Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Immediate and Late Effects of Pulse Widths and Cycles on Bipolar, Gated Radiofrequency-Induced Tissue Reactions in in vivo Rat Skin
Authors Kim HK, Kim HJ, Kim JY, Ban MJ , Son J , Hwang Y, Cho SB
Received 12 January 2023
Accepted for publication 21 March 2023
Published 25 March 2023 Volume 2023:16 Pages 721—729
DOI https://doi.org/10.2147/CCID.S404631
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Hee Kyung Kim,1,* Hyun-Jo Kim,2,* Jae Yun Kim,3 Myung Jin Ban,4 Jiwon Son,5 Yongsung Hwang,5,6 Sung Bin Cho7
1Department of Pathology, Soonchunhyang University Bucheon Hospital, Bucheon, Korea; 2CNP Skin Clinic, Cheonan, Korea; 3Department of Dermatology, Soonchunhyang University Hospital, Cheonan, Korea; 4Department of Otorhinolaryngology-Head and Neck Surgery, Soonchunhyang University Hospital, Cheonan, Korea; 5Soonchunhyang Institute of Medi-Bio Science (SIMS), Soonchunhyang University, Cheonan, Korea; 6Department of Integrated Biomedical Science, Soonchunhyang University, Cheonan, Korea; 7Yonsei Seran Dermatology and Laser Clinic, Seoul, Korea
*These authors contributed equally to this work
Correspondence: Sung Bin Cho, Yonsei Seran Dermatology and Laser Clinic, Geumcheon REMAIN CITY 6F, 224 Siheung-daero, Geumcheon-gu, Seoul, 08628, Korea, Tel +82 2-2135-1375, Fax +82 70-8250-1375, Email [email protected]
Background: Single to multiple pulse packs of bipolar, alternating current radiofrequency (RF) oscillations have been used for various medical purposes using invasive microneedle electrodes. This study was designed to evaluate the effects of pulse widths and cycles of RF pulse packs on immediate and delayed thermal tissue reactions in in vivo rat skin.
Methods: RF energy at the frequency of 1 MHz and power of 70 W was delivered at each experimental setting into in vivo rat skin at 1.5-mm microneedle penetration, and then, tissue samples were obtained after 1 h and 3, 7, 14, and 21 days and histologically analyzed.
Results: A single-pulse-pack RF treatment generated coagulative necrosis zones in the dermal peri-electrode area and zones of non-necrotic thermal reactions in the dermal inter-electrode area. Multiple pulse-pack, RF-treated rat skin specimens revealed that the number and size of peri-electrode coagulative necrosis were markedly decreased by increasing the number of pulse packs and accordingly decreasing the conduction time of each pulse pack. The microscopic changes in RF-induced non-necrotic thermal reaction in the inter-electrode area were more remarkable in specimens treated with RF of 7 or 10 pulse packs than in specimens treated with RF of 1– 4 pulse packs.
Conclusion: The gated delivery of multiple RF pulse packs using a bipolar, alternating current, 1-MHz RF system using insulated microneedle electrodes efficiently generates non-necrotic thermal tissue reactions over the upper, mid, and deep dermis and subcutaneous fat in the inter-electrode areas.
Keywords: radiofrequency, bipolar, alternating current, gated pulse, tissue reaction, rat
Introduction
Bipolar, alternating current, radiofrequency (RF) oscillations can be delivered as a single or multiple pulse packs to target tissues using invasive microneedle electrodes according to the therapeutic purposes and expected clinical outcomes.1–4 RF treatment induces various hyperthermic tissue reactions by regulating the modes and parameters of RF delivery. Moreover, RF-induced cellular and subcellular reactions have been found to depend on the efficiency of RF delivery during achieving and maintaining aimed tissue temperatures.1,5 Low-temperature hyperthermic conditions at temperatures of 40–45°C by RF treatment induce non-necrotic thermal reactions; however, cell damage therein is reversible.1 Meanwhile, high-temperature hyperthermic conditions at temperatures >60°C generate tissue reactions of RF-induced coagulative necrosis, and cytotoxic damage therein is irreversible.1 Moreover, hyperthermic conditions at temperatures >100°C result in tissue desiccation around microneedle electrodes and increase exponentially local impedance and significantly decrease RF delivery efficiency.1,5
Our recent ex vivo pilot study demonstrated that the thermometric effects of bipolar gated RF treatments were significantly associated with the power and pulse widths and cycles of pulse packs.6 Therein, the effects of variables, including RF frequency, power, the number of pulse packs, conduction time/pulse, off-time between pulse packs, total conduction time, total off-time, and total treatment time, on RF-induced thermal tissue reactions were statistically analyzed using univariate and multivariate linear regression.6 Interestingly, the thermometric data of an ex vivo bovine liver model treated with 1-MHz RF was significantly affected by regulating the level of power, the numbers of pulse packs, conduction time/pulse pack, and off-time between pulse packs.6 Meanwhile, the experimental setting of total treatment time, which referred to the sum of total RF conduction time and total off-time, was the single most important factor for generating 2-MHz RF-induced thermal reactions in an ex vivo bovine liver tissue model.6
In this observational, descriptive study, we analyzed gated RF-induced thermal tissue reactions in in vivo rat skin at various pulse widths and cycles of pulse packs. Therefore, the RF frequency of 1 MHz was chosen because the thermometric outcomes of 1-MHz RF treatments were closely associated with the pulse widths and cycles of pulse packs as shown in our ex vivo pilot study.6 Then, the total treatment time was experimentally regulated to 200, 400, 600, and 800 ms, and the number of pulse packs therein was set to 1, 4, 7, and 10. Once the microneedle electrodes were inserted into the in vivo rat skin using a step motor at the penetration depth of 1.5 mm, RF was delivered into the dermis with single to multiple cycles of pulse packs. Then, specimens, which were obtained at the time points of 1 h and 3, 7, 14, and 21 days after RF treatment, were histologically evaluated by hematoxylin and eosin and Masson’s trichrome staining.
Materials and Methods
Bipolar RF Device
An invasive bipolar, alternating current, gated RF device at frequencies of 0.5, 1, and 2 MHz (VIRTUE RFTM; Shenb Co., Ltd., Seoul, Korea) was used to analyze RF tissue reactions in in vivo rat skin. The treatment parameters thereof, including the power, number of pulse packs, and pulse widths of on-time/pulse pack and off-time between pulse packs, were experimentally controlled to fit the study purpose. The device delivers RF energy through 36 insulated microneedle electrodes (24K gold-plated surgical stainless steel) in a 10 mm × 10 mm disposable tip (Smart RFTM 36 tip; Shenb Co., Ltd.), and the electrodes therein are uniformly arranged in a 6 × 6 pattern at the nearest distance between microneedles (1.75 mm). The diameter of insulated microneedles was measured as 300 ± 5 μm, and the length of a non-insulated pointed microneedle tip was 300 μm.
Treatment of Rat Skin with Bipolar RF via Insulated Microneedle Electrodes
Sixteen 6-week-old male Sprague Dawley rats were purchased (Orient Bio Corp., Seongnam, Korea), and the in vivo experiments were performed at the age of 14 weeks at weights of 450–500 g. General anesthesia was administered by intraperitoneally injecting bolus tiletamine/zolazepam (5 mg/kg) and xylazine (2 mg/kg). After the gentle removal of the hairs on the back, the lesions were cleansed with a mild soap and 70% alcohol. The skin was marked using black ink to outline grids for each experimental setting (1 cm in diameter/grid; 18 grids/rat; a total of 288 grids). Then, RF treatment at a frequency of 1 MHz was performed on each grid of the experimental rat at a power of 70 W and an electrode penetration depth of 1.5 mm over one pass. The total treatment time, which refers to the sum of the total RF conduction time and total off-time, was experimentally regulated to 200, 400, 600, and 800 ms (Figure 1). Therein, the number of pulse packs was set to 1, 4, 7, and 10 at each experimental setting of the total treatment time. Neither pretreatment topical anesthesia nor posttreatment cooling was applied. No prophylactic topical and systemic antibiotics or steroids were used after RF treatment. All experiments were performed thrice. All experimental protocols were approved by the Ethics Committee of the Soonchunhyang University Institutional Animal Care and Use Committee in Cheonan, Korea (SCH22-0096). All animal experiments were performed in accordance with the approved guidelines and the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
 |
Figure 1 Schematic illustrations of experimental parameters for 1-MHz, invasive bipolar radiofrequency (RF) treatment on in vivo rat tissue. |
Histological Evaluation
The experimental rats were euthanized to humanely sample the treated tissue according to standard protocols. One hour and 3, 7, 14, and 21 days after treatment, full-thickness tissue specimens, including the epidermis, dermis, subcutaneous fat, muscle, and fascia, were obtained for microscopic evaluation. Each sample was fixed in 10% buffered formalin and embedded in paraffin. Then, serial tissue sections of 4-μm thickness for each treatment setting were prepared and stained with hematoxylin and eosin and Masson’s trichrome.
Results
Single RF Pulse Pack-Induced Immediate Tissue Reactions
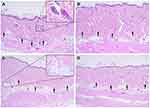
The rat skin specimens, which were obtained 1 h after treatment with a single RF pulse pack at conduction times of 200, 400, 600, and 800 ms, exhibited two zones of thermal tissue reactions: 1) coagulative necrosis zones in the dermis of peri-electrode areas and 2) zones of non-necrotic thermal reactions in the dermis of inter-electrode areas. By increasing the RF conduction time, the size of peri-electrode coagulative necrosis and the degree of tissue necrosis thereof were increased (Figure 2). Moreover, inter-electrode non-necrotic thermal reactions became more obvious in the wider areas of the dermis by increasing the RF conduction time.
In the peri-electrode areas adjacent to the follicular structures, tissue coagulation necrosis was observed around the proximal parts of the microneedles. Therein, remarkable thermal tissue reactions were generated in the mid to deep dermis of inter-electrode zones without coagulation necrosis or carbonization. Meanwhile, in the peri-electrode areas without hair follicles, coagulation necrosis zones were found around the distal parts of the microneedles, and microscopically remarkable but non-necrotic thermal tissue reactions were predominantly obvious in the upper and mid dermis of inter-electrode zones.
Delayed Tissue Reaction After Single-Pulse-Pack RF Treatment
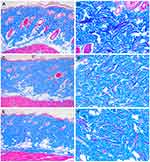
The peri-electrode coagulative necrosis zones became unnoticeable 3 and 7 days after treatment at conduction times of 200 and 400 ms, whereas coagulative necrosis zones remained noticeable microscopically 7 days after treatment at conduction times of 600 and 800 ms (Figure 3). The zones of inter-electrode non-necrotic thermal reactions exhibited remarkable erythematous, irregularly arranged collagen bundles throughout the dermis and subcutaneous fat layers at all settings of RF conduction times 3 and 7 days after treatment. The sebaceous glands in the inter-electrode areas 3 days after treatment shrunk with grayish discoloration, which was suggested to result from RF-induced thermal reaction. The histological features of neocollagenesis therein were mainly found in the upper and mid dermis at conduction times of 200 and 400 ms and in the mid to deep dermis at conduction times of 600 and 800 ms 14 and 21 days after treatment.
Multiple RF Pulse Pack-Induced Immediate Tissue Reactions
The rat skin specimens, which were treated with multiple RF pulse packs (ie, 4, 7, and 10 pulse packs) at treatment times (total conduction time + total off-time) of 200, 400, 600, and 800 ms, exhibited coagulative necrosis and thermal non-necrotic reaction zones (Figure 4). Nonetheless, by increasing the number of pulse packs, the number and size of coagulative necrosis zones were remarkably decreased around peri-electrode areas. In each experimental setting of pulse packs, the size and degree of peri-electrode coagulative necrosis were increased by increasing the RF conduction time. Meanwhile, non-necrotic thermal reactions became more obvious, which were distributed in the wider and deeper areas of inter-electrode areas in the dermis, by increasing the number of pulse packs and RF conduction time.
The total conduction time was shorter, and the total off-time was longer in the experimental setting of 7 or 10 pulse packs than those in the experimental setting of 1 or 4 pulse packs under the same total treatment time. Nonetheless, the microscopic changes in the RF-induced thermal non-necrotic reactions were more remarkable in the specimens treated with RF with 7 or 10 pulse packs in the inter-electrode areas. Moreover, the zones of non-necrotic thermal reactions therein were observed in the dermoepidermal junction and superficial papillary dermis deep into the reticular dermis or subcutaneous fat (Figure 5). The patterns of thermal reactions therein varied depending on the experimental settings and the presence of peri-electrode hair follicles. Particularly, at the experimental settings of 7 and 10 pulse packs, exhibiting thermal reactions in the inter-electrode areas of the upper and mid dermis, the sebaceous glands presented homogeneous grey to black thermal reactions of the sebocytes without noticeable carbonization.
Delayed Tissue Reaction After Multiple-Pulse-Pack RF Treatment
The peri-electrode coagulative necrosis zones became unnoticeable 3 and 7 days after treatment at all pulse pack settings and conduction times (Figures 4 and 5). The zones of inter-electrode non-necrotic thermal reactions exhibited remarkable erythematous irregularly arranged collagen bundles throughout the dermis and subcutaneous fat layers at all settings of RF conduction times 3 and 7 days after treatment. The histological features of neocollagenesis were found from the upper to deeper dermis, particularly at the settings of higher numbers of pulse packs 14 and 21 days after treatment. Moreover, the degree of histological changes in collagen fibers and dermal fibroblasts was markedly greater by increasing RF conduction time at each pulse pack setting (Figure 6). At the same total treatment time, the pulse pack setting of 4 produced greater changes in collagen fibers and dermal fibroblasts than the pulse pack setting of 7. However, the zones of histological changes were notably limited to smaller areas at the pulse-pack setting of 4 than at the pulse pack setting of 7. Meanwhile, under the experimental condition of the same number of pulse packs, the histological changes of increased collagen fibers and dermal fibroblasts were more remarkable at a total treatment time of 600 ms than at a total treatment time of 200 ms.
Discussion
In this study, we demonstrated gated RF-induced thermal tissue reactions in in vivo rat skin at various pulse widths and cycles of pulse packs. We used an invasive bipolar, alternating current, RF device using microneedle electrodes. A previous in vivo porcine skin model study of an invasive bipolar, alternating current, 1-MHz RF device using 49 insulated microneedle electrodes revealed that well-demarcated coagulative necrosis zones were generated in the dermis, the locations of which were suggested to be associated with the non-insulated tips of individual microneedles.7 The authors also suggested that the size of coagulation necrosis zones was notably associated with the RF conduction time and penetration depth of electrodes; meanwhile, the degree of thermal injury was associated with power settings.7 Another experimental study on an in vivo porcine skin model using an invasive bipolar, alternating current, 2-MHz RF device using 25 non-insulated microneedle electrodes demonstrated similar patterns of coagulation necrosis and thermal non-necrotic reactions in the dermis as shown in the 1-MHz RF study using insulated microneedles.7,8
A comparative study using an in vivo porcine skin model histologically analyzed the characteristics of 1-MHz RF-induced thermal tissue reactions and compared them with those of 2-MHz RF-induced thermal tissue reactions using an invasive, bipolar, alternating current RF device with 36 insulated and non-insulated microneedles.9 This study demonstrated that when using insulated microneedles, the areas of peri-electrode coagulative necrosis were more concentrated and narrower in 2-MHz RF-treated specimens than those in 1-MHz RF-treated specimens.9 Moreover, similar patterns of peri-electrode tissue coagulation were observed between 1-MHz RF treatment using non-insulated microneedles and 2-MHz RF treatment using insulated microneedles.9
In this study, we used an in vivo rat skin model, which had numerous follicular structures in the skin compared with the micropig skin or hairless mouse skin models used in previous studies.7–10 We administered 1-MHz single-pulse-pack RF treatment using insulated microneedles, which generated two zones of tissue reactions, including peri-electrode coagulative necrosis and inter-electrode non-necrotic thermal reactions. Moreover, we observed that a longer RF conduction time results in a larger peri-electrode coagulative necrosis zone and greater inter-electrode thermal reactions. However, the results of our in vivo rat study interestingly demonstrated that the peri-electrode coagulative necrosis zones were not always confined to the non-insulated tips of insulated microneedles. We believe that numerous hair follicles in the peri-electrode areas theoretically interfere with the flow of RF energy and heat dispersion to the inter-electrode dermal areas; thus, the coagulative necrosis zones could have developed in the upper to mid dermis. In those cases, because RF oscillations in the microneedle tips are suggested to flow preferentially through the relatively barrier-free areas of the deeper dermis or subcutaneous fat, the coagulative necrosis zones at the microneedle tips could be microscopically unremarkable.
An observational pilot study analyzed the microscopic and electron microscopic effects of pulsed-type RF delivery on in vivo hairless mouse skin using an invasive, bipolar, alternating current 2-MHz RF device.10 Therein, RF oscillations under two experimental conditions, including three pulse packs at 40 ms/pulse pack and five pulse packs at 30 ms/pulse pack, were delivered into the hairless mouse skin using 25 non-insulated microneedles.10 A few small coagulative necrosis zones could be observed only under the experimental condition of three pulse packs at 40 ms/pulse pack in the upper dermis.10 Nonetheless, pulsed-type RF treatment effectively induced inter-electrode non-necrotic thermal reactions that resulted in the thickening of the basement membrane and histological changes in the collagen fibers and vascular components in the dermis.10 Clinically, pulsed-type RF treatments have been applied for treating senescent fibroblasts without generating necrotic thermal reactions.11,12 Nonetheless, data from various conditions of pulse cycles and widths in pulsed-type RF treatments are insufficient.
In this study, we controlled the settings of on-time/pulse pack and off-time between pulses for this experiment to compare the histological effects. Our previous thermometric pilot study using an ex vivo bovine liver model revealed that longer off-times between pulses increased tissue temperature and resulted in greater histological thermal tissue reactions than shorter off-times.6 The thermometric study used RF conduction times ranging from 100 to 800 ms for each pulse pack.6 In this study, we found that shorter conduction times with multiple pulse packs and longer off-times between pulse packs generated remarkable non-necrotic thermal tissue reactions, which were associated with increased collagen bundles in the upper and deeper dermis. Particularly, only a few histological preparations exhibited peri-electrode coagulative necrosis zones. Therefore, the efficacy of pulsed-type RF treatment is suggested to be more significantly associated with RF-induced inter-electrode non-necrotic thermal reactions.
Clinically, we suggest that when treating skin with numerous thick hair follicles, treatment with more pulse packs with a shorter individual conduction time may efficiently deliver RF energy into the entire dermis. These settings also reduce the risk of generating coagulative necrosis in the upper papillary dermis. Moreover, highly efficient RF delivery has been supposed to effectively treat wider and deeper areas of the target tissue. Therefore, practitioners can choose shallower penetration depths of invasive microneedle electrodes that may reduce pain during RF treatments.
Conclusion
In this study, we evaluated gated RF-induced thermal tissue reactions using an invasive bipolar, alternating current RF device in in vivo rat skin at various pulse widths and cycles of pulse packs. Rat skin specimens treated with multiple-pulse-pack RF in this study revealed that the number and size of peri-electrode coagulative necrosis zones were markedly decreased by increasing the number of pulse packs and accordingly decreasing the conduction time of each pulse pack. Moreover, the microscopic changes in RF-induced non-necrotic thermal reaction were more remarkable in the inter-electrode area in the specimens treated with RF settings of 7 or 10 pulse packs over the upper, mid, and deep dermis and subcutaneous fat than in the inter-electrode area in the specimens treated with RF settings of 1 or 4 pulse packs. Nonetheless, this study has several limitations: 1) the design of our pilot study was observational and descriptive investigation; 2) a histological study was conducted on an in vivo rat skin model, not on a human skin model; and 3) only the representative parameters of RF treatment were used in this study. Therefore, further controlled in vivo clinical studies are necessary to confirm the gated RF-induced skin reactions and develop the optimal treatment parameters according to medical purposes.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Statement of Ethics
All experimental protocols were approved by the Ethics Committee of the Soonchunhyang University Institutional Animal Care and Use Committee (Cheonan, Korea).
Acknowledgments
We would like to thank Sunny Kang (Shenb Co., Ltd., Seoul, Korea), Min Choi (Shenb Co., Ltd.), and Bora Kim (Shenb Co., Ltd.) for their technical support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received for this article.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:
2. Golberg A, Bruinsma BG, Uygun BE, Yarmush ML. Tissue heterogeneity in structure and conductivity contribute to cell survival during irreversible electroporation ablation by “electric field sinks”. Sci Rep. 2015;5:8485. doi:10.1038/srep08485
3. Yarmush ML, Golberg A, Serša G, Kotnik T, Miklavčič D. Electroporation-based technologies for medicine: principles, applications, and challenges. Annu Rev Biomed Eng. 2014;16:
4. Golberg A, Yarmush ML. Nonthermal irreversible electroporation: fundamentals, applications, and challenges. IEEE Trans Biomed Eng. 2013;60:
5. Zhang B, Moser MAJ, Zhang EM, Luo Y, Zhang W. A new approach to feedback control of radiofrequency ablation systems for large coagulation zones. Int J Hyperthermia. 2017;33:367–377. doi:10.1080/02656736.2016.1263365
6. Choi M, Lee HS, Kim HJ, Kim H, Kim B, Cho SB. Effect of pulse widths and cycles on invasive, bipolar, gated radiofrequency-induced thermal reactions in ex vivo bovine liver tissue. Clin Cosmet Investig Dermatol. 2023;16:87–97. doi:10.2147/CCID.S395072
7. Zheng Z, Goo B, Kim DY, Kang JS, Cho SB. Histometric analysis of skin-radiofrequency interaction using a fractionated microneedle delivery system. Dermatol Surg. 2014;40:134–141. doi:10.1111/dsu.12411
8. Na J, Zheng Z, Dannaker C, Lee SE, Kang JS, Cho SB. Electromagnetic initiation and propagation of bipolar radiofrequency tissue reactions via invasive non-insulated microneedle electrodes. Sci Rep. 2015;5:16735. doi:10.1038/srep16735
9. Wootten S, Zawacki ZE, Rheins L, Meschter C, Draelos ZD. An evaluation of electrocoagulation and thermal diffusion following radiofrequency microneedling using an in vivo porcine skin model. J Cosmet Dermatol. 2021;20:1133–1139. doi:10.1111/jocd.13690
10. Cho SB, Na J, Zheng Z, et al. In vivo skin reactions from pulsed-type, bipolar, alternating current radiofrequency treatment using invasive noninsulated electrodes. Skin Res Technol. 2018;24:318–325. doi:10.1111/srt.12433
11. Yoon JE, Kim Y, Kwon S, et al. Senescent fibroblasts drive ageing pigmentation: a potential therapeutic target for senile lentigo. Theranostics. 2018;8:4620–4632. doi:10.7150/thno.26975
12. Kim M, Kim SM, Kwon S, Park TJ, Kang HY. Senescent fibroblasts in melasma pathophysiology. Exp Dermatol. 2019;28:719–722. doi:10.1111/exd.13814
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.