Back to Journals » Journal of Blood Medicine » Volume 14
Immunohematological Outcome Among Adult HIV Patients Taking Highly Active Antiretroviral Therapy for at Least Six Months in Yabelo Hospital, Borana, Ethiopia
Authors Ashenafi G , Tibebu M , Tilahun D , Tsegaye A
Received 17 May 2023
Accepted for publication 16 October 2023
Published 20 October 2023 Volume 2023:14 Pages 543—554
DOI https://doi.org/10.2147/JBM.S419414
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Girma Ashenafi,1 Melatwork Tibebu,2 Dagnamyelew Tilahun,1 Aster Tsegaye2
1Department of Medical Laboratory Sciences, Bule Hora University, Bule Hora, Oromia, Ethiopia; 2Department of Medical Laboratory Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Melatwork Tibebu, Email [email protected]
Background: Immunohematological abnormalities among human immunodeficiency virus-infected patients are common abnormalities associated with severe depletion of the immune system, covering a stage of acute syndrome to an advanced disease. The greatest impact was observed in the low- and middle-income countries. However, in Ethiopia, little attention has been paid, and only limited published information exists regarding immunohematological abnormalities among individuals receiving highly active antiretroviral treatment.
Objective: This study aimed to assess changes in immunological and hematological parameters in HIV-infected patients receiving HAART for at least six months at the antiretroviral therapy clinic of Yabelo Hospital, Borena, Ethiopia.
Methods: A cross-sectional study was conducted from February to July 2021 using convenient sampling to recruit 333 participants. Sociodemographic data and clinical characteristics were collected using a pretested questionnaire. Baseline data were extracted from medical records and after six month immunohematological measurements were performed on blood samples collected during the study period. Data analysis was performed using SPSS version 25. Descriptive analysis was performed, and the results are presented as numbers and percentages or means ± SD. A paired t-test was used to compare the mean values of the immunohematological parameters before and after six of taking HAART. Statistical significance was set at P < 0.05.
Results: The prevalence of anemia, leucopenia, neutropenia, lymphopenia and thrombocytopenia were 47.4%, 73.3%, 58.3%, 76.9% and 3.3% before initiation of HAART and 23.1%, 36.4%, 23.4%, 35.7% and 2.4% after initiation of HAART, respectively; Compared to baseline, there was also a significant decrease in the rate of Immunosuppression (CD4 < 350) from 62.2% at base line to 20.7% after HAART initiation.
Conclusion: Immunohematological profile of the patients improved after the initiation of HAART. The observation of large proportion of immunosuppressed individuals at baseline warrants advocating for HIV testing in the pastoralist community so that infected patients could benefit from early initiation of HAART.
Keywords: HIV, highly active antiretroviral therapy, immunohematological abnormalities
Introduction
Human immunodeficiency virus (HIV) infection is characterized by severe depletion of the immune system, covering the stages of acute syndrome to an advanced disease (Acquired Immunodeficiency Syndrome-AIDS). The greatest impact is observed in low- and middle-income countries where an estimated 90% of the global HIV-infected population live; sub-Saharan Africa is the most affected area of world while South-East Asia is the second most affected.1–3
Immunohematological abnormalities in HIV patients are due to diverse reasons which include immune-mediated destruction of cells, direct cytopathic effects of the virus, secondary to various infections, neoplasms, and drug toxicity.4 The most prevalent haematological disorder observed in adults with HIV infection is anemia. Incidences of anemia are particularly high in patients with late stages of the disease and a reduced CD4+ T cell count. The virus replicates in T cells and in turn reduces the growth of bone marrow progenitors, thus suppressing hemopoiesis.5 Anemia and neutropenia are generally caused by inadequate blood cell production because of bone marrow suppression by HIV infection mediated by abnormal cytokine expression and alteration of the bone marrow microenvironment.6,7
Among hematological abnormalities, neutropenia is commonly observed after development of AIDS, and has been associated with types of HAART medications used to treat HIV infection.8 Colony growth hormones of the progenitor cells decreased in patients taking HAART, this leads to decreased production of granulocyte and monocytes produced by the infected cells known to suppress neutrophil production.9 On the other hand, thrombocytopenia could be seen from increased platelets destruction and decreased platelet production by the HIV-infected megakaryocytic cells. Like other immunohematological abnormalities, decreased CD4 count also manifest due to HIV using CD4 receptor as site of attachment and replication.8–10
Understanding immunohematological outcomes is essential for evaluating treatment and prognosis during the follow-up of patients with HIV. Highly active antiretroviral therapy (HAART) is a combined therapy used in HIV-positive patients to radically lengthen the time to AIDS development and/or progression to death in HIV-infected people. The major mechanism of action of HAART is the inhibition of plasma HIV levels, which promotes an increase in CD4 T-lymphocyte count and function. The aim is inhibiting viral replication while minimizing the toxicities and side effects associated with the available drugs. Using HAART, proper growth in children can be promoted, and the survival of all HIV-infected patients can be prolonged by reducing their level of illness and improving their quality of life.11
HAART significantly improves immunohematological changes in HIV-infected individuals. Published data on this outcome are scarce in the Southern Region of Ethiopia, particularly in the Borana Zone. Distinguishing the changes in the immunohematological profiles during treatment can help caregivers and physicians prevent most adverse effects and adequately manage patients. Moreover, our findings can serve as reference and input material for policymakers in the study area, not to mention further research conducted in the region as well as around the globe.
Materials and Methods
Study Setting, Design and Study Population
This cross-sectional study was conducted at the Yabelo General Hospital, Borana, Ethiopia, from February to July 2021. Borena is located at 567 km South of Addis Ababa and its capital is Yabelo town. There are one governmental general hospital and one health center in the town. The hospital provides ART service for HIV positive individuals. Primary and secondary clinical data of the study participants were collected using a pretested standardized questionnaire. The sample size was calculated using a single-population proportion formula and a correction formula for a finite population, to recruit 333 study participants using a convenient sampling technique. HIV-positive patients who were receiving HAART for at least six months and provided informed consent were included in the study. However, patients taking vitamins and iron supplements at the time of sampling, pregnant women, and those who had received a blood transfusion within the last 4 months were excluded.
Data Collection and Quality Assurance
Baseline complete blood count, CD4 and other clinical and demographic data of the study participants before HAART initiation were extracted from the medical record books. Socio-demographic and clinical data pertinent to our study after at least 6 months of initiation of HAART were collected using a trained data collector. Blood samples were collected once in the mornings to minimize diurnal variation when patients come for their routine care using Ethylene diamine tetra acetic acid (EDTA) tube and complete blood count (CBC) was analyzed using Sysmex XE-2100 hematology analyzer (Sysmex, Kobe, Japan). BD FACS PRESTO machine (BD Biosciences, San Jose, California) was used to count CD4+ T cells to detect the immunological abnormalities. The performance of both analyzers was verified by running quality control samples. The quality of the data was assured by designing a pretested standardized questionnaire that was prepared in English and translated to Afan Oromo (native language for the people in Borana, Ethiopia). Besides, standard operating procedures (SOPs) were strictly followed and all reagents were checked for expiration dates.
Statistical Analysis
For analysis, the data were coded, cleaned, entered into EpiData version 3.1 and analyzed using SPSS version 25 (IBM® SPSS®, IBM Corp., Armonk, NY, USA). Descriptive analysis was performed, and the results are presented as numbers and percentages or means ± SD. A paired t-test was used to compare the mean values of the immunohematological parameters before and after six months of taking HAART. Bivariate and multivariable analyses were used to determine the associations between parameters and factors. Statistical significance was set at p < 0.05.
Ethical Consideration
This study was approved by the Department Research and Ethics Review Committee (DRERC) of the Department of Medical Laboratory Sciences, Addis Ababa University. After a letter of cooperation was sent to Borana Health Bureau, the Institutional Review Board of the Borana Health Bureau also approved the study and wrote support letter to Yabelo General Hospital for the study to proceed. Written informed consent was obtained from all the participants. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Results
Socio-Demographic Characteristics
A total of 333 individuals aged 18 years and above, of whom 170 (51.1%) male and 163 (48.9%) females were included in the study, with 100% response rate. The majority of these participants were between the ages of 29–38 followed by 18–28 years with a mean age and standard deviation of 32.83 and 9.48 years. In regards to the marital status, educational level, and occupation of the participants; 151 (45.3%) of them were married, 112 (33.6%) had no formal education, and 85 (25.5%) were housewives. More than half of them were urban residents 195 (58.6%) (Table 1).
 |
Table 1 Socio-Demographic Characteristics of HIV Patients Taking HAART for at Least 6 Months at Yabelo General Hospital, Borana Ethiopia, 2021 |
Clinical Characteristics
Before initiation of HAART, approximately 151 (45.3%) of the study participants were in WHO clinical stage I, while the rest of the patients were 95 (28.5%), 61 (18.3%), and 26 (7.8%) were in WHO clinical stages II, III, and IV, respectively. However, after taking HAART for at least six months, almost all of the study participants were in WHO clinical stage I, 330 (99.1%), the remaining 3 patients (0.9%) were in Stage II. The results revealed an increase in the mean BMI of the study participants; 20.14 before starting HAART and 22.19 after taking HAART for at least six months. It was found that 271 (81.6%) participants had no history of opportunistic infections. Of these patients, 31 (50%) had pulmonary tuberculosis, 18 (29.04%) had severe bacterial pneumonia, and 13 (20.96%) had extra pulmonary tuberculosis. Functional status at base line indicated that 278 (83.5%) were working patients. Treatment interruptions were 114 (34.2%) at different points of follow-up (Table 2).
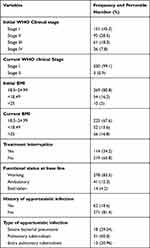 |
Table 2 Clinical Characteristics of Adult HIV Patients Taking HAART for at Least Months at Yabelo General Hospital, Borana, Ethiopia, 2021 |
Hematological and Immunological Values Before and After at Least Six Months of HAART
WBC has changed from 4.106 ±0.970 to 4.995 ±1.34 (109/l); there was an increase in Hgb concentration from 12.38 ± 1.77 to 13.22 ± 1.81 (g/d[); MCV has changed from 87.57 ± 11.63 to 89.99 ± 8.87 (fl); also platelet shows change from 243.3 ± 52.59 to 292.18 ± 60.539 (109/l) and CD4 changed from 356.56 ± 212.32 to 583.7 ± 238.25 cells/mm3 resulting in a mean change of 227.14 cells/μL.
The study revealed a significant change in hematological parameters (WBC, Hgb, MCV, and CD4) after the use of HAART for at least six months of initiation of HAART (P = 0.0001), with the exception of MCHC (p = 0.706) (Table 3).
 |
Table 3 Hematological and Immunological Parameters of Adult HIV-Positive Individuals Before and After Initiation of HAAART at Yabelo General Hospital, South Ethiopia, 2021 |
Hematological Abnormalities Before and After at Least Six Months of HAART
According to WHO classification of anemia based on Hgb concentration, 158 (47.4%) were anemic at baseline, and the magnitude decreased to 77 (23.1%) after at least 6 months of HAART initiation. When data were analyzed by severity, at base line of the 158 anemic study participants majority of them had mild 142 (89.9%) anemia, while 10 (6.3%) moderate, and 6(3.8%) severe. However, after taking HAART for at least six months, while the prevalence of anemia decreased from 47.4% to 23.1%, the severity remained almost same where 71 (92.2%), 2(2.6%),4 (5.2%) had mild, moderate, and severe anemia, respectively (Table 4).
 |
Table 4 Prevalence of Immunohematological Abnormalities of Adult HIV Patients Taking HAART for at Least 6 Months at Yabelo General Hospital Borana, Ethiopia, 2021 |
Other hematological abnormalities like leukopenia (WBC < 4000cells/μL), Neutropenia (Neutrophil < 1500cells/μL; <40%), lymphopenia (lymphocyte <1000cells/μL; <20%) and thrombocytopenia (PLT < 150,000cells/μL) has shown changes in prevalence between baseline and at least 6 months after therapy. Accordingly rates of leukopenia is reduced from 244 (73.3%) to 121 (36.3%), lymphopenia from 256 (76.9%) to 119 (35.7%), and thrombocytopenia from 11 (3.3%) to 8(2.4%). In contrast, Neutropenia increased from 78 (23.4%) to 194 (58.3%) (Table 4).
Baseline Immunosuppression Level by Age Category in HIV Patients Before and After at Least Six Months of HAART
Of the total study participants, 207 (62.2%) had immune suppression (decrements in CD4 count < 350 cells/µL) at initiation of HAART, of which 95 (45.9%) had severe immune suppression as defined by CD4 < 200 cells/μL. There was also increment in CD4 count after initiation of HAART, which revealed a decreased immune suppression 69 (20.7%) while severe immunodeficiency decreased from 45.9% to 20.7% (Table 4).
The majority of the study participants had a CD4 count of <350 cells/μL, regardless of age category. Specifically, 12.9%, 11.4%, 6.9% of the participants in the age groups 29–38, 18–28, and 39–48 years, respectively, were in the CD4 count 200–349 cells/μL category. Severe immunodeficiency (CD4 < 200 cells/μL) was predominant in the older age groups of 49–58 and >59 years.
In contrast to the baseline data, the majority of the participants after taking HAART for at least 6 months were found in the CD4 > 500 cells/μL category, irrespective of age category, although the highest proportion was observed in the age group 18–28 years where 71.3% were in the CD4 > 500 cells/μL category. The proportion of patients with severe immunosuppression of CD4 < 200 cells/μL increased with increasing age category which is 12.3%, 21.7%, 24.5%, 33.3%, and 40.0% for the age groups 18–28, 29–38, 39–48, 49–58 and >59 years, respectively (Figure 1).
 |
Figure 1 Prevalence of immunosuppressed Adult HIV patients with age category that had been taking HAART for at least 6 months at Yabelo General Hospital, Borana, Ethiopia, 2020. |
Factors Associated with Anemia
We further analyzed factors associated with current anemia using the logistic regression model. Those variables with P ≤0.20 in the bivariate analysis were used as a cutoff value to determine candidates for multivariable logistic regression. Based on the above assumption, treatment interruption, type of opportunistic infection, BMI, sex, WHO clinical staging, residence, and educational status were candidate variables for multivariable analysis. Among the variables included in the final model, sex and treatment interruption were associated with current anemia. Those individuals with BMI less than 18.49 are more likely to develop anemia (5.4 times) [OR=5.428) 95% CI, 1.425–20.674] when compared to those with normal BMI, and female patients were more likely to have anemia when compared to male patients [OR=2.02 (95% CI, 1.164–3.503]. Besides, those patients with treatment interruption were more likely to be anemic than those that do not [OR=1.835 (95% CI, 1.003–3.359)] (Table 5).
 |
Table 5 Factors Associated with Anemia After Taking HAART for at Least Six Month at Yabelo General Hospital, South Ethiopia, 2021 |
Discussion
Our study investigated immunohematological changes among HIV-positive adults after initiation of HAART for more than six months at the Yabelo General Hospital. The study revealed a significant change in hematological parameters after the use of HAART for at least six months, with the exception of MCHC. In addition, there was an average increase of 227.14 CD4 cells/μL after treatment. The most common abnormalities include anemia, thrombocytopenia, leukopenia, lymphopenia, immunosuppression, and neutropenia. Low CD4 count is the most important immunological abnormality. Studies have revealed that HAART for at least six month has decreased the prevalence of both hematological and immunological abnormalities.12–24
Our finding of anemia at baseline is in line with a study conducted in Addis Ababa, Ethiopia, by Woldeamanuel et al in 2018 at Black Lion Specialized Hospital (41.9%) and Assefa et al in 2015 at Zewditu Memorial Hospital (42.9%)22,25 indicating the effect of HIV infection in the country. The prevalence of anemia before HAART initiation in this study was higher than other studies conducted in other parts of Ethiopia in 2014 by Enawgaw et al (29.7%), Tsegaye et al in 2018 at Ras Desta Memorial Hospital (24.1%), Daka et al in 2013 at Hawassa University Referral Hospital (23.4%), Damtie et al in 2021 at Debre Tabor Comprehensive Specialized Hospital (31.8%), and Uguma et al in 2021 at Goba referral Hospital (37.1%).10,13,26–28 This discrepancy might be due to the differences in life style of ART patients, the presence of opportunistic infections, and nutritional status of the study participants in the other studies.
The baseline prevalence of anemia in our study was lower than the prevalence reported in 2013 by Denue et al in Nigeria (57.5%), Owiredu et al in Ghana with 63% before HAART, and Saha et al with 46% after HAART in 2015 in India.18,29,30 This discrepancy might be attributed to the differences in the life style, study population, sample size, socio-demographic characteristics of study subjects, and variability in the definition of anemia. Relating to the prevalence of anemia after receiving HAART for at least six months, our findings were 23.1%. This result is in agreement with study conducted in Addis Ababa (20.9%), Debre Tabor (17.4%), and Nigeria (24.3%).23,25,29 This prevalence is higher than that reported in other parts of Ethiopia, such as Gondar (11.7%), Addis Ababa (11.4%), and Goba (14.6%).4,22,28 This difference in prevalence may be attributed due to life style, altitude, study population, sample size, sociodemographic characteristics of study subjects.
In the current study, the prevalence of anemia after receiving HAART for at least six months was significantly associated with treatment interruption, sex, and BMI greater than 18.99. Female patients were more likely to have anemia when compared to male patients which is supported by study done in Dessie, Ethiopia.28 The result of this study has shown that leucopenia was the most common hematological abnormality with higher prevalence at before Starting of HAART than after taking HAART for at least 6 months (73.3% vs 36.35%), this finding is consistent with the reports of various studies affirming the knowledge that the HIV attacks white blood cells of patients.22,29,30
In the current study, the increase in mean WBC count after six months of HAART initiation indicated boosting of the immune system (4.106 ±0.970 to 4.995 ±1.34). The finding was supported, by study in Makurdi, Benue state of Nigeria which indicated that HAART brings statistically significant increment in WBC from 4.07 ± 0.25×103 /μL at baseline to 4.76 ± 0.15×103 /μL after ART.29 The increase in WBC count after HAART initiation might be due to an increase in hematopoietic progenitor cell growth following a decrease in HIV viral load.26
In contrast with the current findings, other reports have shown a decrease in WBC count after HAART.27 This difference may be attributed to the short duration of HAART intake in other studies, as in the initial stage of ART initiation; WBC count will be low, which gradually adjusts itself over time.
The prevalence of Neutropenia In the present study was 23.4% before HAART initiation and 58.3% after taking HAART for at least six months. This finding contradicts with reports from Gondar (14.8%) and South Korea (10%) at base line and 28.3% in Gondar5 and 8.9% in South Korea8 after initiation of HAART. The higher prevalence of neutropenia might be attributed to opportunistic and other infections observed in our study.
The present study also revealed differences in mean CD4+ T cell counts before and after HAART initiation (356.56±212.32 vs 583.7±238.25cells/μL), with an average increase of 227.14 CD4 cells/μL following treatment. In contrast, previous studies conducted at Zewditu Memorial Hospital reported limited increments after HAART (116 ± 69.4 vs 112 ± 67 cells/μL).6 This can be explained by the policy change since the research was conducted with regard to ART initiation, where treatment is administered regardless of CD4 count in recent years, so that the participants of the current study had better initial CD4 counts compared to the earlier studies.
The lower CD4 count at baseline is due to the HIV infection, which decreases the production of CD4+ T cells by preventing the maturation of lymphoid precursors. Inhibition of viral replication by HAART is therefore associated with improved production of CD4+ T cells.4,17,19,20 Highly active antiviral treatment decreases the plasma viral load after taking the medication for at least six months which then increases the CD4 count.5
The prevalence of thrombocytopenia in the present study was high at baseline compared to that in patients on HAART for at least six months. Thrombocytopenia was reduced from 3.3 to 2.4% after at least six months of HAART. This result is consistent with the findings of a study in India.18 The improvement in thrombocytopenia might be attributed to the effect of treatment, in which after HAART initiation, abnormalities of hematopoietic, opportunistic infections, and immune causes related to HIV leading to low platelet count could be reverted.27,29
Also, research in Gondar (9% to 4.1%) and Bale (Goba Referral Hospital) (11.4% to 4.5%) has revealed decrease in thrombocytopenia after they took HAART for at least six months, but the results were slightly higher than the current findings.4,28 The variations in the results observed in this study were attributed to variations in the definition of thrombocytopenia, size of the study population, and study design used.
In summary, this study was conducted with the objective of identifying changes in Immuno-hematological parameters among adult HIV-positive patients receiving HAART for at least six months in Borana, Yabelo General Hospital. As shown in these studies, participants at baseline had significantly higher rates of anemia, thrombocytopenia, and CD4+ T lymphocytopenia, which improved dramatically after HAART initiation. The finding of a significant number of immunodeficiency, 207 (62.2%) (CD4 count < 350 cells/µL) at initiation of HAART, and severe immunodeficiency in 95 (45.9%) of them as defined by CD4 < 200 cells/μL indicated quite large number of individuals were living with the virus in the study area. This needs critical attention of the local health authorities to encourage people to get HIV tested so that the benefit of HAART can be maximized as it is given to those tested positive irrespective of CD4 count.
Conclusion
This study showed that the most common hematologic abnormalities (outcomes) in HIV/AIDS patients were anemia, thrombocytopenia, leucopenia, lymphopenia, neutropenia, and thrombocytopenia. Anemia after HAART initiation is linked to a number of factors, including treatment interruption, current BMI and sex. In order for infected patients to benefit from early commencement of HAART, it is necessary to promote HIV testing and early HAART initiation in the pastoralist population due to the observation of a significant percentage of immunosuppressed people at baseline.
Abbreviations
AIDS, Acquired immune deficiency syndrome; AOR, Adjusted odds ratio; ART, Antiretroviral therapy; BMI, Body Mass Index; CBC, Complete blood count; CD4, Cluster of differentiation 4; HAART, Highly active antiretroviral treatment; Hgb, Hemoglobin; HIV, Human immunodeficiency virus; MCH, Mean Corpuscular Hemoglobin; MCHC, Mean Corpuscular Hemoglobin Concentration; MCV, Mean Corpuscular Volume; OI, Opportunistic infection; OR, Odds ratio; PLT, Platelets; RBC, Red blood cell; SD, Standard Deviation; SPSS, Statistical Package for Social Sciences; WBC, White blood cell; YGH, Yabelo general hospital; WHO, World health organization.
Acknowledgments
We would like to thank Addis Ababa University, the study participants and Yabelo General Hospital staff, especially the data collectors and laboratory staff, for their valuable contributions.
Disclosure
The authors declare no conflicts of interest in this work. This paper is based on the thesis by Girma Ashenafi. It has been published on the institutional website: http://etd.aau.edu.et/handle/123456789/28526.
References
1. Patwardhan M, Golwilkar A, Abhyankar JR, Atre M. Hematological profile of HIV positive patients. Indian J Pathol Microbiol. 2002;45(2):
2. Clark S. Experts predict global devastation due to HIV/AIDS. Lancet. 2002;360(9327):145. doi:10.1016/S0140-6736(02)09439-4
3. Stover J, Walker N, Garnett G. Can we reverse the HIV/AIDS pandemic with an expanded response. Lancet. 2002;360(9326):373–377.
4. Geletaw T, Tadesse MZ, Demisse AG. Hematologic abnormalities and associated factors among HIV infected children pre- and post-antiretroviral treatment, North West Ethiopia. J Blood Med. 2017;8(8):99–105. doi:10.2147/JBM.S137067
5. World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. World Health Organization; 2007.
6. Mathews S, Bala DS, Sharma A. Association of hematological profile of human immunodeficiency virus-positive patients with clinicoimmunologic stages of the disease. J Lab Physicians. 2013;5(1):34–37. doi:10.4103/0974-2727.115929
7. Gifford RJ, De-oliveira T, Rambaut A. UK collaborative group on HIV drug resistance: phylogenetic surveillance of viral genetic diversity and the evolving molecular epidemiology of human immunodeficiency virus type 1. J Virol. 2007;81(23):13050–13056. doi:10.1128/JVI.00889-07
8. UNAIDS. AIDS epidemic Gap report; 2020.
9. Choi SY, Kim I, Kim NJ, Lee S, Choi Y, Bae J. Hematological manifestations of human immunodeficiency virus infection and the effect of highly active antiretroviral therapy on cytopenia. Korean J Hematol. 2011;46(4):253–257. doi:10.5045/kjh.2011.46.4.253
10. Enawgaw B, Alem M, Addis Z, Melku M. Determination of hematological and immunological parameters among HIV positive patients taking highly active antiretroviral treatment and treatment naïve in the antiretroviral therapy clinic of Gondar University Hospital, Gondar. BMC Hematol. 2014;14(1):14–18. doi:10.1186/2052-1839-14-14
11. Lu DY, Wu HY, Yarla NS, Xu B, Ding J, Lu TR. HAART in HIV/AIDS Treatments: future Trends. Infect Disord Drug Targets. 2018;18(1):15–22. doi:10.2174/1871526517666170505122800
12. Derbe M, Monga D, Daka D. Immunological response among HIV/AIDS patients before and after ART therapy at Zewuditu Hospital Addis Ababa, Ethiopia. Am J Res Commun. 2013;1(1):103–115.
13. Tsegaye A, Desta K, Oma D, et al. Prevalence of anemia before and after initiation of antiretroviral therapy on HIV infected patients at Ras Desta Damtew Memorial Hospital, Addis Ababa, Ethiopia. RRJMHS. 2018;7(4):24–30.
14. HIV status in Ethiopia. UNAIDS; 2018. Available from: https://www.unaids.org/en/regionscountries/countries/ethiopia.
15. Huruy K, Kassu A, Mulu A, Wondie Y. Immune restoration disease and changes in CD4+ T cell count in HIV-infected patients during highly active antiretroviral therapy at Zewditu memorial hospital, Addis Ababa, Ethiopia. AIDS Res Ther. 2010;7(1):1–7. doi:10.1186/1742-6405-7-46
16. Adane A, Desta K, Bezabih A, Gashaye A, Kassa D. HIV-associated anemia before and after initiation of antiretroviral therapy at ART Centre of Minilik II Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2012;50(1):13–21.
17. Gedefaw L, Yemane T, Sahlemariam Z, Yilma D. Anemia and risk factors in HAART naive and HAART experienced HIV positive persons in south west Ethiopia: a comparative study. PLoS One. 2013;8(8):e72202. doi:10.1371/journal.pone.0072202
18. Saha D, Kini J, Subramaniam R. A study of the hematological profile of human immunodeficiency virus positive patients in coastal Indian region. Med sci. 2015;35(5):190–193.
19. Qazi RA, Bashir N, Daud Y. Correlation of CD4 lymphocyte count with haemoglobin concentration in HIV infected patients at HIV treatment centre. Med sci. 2013;9(3):138–140.
20. Robinson O, Obianime AW, Tamun I. Immunological and Hematological profile of HIV patients on antiretroviral therapy in port harcourt, River state Nigeria. Int Blood Res. 2017;7(4):1–12.
21. Addis Z, Yitagezu G. Tacheble B. prevalence of some hematological abnormalities among HIV positive patients on their first visit to tertiary health institution. Int Blood Res. 2014;2(6):270–277. doi:10.9734/IBRR/2014/11137
22. Woldeamanuel G, Wondimu H. Prevalence of anaemia before and after initiation of antiretroviral therapy among HIV infected Patients at black lion specialized hospital Addis Ababa, Ethiopia. BMC Hematol. 2018;18:7. doi:10.1186/s12878-018-0099-y
23. Fenetahun Y, Fentahun T. Socio-economic profile of arid and semi-arid agro-pastoral region of Borana rangeland Southern Ethiopia. MOJ Eco Environ Sci. 2020;5(3):113–122.
24. Negesse A, Getaneh T, Temesgen H. Prevalence of anemia and its associated factors in human immuno deficiency virus infected adult individuals in Ethiopia. A systematic review and meta-analysis. BMC Hematol. 2018;8:32. doi:10.1186/s12878-018-0127-y
25. Assefa M, Abegaz WE, Shewamare A, Medhin G, Belay M. Prevalence and correlates of anemia among HIV infected patients on highly active antiretroviral therapy at Zewditu Memorial Hospital, Ethiopia. BMC Hematol. 2015;15(1):1–6. doi:10.1186/s12878-015-0024-6
26. Daka D, Lelissa D, Amsalu A. Prevalence of anemia before and after the initiation of antiretroviral therapy at ART center of Hawassa University Referral Hospital, Hawassa, South Ethiopia. Sch J Med. 2013;3(1):1–6.
27. Damtie S, Lemma W, Teklehaimanot K, Tahir E, Tegenaw T. Hematological abnormalities of adult HIV-infected patients before and after initiation of highly active antiretroviral treatment at Debre Tabor Comprehensive Specialized Hospital, Northcentral Ethiopia: a Cross-Sectional Study. HIV/AIDS Res Palliat Care. 2021;4(13):477–484.
28. Uguma N, Kiya GT, Maleko WA, Bimerew LG. Hematological parameters abnormalities and associated factors in HIV-positive adults before and after highly active antiretroviral treatment in Goba Referral Hospital, South-East Ethiopia. SAGE Open Med. 2021;9:1–12.
29. Denue BA, Kida IM, Hammagabdo A, Dayar A, Sahabi MA. Prevalence of anemia and immunological markers in HIV-infected patients on highly active antiretroviral therapy in Northeastern Nigeria. Infect Dis Res Treat. 2013;6:25–33.
30. Owiredu WK, Quaye L, Amidu N, Addai-Mensah O. Prevalence of anaemia and immunological markers among Ghanaian HAART-naïve HIV-patients and those on HAART. Afr Health Sci. 2011;11(1):2–15.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
