becomes imperative to thoroughly study and evaluate the suitable exercise intensity and duration, a task that imaging methods can facilitate.24 The role of nutritional intervention in preventing and treating sarcopenia is less straightforward; nevertheless, some data suggest that adopting healthier dietary patterns, including adequate amounts of protein, vitamin D, antioxidants, and long-chain polyunsaturated fatty acids, could confer benefits.25–27
Muscle Mass and Hypoglycemic Medication Interaction
Given the diverse effects of hypoglycemic medications on muscle mass (Figure 2), the choice of such medications should consider not only blood glucose levels and cardiovascular conditions but also the risk of sarcopenia. When compared with patients with T2DM but without sarcopenia, those with both conditions exhibit elevated blood glucose levels and inadequate insulin production. Elevated levels of HbA1c and mean blood glucose contributed to the increased risk of sarcopenia. Metformin, the primary choice for T2DM treatment, might enhance strength and muscle growth.28 However, a study29 indicated that metformin might attenuate the typical increase in muscle density associated with progressive resistance exercise. Long-term effects of sodium-glucose transport protein 2 inhibitors (SGLT2i) might enhance insulin sensitivity, thereby mitigating muscle catabolism and influencing the quality and functionality of the skeletal muscle.30,31 Epidemiological research indicates a link between thiazolidinediones and reduced loss of muscle mass as well as improved walking pace among patients with T2DM.28,32 Nonetheless, clinical trial data remain limited and contradictory.33 The notable advantage for muscle preservation seems to be the use of glucagon-like peptide-1 (GLP-1) receptor agonists34,35 and dipeptidyl peptidase-4 inhibitors (DPP-4i),36–38 which not only minimally affects muscle mass loss but also stimulates muscle contraction and aid in muscle recovery. A novel agent, tirzepatide, a glucose-dependent insulin polypeptide and GLP-1 receptor agonist, exhibits approximately five times higher binding affinity to the GLP-1 receptor than natural GLP-1 does,39 leading to reduced blood sugar levels in patients with T2DM, particularly those who are obese. It also results in clinically relevant weight loss and effectively improved overall body function.40 Conversely, sulfonylureas and glinides appear to consistently have a negative effect on skeletal muscle.41,42 While existing clinical trial and epidemiological data demonstrate a positive correlation between insulin use and measures related to sarcopenia,43–45 the effect of insulin on overall body weight cannot be disregarded. In light of this, clinicians should exercise caution in prescribing these medications to diabetic patients who are susceptible to sarcopenia, shifting focus instead to monitoring changes in muscle mass. This underscores the crucial role of imaging methods in promptly and accurately assessing the muscle mass of patients.
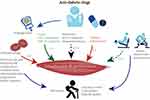 |
Figure 2 Effects of hypoglycemic drugs and exercise on muscle mass and performance. |
Imaging Techniques for Sarcopenia in Type 2 Diabetes Mellitus
Dual-Energy X-Ray Absorptiometry
DXA can be used to assess the adipose tissue, bone mineral content, and nonbony lean tissue in the entire body or specific anatomical regions.46 The appendicular lean mass (ALM) value, which represents the combined muscle mass of the upper and lower limbs as measured by DXA, is commonly utilized for assessing muscle mass. This value is then standardized to height by calculating the appendicular lean mass index (ALMI = ALM/height2). The recent guidelines from EWGSOP have made slight modifications to previous diagnostic cutoff values, proposing an ALMI < 5.5 kg/m2 in women and ALMI < 7.0 kg/m2 in men be used as criteria for defining low muscle mass (1). Additionally, the AWGS suggests an ALMI < 5.7 kg/m2 in women and ALMI < 7.0 kg/m2 in men, taking into account the anthropometric characteristics of the Asian population (2). Through a comparison of data across different age groups and DXA-based sarcopenia assessments, other researchers47 identified significant differences in skeletal muscle mass between older and middle-aged patients with diabetes. Notably, the prevalence of sarcopenia among older adults with T2DM was markedly higher than that in their middle-aged diabetic counterparts. The link between sarcopenia and diabetes was more pronounced among individuals over the age of 75 years. Lean tissue, as determined by DXA, encompasses skin, fibrous connective tissue, body water in addition to the muscle. Consequently, estimating muscle mass solely based on lean tissue could yield inaccurate results.48 Bredella et al49 compared DXA with CT and observed a tendency of DXA to overestimate thigh muscle mass, especially in severely obese women, due to the influence of body thickness and level of hydration on DXA measurements, which suggests potential limitations in quantifying body composition. Obesity contributes to an increased risk of both T2DM and insulin resistance.50 In cases of obese patients with sarcopenia and T2DM, DXA measurements might overstate muscle mass, potentially leading to treatment delays. Additionally, the accuracy of measurements could be compromised if a subject’s body length exceeds the scanning area’s dimensions or if their arm spacing exceeds the scanning area’s width due to design constraints.
Computed Tomography
Although Steven Heymsfield utilized CT for analyzing body composition in the 1980s,51 Shen et al52 did not present a method for calculating whole-body skeletal muscle and adipose tissue from a single abdomen CT cross-sectional scan until 2004. A commonly used approach for evaluating sarcopenia involves quantifying the skeletal muscle area (SMA) at the lumbar 3 (L3) level by delineating regions of interest (ROIs) and applying standardized thresholds (−29 Hounsfield units (HU)/+150 HU) to identify the muscle tissue, thereby obtaining the cross-sectional area (CSA) of the muscle.53,54 The skeletal muscle index (SMI) is typically calculated by dividing the cross-sectional area (CSA) by height squared (CSA/height2). According to a recent meta-analysis, the commonly used SMI cutoff values for assessing muscle mass on CT scans range from 39 to 41 cm2/m2 for females and from 52 to 55 cm2/m2 for males.55 A study56 utilized CT to analyze the body composition of 1787 healthy individuals across four northern Chinese cities and established diagnostic thresholds for skeletal muscle mass loss based on 700 younger healthy adults. In a prospective study of Japanese Americans, Han et al57 used CT to investigate the relationship between cumulative changes in visceral fat, thigh muscle cross-sectional area, and the occurrence of T2DM over 5 years. Their findings indicated that simultaneous changes in visceral fat and thigh muscle area were associated with an increased risk of T2DM. These reference values and research outcomes could aid doctors in predicting outcomes, enhancing nutritional therapy, and diagnosing malnutrition, though further research is necessary to determine the predictive effect of these reference values across various disease groups. However, because of variations in population characteristics and criteria, there is no universally recognized standard for CT-based sarcopenia diagnosis.58 Moreover, the high radiation dose, cost, and operational complexity of CT for assessing skeletal muscle quality limit its practical applicability.
The exploration of the link between sarcopenia, osteoporosis, and diabetes is facilitated by quantitative CT (QCT), which mitigates the impact of unstable CT results on measurements and quantifies true volumetric bone mineral density. Peripheral QCT (pQCT), based on peripheral body layer imaging, is a compact scanning device that gauges muscle area, muscle density, and intra-muscular fat content in limb cross sections. Researchers59 investigated longitudinal changes in pQCT-measured calf myosteatosis and skeletal muscle density. Intermuscular adipose tissue (IMAT) has been associated with alterations in lipid and glucose metabolism, emerging as a noteworthy concern. Studies suggest that increased intermuscular fat with aging correlates with the onset of T2DM, raising the possibility that intermuscular fat could serve as an independent predictor of T2DM in males of African descent.60 pQCT offers advantages over standard CT, including reduced radiation exposure, shorter scan times, and lower costs.60 The main drawbacks of pQCT include the lack of image acquisition and processing protocol homogeneity and limitations in scanning range, potentially leading to measurement errors.61
Magnetic Resonance Imaging
Through the manipulation of radiofrequency pulse sequences, MRI technology can evaluate the adipose tissue and fat-free tissue by assessing the absorption and release of radiofrequency energy from hydrogen nuclei under the influence of an external magnetic field.62 MRI can diagnose sarcopenia and is considered the gold standard for determining body composition, similar to CT. It not only provides data on fat infiltration akin to CT but also offers additional insights into muscle quality by detailing muscle edema, fiber infiltration, fiber contraction, and elasticity. These aberrant changes can lead to reduced muscle mass and strength, both crucial components of sarcopenia.63–65 However, studies on MR have been conducted on various muscular regions without standardized imaging protocols and biomarkers.66 Studies have correlated skeletal muscle fat infiltration with insulin resistance, diabetes, and sarcopenia.67 In a study,68 whole-body MRI quantified intermuscular adipose tissue (IMAT), visceral adipose tissue (VAT), subcutaneous adipose tissue, and total adipose tissue to determine changes in body composition associated with aging. The results revealed a decline in skeletal muscle mass coupled with an increase in VAT and IMAT. Animal studies have shown69 that diabetes mellitus leads to reduced skeletal muscle weight, strength, and cross-sectional area of muscle fibers, while intramyocellular (IMCL) lipid content increases. The rise in IMCL correlates with the loss of skeletal muscle mass.68 Researchers70 suggest that specific patterns of fat infiltration, rather than overall fat content, might serve as more significant risk factors for T2DM and metabolic syndrome. Consequently, changes in skeletal muscle fat content and infiltration represent a pivotal focus in sarcopenia and T2DM research.
Iterative Decomposition of Water and Fat with Echo Asymmetry and Least-Squares Estimation (IDEAL) is one of the most advanced hydro-lipid imaging methods available.71 In light of clinical study stability criteria, imaging techniques advocate employing the three-point Dixon approach for quantifying muscle fat content. Precise measurement of muscle72 cell lipid content can be achieved through 1H magnetic resonance spectroscopy (1H MRS).73 This technique quantifies IMCL and extracellular (EMCL) lipids, minimizing potential errors related to manual EMCL adipose tissue removal.74 The model provides a non-invasive assessment method and can also indicate muscle lipid metabolism during exercise.75 Magnetization transfer 31P magnetic resonance spectroscopy (31P MRS) can measure ATP synthase rates and forward creatine kinase activity in humans and animals. ATP levels and other phosphorus metabolites are linked to metabolic changes in insulin-resistant T2DM.76 Using saturation transfer 31P MRS, ATP production rates were found to be reduced in muscles of the older adults and offspring of patients with T2DM.77,78 Thus, 31P MRS assessment of enzyme activity and mitochondrial function plays a crucial role in studying metabolic shifts, aging, and diseases.
The MRI technique called diffusion tensor imaging (DTI) has shown sensitivity to muscle microstructure alterations.79,80 In skeletal muscles, fiber size significantly influences λ2, λ3, mean diffusion, and fractional anisotropy (FA). DTI serves as a sensitive tool for tracking muscle atrophy, although its applicability in measuring muscles with larger fibers may be limited.81 Blood oxygen level–dependent imaging (BOLD) can assess skeletal muscle oxygenation and microcirculation. In a study by Liu et al,82 adult nonobese T2DM rats exhibited lower BOLD signal intensity in the gastrocnemius muscle during the ischemia-reperfusion period compared to the control group.
MRI stands as the most modern and dependable method for studying body composition. However, challenges such as high costs, complexity, and limited availability restrict its clinical usage, making it primarily applicable in research settings.
Ultrasound
US of the musculoskeletal system offers advantages of high resolution, noninvasiveness, portability, and safety. It can be employed for both static and dynamic assessments, making it a reliable tool for evaluating muscle quality. Key metrics used by US to assess muscle mass include skeletal muscle thickness, cross-sectional area, fascicle length, and pennation angle.83 Additionally, new parameters have been introduced, such as muscle volume, stiffness, contraction potential, and microcirculation.
Significant reduction in total muscle mass occurs relatively late in the natural progression of sarcopenia, even after the loss of physical capabilities. Muscle mass loss varies across different body areas as we age. Sarcopenia predominantly affects lower limb muscles rather than upper limb muscles.84 Studies have indicated that rapidly contracting type II muscle fibers are more affected in patients with sarcopenia compared with type I fibers.85 Utilizing high-frequency musculoskeletal ultrasonography to assess the thickness of plantar tissue and intrinsic foot muscles under the metatarsals, patients with T2DM exhibited notably thinner plantar tissue and intrinsic foot muscles compared with people without diabetes.86 Although IMAT accounts for only 5% of total thigh fat, research has linked it to insulin resistance.87 IMAT appears hyperechoic (white) on US, while skeletal muscle appears hypoechoic (black).88 Standardizing ultrasonic measurement techniques may assist in identifying causal links or predicting disease changes. Nevertheless, relying solely on US for measuring IMAT lacks accuracy, and consistency in the results is compromised because of scanner subjectivity, probe angle, pressure, and other factors. US-guided skeletal muscle biopsy can enhance IMAT extraction from the body and serve as an effective imaging method for monitoring precise IMAT metabolic activity.89
From a technical standpoint, image acquisition is quick, straightforward, and does not demand advanced operator expertise. The integration of portable US with smartphones makes this technology suitable for predicting sarcopenia in community and inpatient settings. Studies with limited sample sizes have demonstrated a strong correlation between DXA,90 MRI91 measurements, and US-based muscle mass measurements.
Shear-wave elastography (SWE) is a fully quantitative technique for determining the absolute elasticity of soft tissues.92 Changes in muscle hardness can be influenced by age-related alterations in muscle structure and organization, including muscle fat infiltration, muscle fiber tissue growth, collagen tissue loss, increased collagen fiber, and enhanced muscle glycosylation.93,94 Thus, elastic ultrasonography can identify early sarcopenia-related changes in muscle function. A study evaluated the effects of aging on skeletal muscle stiffness in relaxed and contracted status using SWE. The average stiffness values of the medial head of the gastrocnemius muscle were 12.51 ± 2.56 kPa and 81.74 ± 15.77 kPa in the relaxed and contracted states,95 respectively. Chen et al96 developed a diagnostic model with excellent performance for identifying T2DM individuals with sarcopenia. The skeletal muscle microcirculation, the body’s largest and most crucial capillary system, facilitates nutrient, oxygen, and hormone exchange, especially during physical activity. Although individual circulatory capacity decreases with age, physical activity can enhance microcirculation.97 Contrast-enhanced ultrasonography (CEUS) can reveal muscle microcirculation changes. T2DM affects both macro- and microvessels, yet comprehensive CEUS studies on T2DM with sarcopenia remain lacking. Analyzing microcirculation under CEUS during different sarcopenia phases under appropriate CEUS conditions holds diagnostic potential that can be fully utilized.
Additionally, bioelectrical impedance analysis (BIA) has been recognized as a tool for assessing skeletal muscle mass (quantity).98 As US, BIA is non-invasive, simple and less expensive than other techniques. However, it is important to note that BIA does not directly measure muscle mass. BIA relies on the correlation between total body water and electrical body impedance. Nevertheless, it is widely acknowledged that numerous physiological factors (such as gender, race, age, pregnancy, hormonal cycles, and exercise) as well as pathological conditions (including obesity, medications, and various diseases) can influence hydration status.99,100 Given the potential for significant alterations in body fluids among older adults with T2DM due to diabetes-related complications and treatments,101,102 the accuracy of BIA in assessing body composition could be substantially impacted.
Future Perspectives and Conclusions
Prediabetes is believed to be reversible.103 The combination of exercise and medication can prevent the progression from prediabetes to T2DM by enhancing muscle blood flow, regulating muscle insulin sensitivity, and reducing chronic low-grade inflammation in order to optimize muscle mass. These hypotheses should be tested in both animal models and human subjects using imaging techniques to monitor the impact of a comprehensive intervention approach encompassing physical activity, dietary control, nutritional supplementation, and medication on preserving muscle mass and strength while safeguarding against age-related muscle loss.
The realm of imaging-based diagnostic methods for sarcopenia presents variations in terms of diagnosis techniques, standards, and clinical applications. Addressing these discrepancies necessitates further investigation. Artificial intelligence holds promise as a valuable tool in sarcopenia detection. Similar to commercial systems employing AI algorithms for automated osteoporosis detection on CT59 scans, AI could prove beneficial in detecting sarcopenia. Another emerging avenue of exploration is radiomics, a field utilizing high-throughput methodologies to extract and analyze intricate quantitative imaging features from medical images like DXA, CT, and MRI. The integration of supplementary imaging omics focused on sarcopenia has the potential to refine existing diagnostic criteria, improve predictions of clinical outcomes, and deepen our understanding of the impact of sarcopenia. Before imaging techniques can be effectively employed in clinical settings, it is imperative to establish reference values for various stages of diabetes and for healthy blood glucose levels. Moreover, there should be a heightened focus on comprehending how fluctuations in blood sugar and other pertinent factors influence muscle size and function. The early identification of predilections toward sarcopenia in patients with T2DM and tailored exercise and treatment regimens to mitigate, halt, or reverse the loss of muscle mass, strength, or quality can greatly enhance the well-being of patients in their later years.
Acknowledgments
The authors wish to thank all hands and minds involved in this review.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no external funding.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31.
2. Chen LK, Woo J, Assantachai P, et al. Asian working group for sarcopenia: 2019 Consensus update on sarcopenia diagnosis and treatment. J Am Med Directors Assoc. 2020;21(3):300–307.
3. Anagnostis P, Gkekas NK, Achilla C, et al. Type 2 diabetes mellitus is associated with increased risk of sarcopenia: a systematic review and meta-analysis. Calcif Tissue Int. 2020;107(5):453–463.
4. American Diabetes Association Professional Practice Committee. 13. Older adults: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S195–s207.
5. Liccini A, Malmstrom TK. Frailty and sarcopenia as predictors of adverse health outcomes in persons with diabetes mellitus. J Am Med Directors Assoc. 2016;17(9):846–851.
6. Trierweiler H, Kisielewicz G, Hoffmann Jonasson T, Rasmussen Petterle R, Aguiar Moreira C. Sarcopenia: a chronic complication of type 2 diabetes mellitus. Diabetol Metab Syndr. 2018;10:25.
7. Park SW, Goodpaster BH, Lee JS, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32(11):1993–1997.
8. Leenders M, Verdijk LB, van der Hoeven L, et al. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Directors Assoc. 2013;14(8):585–592.
9. Chen S, Yan S, Aiheti N, et al. A bi-directional Mendelian randomization study of sarcopenia-related traits and type 2 diabetes mellitus. Front Endocrinol. 2023;14:1109800.
10. Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9(12):e113637.
11. Hovanec N, Sawant A, Overend TJ, Petrella RJ, Vandervoort AA. Resistance training and older adults with type 2 diabetes mellitus: strength of the evidence. J Aging Res. 2012;2012:284635.
12. Doherty TJ Invited review: aging and sarcopenia. J Appl Physiol. 2003;95(4):1717–1727.
13. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990s–1s.
14. Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–759.
15. Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Directors Assoc. 2014;15(2):95–101.
16. Merz KE, Thurmond DC. Role of skeletal muscle in insulin resistance and glucose uptake. Compr Physiol. 2020;10(3):785–809.
17. DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30(12):1000–1007.
18. Saini A, Faulkner S, Al-Shanti N, Stewart C Powerful signals for weak muscles. Ageing Res Rev. 2009;8(4):251–267.
19. Thiebaud D, Jacot E, DeFronzo RA, Maeder E, Jequier E, Felber JP. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982;31(11):957–963.
20. Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol Ser A. 2002;57(5):M326–32.
21. Heber D, Ingles S, Ashley JM, Maxwell MH, Lyons RF, Elashoff RM. Clinical detection of sarcopenic obesity by bioelectrical impedance analysis. Am J Clin Nutr. 1996;64(3 Suppl):472.
22. Taaffe DR, Pruitt L, Pyka G, Guido D, Marcus R. Comparative effects of high- and low-intensity resistance training on thigh muscle strength, fiber area, and tissue composition in elderly women. J Physiol. 1996;16(4):381–392.
23. Kalapotharakos VI, Michalopoulou M, Godolias G, Tokmakidis SP, Malliou PV, Gourgoulis V. The effects of high- and moderate-resistance training on muscle function in the elderly. J Aging Phys Act. 2004;12(2):131–143.
24. Otsuka Y, Yamada Y, Maeda A, et al. Effects of resistance training intensity on muscle quantity/quality in middle-aged and older people: a randomized controlled trial. J Cachexia Sarcopenia Muscle. 2022;13(2):894–908.
25. Robinson SM, Reginster JY, Rizzoli R, et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin Nutr. 2018;37(4):1121–1132.
26. Bloom I, Shand C, Cooper C, Robinson S, Baird J. Diet quality and sarcopenia in older adults: a systematic review. Nutrients. 2018;10:3.
27. van Dronkelaar C, van Velzen A, Abdelrazek M, van der Steen A, Weijs PJM, Tieland M. Minerals and sarcopenia; the role of calcium, iron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: a systematic review. J Am Med Directors Assoc. 2018;19(1):6–11.e3.
28. Lee CG, Boyko EJ, Barrett-Connor E, et al. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care. 2011;34(11):2381–2386.
29. Long DE, Peck BD, Tuggle SC, et al. Associations of muscle lipid content with physical function and resistance training outcomes in older adults: altered responses with metformin. GeroScience. 2021;43(2):629–644.
30. Sasaki T. Sarcopenia, frailty circle and treatment with sodium-glucose cotransporter 2 inhibitors. J Diabetes Invest. 2019;10(2):193–195.
31. Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124(2):509–514.
32. Lee CG, Schwartz AV, Yaffe K, Hillier TA, LeBlanc ES, Cawthon PM. Changes in physical performance in older women according to presence and treatment of diabetes mellitus. J Am Geriatr Soc. 2013;61(11):1872–1878.
33. Tahrani AA, Barnett AH, Bailey CJ. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat Rev Endocrinol. 2016;12(10):566–592.
34. Green CJ, Henriksen TI, Pedersen BK, Solomon TP. Glucagon like peptide-1-induced glucose metabolism in differentiated human muscle satellite cells is attenuated by hyperglycemia. PLoS One. 2012;7(8):e44284.
35. Choung JS, Lee YS, Jun HS. Exendin-4 increases oxygen consumption and thermogenic gene expression in muscle cells. J Mol Endocrinol. 2017;58(2):79–90.
36. Giannocco G, Oliveira KC, Crajoinas RO, et al. Dipeptidyl peptidase IV inhibition upregulates GLUT4 translocation and expression in heart and skeletal muscle of spontaneously hypertensive rats. Eur J Pharmacol. 2013;698(1–3):74–86.
37. Sato H, Kubota N, Kubota T, et al. Anagliptin increases insulin-induced skeletal muscle glucose uptake via an NO-dependent mechanism in mice. Diabetologia. 2016;59(11):2426–2434.
38. Takada S, Masaki Y, Kinugawa S, et al. Dipeptidyl peptidase-4 inhibitor improved exercise capacity and mitochondrial biogenesis in mice with heart failure via activation of glucagon-like peptide-1 receptor signalling. Cardiovasc Res. 2016;111(4):338–347.
39. Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metabol. 2018;18:3–14.
40. Venniyoor A. Tirzepatide once weekly for the treatment of obesity. New Engl J Med. 2022;387(15):1433–1434.
41. Mele A, Calzolaro S, Cannone G, Cetrone M, Conte D, Tricarico D. Database search of spontaneous reports and pharmacological investigations on the sulfonylureas and glinides-induced atrophy in skeletal muscle. Pharmacol Res Perspect. 2014;2(1):e00028.
42. Tricarico D, Mele A, Camerino GM, et al. The KATP channel is a molecular sensor of atrophy in skeletal muscle. J Physiol. 2010;588(Pt 5):773–784.
43. Ferrari U, Then C, Rottenkolber M, et al. Longitudinal association of type 2 diabetes and insulin therapy with muscle parameters in the KORA-Age study. Acta diabetologica. 2020;57(9):1057–1063.
44. Ida S, Nakai M, Ito S, et al. Association between sarcopenia and mild cognitive impairment using the Japanese version of the SARC-F in elderly patients with diabetes. J Am Med Directors Assoc. 2017;18(9):809.e9–e13.
45. Bouchi R, Fukuda T, Takeuchi T, et al. Insulin treatment attenuates decline of muscle mass in Japanese patients with type 2 diabetes. Calcif Tissue Int. 2017;101(1):1–8.
46. Bazzocchi A, Ponti F, Albisinni U, Battista G, Guglielmi G. DXA: technical aspects and application. Eur J Radiol. 2016;85(8):1481–1492.
47. Koo BK, Roh E, Yang YS, Moon MK. Difference between old and young adults in contribution of β-cell function and sarcopenia in developing diabetes mellitus. J Diabetes Invest. 2016;7(2):233–240.
48. Cawthon PM. Assessment of lean mass and physical performance in sarcopenia. J Clin Densitom. 2015;18(4):467–471.
49. Bredella MA, Ghomi RH, Thomas BJ, et al. Comparison of DXA and CT in the assessment of body composition in premenopausal women with obesity and anorexia nervosa. Obesity. 2010;18(11):2227–2233.
50. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846.
51. Heymsfield SB, Wang Z, Baumgartner RN, Ross R. Human body composition: advances in models and methods. Annu Rev Nutr. 1997;17:527–558.
52. Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97(6):2333–2338.
53. van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ. Systematic review and meta-analysis of the impact of computed tomography-assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant. 2016;16(8):2277–2292.
54. van der Werf A, Langius JAE, de van der Schueren MAE, et al. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur J Clin Nutr. 2018;72(2):288–296.
55. Amini B, Boyle SP, Boutin RD, Lenchik L. Approaches to assessment of muscle mass and myosteatosis on computed tomography: a systematic review. J Gerontol Ser A. 2019;74(10):1671–1678.
56. Kong M, Geng N, Zhou Y, et al. Defining reference values for low skeletal muscle index at the L3 vertebra level based on computed tomography in healthy adults: a multicentre study. Clin Nutr. 2022;41(2):396–404.
57. Han SJ, Kim SK, Fujimoto WY, Kahn SE, Leonetti DL, Boyko EJ. Effects of combination of change in visceral fat and thigh muscle mass on the development of type 2 diabetes. Diabetes Res Clin Pract. 2017;134:131–138.
58. Mayhew AJ, Amog K, Phillips S, et al. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. 2019;48(1):48–56.
59. Guerri S, Mercatelli D, Aparisi Gómez MP, et al. Quantitative imaging techniques for the assessment of osteoporosis and sarcopenia. Quant Imaging Med Surg. 2018;8(1):60–85.
60. Miljkovic I, Kuipers AL, Cvejkus R, et al. Myosteatosis increases with aging and is associated with incident diabetes in African ancestry men. Obesity. 2016;24(2):476–482.
61. Erlandson MC, Lorbergs AL, Mathur S, Cheung AM. Muscle analysis using pQCT, DXA and MRI. Eur J Radiol. 2016;85(8):1505–1511.
62. Lustgarten MS, Fielding RA. Assessment of analytical methods used to measure changes in body composition in the elderly and recommendations for their use in Phase II clinical trials. J Nutr. 2011;15(5):368–375.
63. Fischer MA, Pfirrmann CW, Espinosa N, Raptis DA, Buck FM. Dixon-based MRI for assessment of muscle-fat content in phantoms, healthy volunteers and patients with achillodynia: comparison to visual assessment of calf muscle quality. Eur Radiol. 2014;24(6):1366–1375.
64. Grimm A, Nickel MD, Chaudry O, et al. Feasibility of Dixon magnetic resonance imaging to quantify effects of physical training on muscle composition-A pilot study in young and healthy men. Eur J Radiol. 2019;114:160–166.
65. Sinha U, Malis V, Csapo R, Moghadasi A, Kinugasa R, Sinha S. Age-related differences in strain rate tensor of the medial gastrocnemius muscle during passive plantarflexion and active isometric contraction using velocity encoded MR imaging: potential index of lateral force transmission. Magn Reson Med. 2015;73(5):1852–1863.
66. Power GA, Allen MD, Booth WJ, Thompson RT, Marsh GD, Rice CL. The influence on sarcopenia of muscle quality and quantity derived from magnetic resonance imaging and neuromuscular properties. Age. 2014;36(3):9642.
67. Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90(6):1579–1585.
68. Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004;79(5):874–880.
69. Kemmochi Y, Ohta T, Motohashi Y, et al. Pathophysiological analyses of skeletal muscle in obese type 2 diabetes SDT fatty rats. J Toxicol Pathol. 2018;31(2):113–123.
70. Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278(5):E941–8.
71. Costa DN, Pedrosa I, McKenzie C, Reeder SB, Rofsky NM. Body MRI using IDEAL. AJR. 2008;190(4):1076–1084.
72. Carlier PG, Marty B, Scheidegger O. Skeletal muscle quantitative nuclear magnetic resonance imaging and spectroscopy as an outcome measure for clinical trials. J Neuromuscul Dis. 2016;3(1):1–28.
73. Cai Z, Yi P, Tao Q, Feng Y. 1H-MRS、Dixon水脂分离与Z谱成像技术在大鼠棕色脂肪上的量化比较 [Comparison of (1)H-MRS, Dixon fat-water separation and Z-spectral imaging for quantification of brown adipose tissue in rats]. Nan fang yi ke da xue xue bao. 2021;41(5):783–788. Chinese.
74. Hwang JH, Choi CS. Use of in vivo magnetic resonance spectroscopy for studying metabolic diseases. Exp Mol Med. 2015;47(2):e139.
75. Rico-Sanz J, Hajnal JV, Thomas EL, Mierisová S, Ala-Korpela M, Bell JD. Intracellular and extracellular skeletal muscle triglyceride metabolism during alternating intensity exercise in humans. J Physiol. 1998;510(Pt 2):615–622.
76. Szendroedi J, Chmelik M, Schmid AI, et al. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology. 2009;50(4):1079–1086.
77. Phielix E, Szendroedi J, Roden M. Mitochondrial function and insulin resistance during aging: a mini-review. Gerontology. 2011;57(5):387–396.
78. Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140.
79. Heemskerk AM, Drost MR, van Bochove GS, van Oosterhout MF, Nicolay K, Strijkers GJ. DTI-based assessment of ischemia-reperfusion in mouse skeletal muscle. Magn Reson Med. 2006;56(2):272–281.
80. Van Donkelaar CC, Kretzers LJ, Bovendeerd PH, et al. Diffusion tensor imaging in biomechanical studies of skeletal muscle function. J Anatomy. 1999;194(Pt 1):79–88.
81. Berry DB, Regner B, Galinsky V, Ward SR, Frank LR. Relationships between tissue microstructure and the diffusion tensor in simulated skeletal muscle. Magn Reson Med. 2018;80(1):317–329.
82. Liu Y, Mei X, Li J, Lai N, Yu X. Mitochondrial function assessed by 31P MRS and BOLD MRI in non-obese type 2 diabetic rats. Physiol Rep. 2016;4:15.
83. Perkisas S, Bastijns S, Baudry S, et al. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update. Eur Geriatric Med. 2021;12(1):45–59.
84. Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bul. 2010;95:139–159.
85. Kara M, Kaymak B, Ata AM, et al. STAR-sonographic thigh adjustment ratio: a golden formula for the diagnosis of sarcopenia. Am J Phys Med Rehabil. 2020;99(10):902–908.
86. Kumar CG, Rajagopal KV, Hande HM, Maiya AG, Mayya SS. Intrinsic foot muscle and plantar tissue changes in type 2 diabetes mellitus. J Diabetes. 2015;7(6):850–857.
87. Sachs S, Zarini S, Kahn DE, et al. Intermuscular adipose tissue directly modulates skeletal muscle insulin sensitivity in humans. Am J Physiol Endocrinol Metab. 2019;316:5.
88. Yu F, Fan Y, Sun H, Li T, Dong Y, Pan S. Intermuscular adipose tissue in type 2 diabetes mellitus: non-invasive quantitative imaging and clinical implications. Diabetes Res Clin Pract. 2022;187:109881.
89. Sparks LM, Goodpaster BH, Bergman BC. The metabolic significance of intermuscular adipose tissue: is IMAT a friend or a foe to metabolic health? Diabetes. 2021;70(11):2457–2467.
90. Ismail C, Zabal J, Hernandez HJ, et al. Diagnostic ultrasound estimates of muscle mass and muscle quality discriminate between women with and without sarcopenia. Front Physiol. 2015;6:302.
91. Worsley PR, Kitsell F, Samuel D, Stokes M. Validity of measuring distal vastus medialis muscle using rehabilitative ultrasound imaging versus magnetic resonance imaging. Manual Ther. 2014;19(3):259–263.
92. Docking SI, Ooi CC, Connell D. Tendinopathy: is imaging telling us the entire story? J Orthop Sports Phys Ther. 2015;45(11):842–852.
93. Alfuraih AM, Tan AL, O’Connor P, Emery P, Wakefield RJ. The effect of ageing on shear wave elastography muscle stiffness in adults. Aging Clin Exp Res. 2019;31(12):1755–1763.
94. Saito A, Wakasa M, Kimoto M, et al. Age-related changes in muscle elasticity and thickness of the lower extremities are associated with physical functions among community-dwelling older women. Geriatrics Gerontol Int. 2019;19(1):61–65.
95. Şendur HN, Cindil E, Cerit MN, Kılıç P, Gültekin I, Oktar S. Evaluation of effects of aging on skeletal muscle elasticity using shear wave elastography. Eur J Radiol. 2020;128:109038.
96. Chen ZT, Jin FS, Guo LH, et al. Value of conventional ultrasound and shear wave elastography in the assessment of muscle mass and function in elderly people with type 2 diabetes. Eur Radiol. 2023;33(6):4007–4015.
97. Billot M, Calvani R, Urtamo A, et al. Preserving mobility in older adults with physical frailty and sarcopenia: opportunities, challenges, and recommendations for physical activity interventions. Clin Interventions Aging. 2020;15:1675–1690.
98. Sbrignadello S, Göbl C, Tura A. Bioelectrical impedance analysis for the assessment of body composition in sarcopenia and type 2 diabetes. Nutrients. 2022;14:9.
99. Houtkooper LB, Going SB, Lohman TG, Roche AF, Van Loan M. Bioelectrical impedance estimation of fat-free body mass in children and youth: a cross-validation study. J Appl Physiol. 1992;72(1):366–373.
100. Gutiérrez-Marín D, Luque V, Ferré N, Fewtrell MS, Williams JE, Wells JCK. Associations of age and body mass index with hydration and density of fat-free mass from 4 to 22 years. Eur J Clin Nutr. 2019;73(10):1422–1430.
101. Lehrke M, Marx N. Diabetes mellitus and heart failure. Am J Cardiol. 2017;120(1s):S37–s47.
102. Elmahal ME, Ramadan MM. Insulin-induced edema in a patient with type 2 diabetes mellitus. Am J Case Rep. 2021;22:e931960.
103. Tuso P. Prediabetes and lifestyle modification: time to prevent a preventable disease. Perm J. 2014;18(3):88–93.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
