Back to Journals » Research and Reports in Urology » Volume 10
Inflammatory mechanisms and oxidative stress in prostatitis: the possible role of antioxidant therapy
Authors Paulis G
Received 7 April 2018
Accepted for publication 28 June 2018
Published 17 September 2018 Volume 2018:10 Pages 75—87
DOI https://doi.org/10.2147/RRU.S170400
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Gianni Paulis1,2
1Andrology Center, Villa Benedetta Clinic, Rome, Italy; 2Department of Uro-Andrology, Castelfidardo Medical Team, Rome, Italy
Abstract: This article focuses on the role that oxidative stress plays in chronic prostatitis, not only with respect to the known impact on symptoms and fertility but also especially in relation to possible prostate cancer development. Prostatitis is the most common urologic disease in adult males younger than 50 years and the third most common urologic diagnosis in males older than 50 years. If the germ-causing acute prostatitis is not eliminated, the inflammatory process becomes chronic. Persistent inflammation causes ongoing production of large quantities of pro-inflammatory cytokines and both oxygen and nitrogen reactive species, with consequent activation of transcription factor nuclear factor-kappa B (NF-κB) and genes encoding for further production of pro-inflammatory cytokines, chemotactic factors, and growth factors. Confirming the role of oxidative stress in chronic prostatitis, several studies have demonstrated the presence of oxidative stress markers in the genital secretions of patients suffering from the disease. Antioxidants can therefore play an essential role in the treatment of chronic bacterial and non-bacterial prostatitis; in the case of bacterial inflammation, they can be associated with antibiotic therapy. Moreover, due to their anti-inflammatory properties, antioxidants hinder the progression of inflammation and the possible development of prostate cancer.
Keywords: chronic prostatitis, chronic prostatitis treatment, radical oxygen species, nitrosative stress, antioxidant therapy
Introduction
Prostatitis is the most common urologic disease in adult males younger than 50 years and the third most frequent urologic diagnosis in men older than 50 years.1 In the literature, prevalence of the disease varies between 1.8% and 8.2%; in the United States, prostatitis contributes to about 8% of urologist visits and 1% of all general practitioner visits.2,3 It has been estimated that about 50% of men experience symptoms of prostatitis during their lifetime.4 Patients with a previous diagnosis of prostatitis have a 20%–50% risk of developing recurrence.5 About 5%–12% of infertile men have a history of genital inflammation, including prostatitis, epididymitis, and orchitis.6 Although a rarer asymptomatic form is possible, prostatitis symptoms can vary widely and include high fever (only in acute forms or new flares), flu-like symptoms (malaise, arthralgia, and myalgia), dysuria, stinging sensation when urinating, urethral burning (even at rest), strangury, difficulty in voiding, urinary urgency, frequent urination, urethral discharge, pain in one or several areas (urethra, penis, hypogastrium, groin, epididymis and testicle, perineum, anorectal and sacral region, and pelvis), hematospermia, ejaculatory disorders (premature ejaculation, peri- and/or post-orgasmic penile, and/or scrotal pain), very often emotional distress and negative impact on quality of life, and more rarely erectile dysfunction. The cause of prostatic infection, in most cases, is bacterial, and the most common germs are Gram-negative bacteria, particularly Escherichia coli, Enterobacter, Klebsiella, Serratia, Pseudomonas, and Proteus species, but Gram-positive bacteria, particularly Enterococcus, can also be responsible for prostatic infection. Microorganisms responsible for sexually transmitted diseases can also cause prostatic infection; these include Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis, Trichomonas vaginalis, and Gardnerella vaginalis.7–9 The risk factors for prostatic infection are intraprostatic ductal reflux, endourethral diagnostic and surgical procedures, urethral catheterization, unprotected anal intercourse, and phimosis.7
Prostatitis has been classified into the following categories: type I, acute bacterial prostatitis; type II, chronic bacterial prostatitis; type III, chronic non-bacterial prostatitis, also known as chronic pelvic pain syndrome (CPPS), which may be “inflammatory” (category III A) or “non-inflammatory” (category III B); and type IV, asymptomatic inflammatory prostatitis.10
In about 5% of patients with acute bacterial prostatitis, the inflammation can become chronic.7 Recently, the chemical processes involved in oxidative stress have been shown to play an essential role in the pathophysiology of inflammation.11 In the literature, several authors have proven the presence of oxidative stress in chronic prostatic inflammation.12–17 This article focuses on the role that oxidative stress plays in chronic prostatitis, not only with respect to the known impact on symptoms and fertility but also especially in relation to possible prostate cancer development. High concentrations of free radicals are produced in the tissues during chronic prostatitis, leading to marked tissue damage and DNA fragmentation; excessive epithelial proliferation may then occur, with possible development of prostate cancer. Our interest in this topic was reinforced by a number of recent studies which, besides pointing to an increase in risk of developing prostate cancer in men with a history of chronic prostatitis, demonstrated that oxidative stress is associated with the development and progression of prostate cancer.18–27
Oxidative stress and prostatic inflammation
Oxidative stress
In many of our organism’s cellular processes, there is a physiological balance between production and elimination of reactive oxygen species (ROS) (redox homeostasis). This balance is preserved by protective endogenous antioxidant mechanisms. However, redox homeostasis is altered when there is an excessive production of reactive species, leading to the development of a state of oxidative stress.11 Alteration of redox homeostasis causes a series of chemical reactions that play an essential role in the pathophysiology of inflammation. As a proof of this, several studies have shown that oxidative stress can cause the maintenance and progression of chronic inflammation, contributing to the pathophysiology of many chronic diseases, including chronic prostatitis and prostate cancer.11–17,21
Although in moderate concentrations, reactive species ensure that cell processes run normally, in high concentrations, they cause pathological changes in intracellular substances such as proteins, lipids, and DNA.11 Therefore, certain excessive oxidative stress conditions, for instance, inflammation, including prostatitis, can cause cellular death and damage to the extracellular matrix (ECM) with consequent tissue damage and fibrosis.28
Acute inflammation of prostate
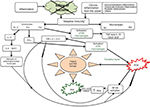
When microbial pathogens (bacteria, viruses, fungi, etc) are detected by pattern recognition receptors (PRRs), the immune system produces an acute inflammatory response (Figure 1) aimed at eradicating the infectious agents.29,30 This first defense line of the body is known as innate immunity (or non-specific immune system or natural immunity). PRRs are expressed by various innate immunity cells (neutrophils, macrophages, monocytes, and dendritic cells) and are able to recognize pathogen-associated molecular patterns (PAMPs), as well as patterns of the external cell walls of bacteria such as the lipopolysaccharides (LPSs) of Gram-negative bacteria (Figure 1) and the lipoteichoic acids and peptidoglycans of Gram-positive bacteria.29–31 After the body has recognized the germ and the innate immune system has been activated, an acute inflammatory response begins with the secretion of various cytokines (tumor necrosis factor alpha [TNF-α] and interleukin [IL]-1) and chemokines (IL-8, monocyte chemoattractant protein-1 [MCP-1], and macrophage inflammatory protein-1-alpha [MIP-1-α]), which stimulate the recruitment of inflammatory cells at the site of prostatic infection.32–35
MIP-1-α is a chemotactic agent for neutrophil granulocytes, monocytes, and macrophages. In particular, MCP-1 acts as a powerful chemotactic agent for circulating monocytes, which turn into macrophages once they have reached the site of inflammation; therefore, MCP-1 regulates the recruitment of monocytes and the formation of macrophage infiltrate. MCP-1 is produced by many types of cells, both by natural gene expression and by growth factors, cytokines, and oxidative stress after induction.
During the inflammatory reaction induced by a bacterial infection, a high concentration of inflammatory cells immediately occurs at the infection site; these cells are mainly neutrocytes (within and around the prostatic acini), followed in time by a stromal infiltration formed by lymphocytes and macrophages; in the following days, the neutrocytes gather mainly in the stroma.36
In the pre-antibiotic era, evolution into prostatic abscess with acute prostatitis was very frequent, whereas this event is rarer today (6%) although it can develop in particular situations (urethral or prostatic obstruction, urethral procedures with catheterization, prostate biopsy procedures, presence of prostatic urethral implants, immunosuppressive conditions, diabetes, chronic kidney failure).37
After the receptors have recognized bacteria and/or viruses and have been “activated,” the cells of the infiltrate, in particular neutrophils and macrophages, undergo degranulation with the release of lysosomal enzymes, and at the same time, a “respiratory burst” occurs, which results in the rapid release of ROS: superoxide anion radical and hydrogen peroxide.11 Leukocyte activation takes place due to the presence of the enzyme nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in the neutrophils and macrophages. NADPH oxidase, catalyzing the chemical reaction, causes formation of the superoxide anion (O2•-).
Production of high amounts of superoxide anion by the inflammatory cells leads to subsequent chemical reactions, with the production of further reactive species such as hydrogen peroxide, hydroxyl radical, hypochlorous acid, and singlet oxygen. In this phase, the production of ROS, mainly superoxide anion, hydroxyl radical, and hypochlorous acid, is aimed at killing the bacteria responsible for the prostatic infection.11,38–40
The cells of the inflammatory infiltrate, in turn, produce the release of inflammatory mediators such as pro-inflammatory cytokines: TNF-α, IL-1, and IL-6. As a consequence, high amounts of the following are released: cyclooxygenase-2 (COX-2), transcription factor nuclear factor-kappa B (NF-κB), and inducible nitric oxide synthase (iNOS). The continuous production of iNOS causes high local concentrations of nitric oxide radical (NO•-), which is able to compete with the enzyme superoxide dismutase (SOD) by stealing a superoxide anion from it and causing the formation of peroxynitrite and a series of reactions that lead to the formation of some of its highly reactive and toxic metabolites and aiming to destroy the microorganism; these metabolites, together with the nitric oxide radical, are called reactive nitrogen species (RNS).11,30,38,41,42
SOD is a part of the endogenous antioxidant defenses and intervenes as primary cellular defense against the superoxide anion.
Besides neutrocytes and macrophages, lymphocytes are also known to be present among the cells of the inflammatory infiltrate.
Recently, it has been ascertained that some T cells (which are part of the adaptive immune system) also contribute to acute inflammation.43 Among these T cells, the most important are T helper 17 (Th17) cells, a subgroup of T helper (Th) cells that produce IL-17, which is able to induce the production of chemokines IL-8 and MCP-1, whose function is to recruit other leukocytes to the inflammatory site.43
After recognition of the pathogen, due to the release of ROS and RNS and activation of redox-sensitive factor NF-κB by ROS and cytokines IL-1 and TNF-α, a process is triggered that causes transcriptional expression of numerous inflammatory mediators, which coordinate the elimination of pathogens and infected cells.11,29,38,44
Furthermore, a downregulation of prostate-specific transcription factor Nkx3-1 occurs during bacterial prostatitis.36 It has been experimentally proven that the loss of factor Nkx3-1 not only leads to a reduction in the gene expression of several antioxidant enzymes but also leads to an increase in the production of pro-oxidant enzymes.36 This results in further accumulation of ROS and consequent amplification of the inflammatory process, due to further activation of redox-sensitive transcription factor NF-κB.11,38
Among many free radicals produced when prostate inflammation is in progress, it is important to mention isoprostane 8-epi prostaglandin-F2α (PGF2α). The production of this isoprostane also increases in other conditions associated with oxidative stress (smoking, consumption of alcohol, diabetes, aging, etc.). Isoprostane 8-epi PGF2α is also used as a urinary marker of oxidative stress in patients with prostatitis.17 Isoprostane 8-epi PGF2α can cause potent smooth muscle contraction, having important effects on bladder muscles, significantly affecting the symptoms of prostatitis (urinary urgency, frequent need to urinate, etc).17,45
During the acute inflammatory process, inflammatory mediators (cytokines, ILs, chemokines, etc) are produced in waves, until the stimulus persists, and because they have a short half-life, they are degraded shortly after their release. Even neutrophils have a short half-life in tissues and die by apoptosis within a few hours. As the acute inflammation progresses, once the germs are eliminated, resolution of the inflammatory process occurs, due to a series of stop signals (arachidonic acid metabolites, IL-10, inhibition of the production of TNF-α, etc.), which lead to the end of the inflammatory reaction. The acute inflammatory process in acute prostatitis ends with the elimination of the germ.
Chronic inflammation of prostate
If the germ is not eliminated, the inflammatory process turns chronic (Figure 2).11,38 Adaptive (specific or acquired) immunity is then triggered, with activation of antigen-specific T and B cells.38 Since the inflammatory process is not interrupted, the production of cytokines, ROS, and RNS persists, as the regulatory mechanisms that should have blocked the production and release were not activated, due to the persistence of the inflammation-causing agent. Thus, the prostatic inflammation becomes chronic.
More rarely, the inflammatory process can also insidiously present right from the start as a chronic inflammatory response, with no typical manifestation of acute inflammation. For example, this can occur with prostate tuberculosis and prostatic syphilis.
The histopathological profile of chronic prostatitis shows the presence of lymphocyte infiltration, more widely present in the stroma immediately adjacent to the prostatic acini and more scarcely present in the epithelium; macrophages are also present in significant numbers both within the glands and in the intervening stroma.7,46,47 Macrophages have a dominant role in chronic inflammations as they contribute to the inflammatory reaction by secreting cytokines and growth factors and activating other cells, in particular T cells. During inflammation, macrophages can be activated both through the classic pathway (M1 macrophages) and through the alternative pathway (M2 macrophages). Macrophage activation through the classic pathway can be caused by microbial endotoxins and cytokine interferon gamma (IFN-γ) produced by T helper 1 (Th1) cells. Alternative macrophage activation, instead, occurs following the action of cytokines IL-4, IL-5, and IL-13 produced by Th2 cells and other types of cells (mast cells, eosinophils, and T CD8+ cells). Macrophages must be divided into M1 and M2 macrophages; M1 macrophages are actively involved in the inflammatory reaction, whereas M2 macrophages suppress inflammation by producing cytokine IL-10 and contribute to tissue repair and remodeling by releasing transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), and epidermal growth factor (EGF). M1 macrophages are inflammatory cells designed to eliminate pathogens and therefore release high amounts of ROS, which are highly cytotoxic; if the production of ROS is excessive and uncontrolled, it can cause tissue damage. M1 macrophages also secrete TNF-α, IL-1, IL-6, and IL-12.38 The T cells in the inflammatory infiltrate are both (cytotoxic) CD8+ T cells and CD4+ Th cells (also called CD4+ T cells or Th cells). In particular, CD4+ T cells 1 or simply Th1 cells produce IFN-γ, a cytokine that causes activation of M1 macrophages.
CD4+ T helper 2 (Th2) cells, besides producing cytokines IL-4, IL-5, and IL-13, cause activation of M2 macrophages. Th17 cells, which represent a subgroup of Th cells, produce IL-17, a cytokine that induces production (on the part of macrophages) of IL-8 and MCP-1, which have chemotactic effects on white blood cells.48,49 In conclusion, there is an important interaction between macrophages and lymphocytes in chronic inflammation, because T cells produce cytokines that recruit macrophages and other cytokines, which activate the macrophages. In turn, the activated macrophages stimulate the response of T cells through cytokines such as IL-12 and IL-23.43 In particular, cytokine IL-12 induces maturation of CD4+ cells toward Th1; IL-12 also stimulates the production of IFN-γ by Th17 cells; cytokine IL-23 induces synthesis of pro-inflammatory cytokines (IL-17) by Th17 cells.43
Persistence of inflammation, therefore, causes ongoing production of high amounts of pro-inflammatory cytokines and hyperproduction of ROS and RNS, with consequent activation of NF-κB and the expression of genes encoding for fibroblast growth factor (FGF), TGF-β, IFN-γ, iNOS, and IL-17.11,15,16,38,47,50–52
The excessive production of iNOS leads to high local levels of ROS and RNS, which can cause cell and tissue damage, ECM damage, even through the lipidic peroxidation.11,15,16,28,53
Cellular death and ECM damage result in the release of several molecules originating from the cells (cytosolic or nuclear proteins, etc) and from ECM degradation. These damage-associated molecular patterns (DAMPs), or “alarmins,” are able to amplify and maintain the inflammatory response, since they are promptly detected by PRRs.11 The inflammatory cascade is reactivated and boosted by the products of the damage caused by lipid peroxidation, thus favoring further progression of chronic inflammation.
Significant tissue damage is, therefore, a hallmark of chronic inflammation.15,16 In prostatitis as in other inflammatory diseases, nitric oxide radical effects depend on its concentrations, so when local concentrations of the radical increase significantly, a high oxidation state occurs; in these conditions, the nitric oxide radical interferes with SOD, leading to high local concentrations of peroxynitrite.38,53 Peroxynitrite is highly toxic and reactive and is capable of causing cell damage (by lipid peroxidation and DNA fragmentation), tissue damage, and depletion of plasma antioxidants. The high concentration of reactive species (ROS and RNS) that occurs during chronic prostatic inflammation is capable of causing direct DNA damage, with consequent sperm DNA fragmentation and its negative effects on fertility.15,16,54–57 As in other forms of chronic inflammation, an important consequence of these abnormal inflammatory phenomena is the tissue damage due to oxidative stress and excessive induction of tissue repair mechanisms. Inflammation is an immunological response the main purpose of which is to eradicate pathogens and to repair damaged tissues, but if the natural response is not appropriate, excessive tissue repair (fibrosis, etc) and persistent oxidative stress can maintain and amplify the inflammatory response.11 Thus, the abnormal inflammatory response becomes dysfunctional and capable of causing significant alterations in tissue and organ functions; this occurs in chronic prostatitis and other more serious conditions, eg, atherosclerosis, chronic heart failure, neurodegenerative diseases, cancer, diabetes.11,58 As in other chronic inflammatory diseases, tissue damage in chronic prostatitis essentially results in fibrosis and calcifications that cause lower urinary tract symptoms (LUTS), (at times obstructive) dysuria symptoms, and frequently recurring infections.59–62 Moreover, the presence of bacteria within prostatic calcifications has been reported; therefore, calcifications represent a permanent receptacle for bacteria and cause bacterial persistence.63
Furthermore, a greater expression of cytokine TNF-α has been observed in patients with prostatic calcifications.64 Considering the excessive inflammatory response occurring in chronic prostatitis, a number of authors have postulated that chronic inflammatory disease of the prostate is the result of an excessive adaptive immune response that can cause activation of an autoimmune process (autoimmune prostatitis).7,65–68
Possible causes of the chronicization of prostatitis
Prostatitis may become chronic as a result of the following:
- failed recognition and diagnosis of the first episode of acute bacterial prostatitis, with consequent failure to treat;
- persistence of the germ in the prostate gland for various reasons (eg, presence of intra-prostatic calcifications), including the frequent possibility of antibiotic resistance;69,70
- insufficient duration of the antibiotic therapy (targeted treatment for 4–6 weeks is needed);7,71,72
- insufficient antibiotic concentration reached in the prostatic tissue;7,73
- asymptomatic prostatitis.7,10
Antioxidants already studied and employed in the treatment of chronic prostatitis
Obviously, in the case of bacterial prostatitis, therapy must principally aim at eradication of the germ, with the use of antibiotics that should ideally be able to penetrate into the prostate and reach high tissue concentrations. Considering the long-term duration of antibiotic therapy, it is always advisable to associate prebiotic supplementation to avoid side effects on the resident’s intestinal microbiota. Antibiotic therapy should also be associated with anti-inflammatory and antioxidant substances, so as to limit the tissue damage and consequent symptoms as much as possible.
In the treatment of chronic prostatitis, several natural substances have been used, but scientific evidence and therapeutic rationale exist for only a few of them.
Serenoa repens
Serenoa repens (SR), or saw palmetto, is very likely the most commonly used plant in the treatment of both prostatitis and benign prostatic hyperplasia. Despite widespread belief that SR acts through an antiandrogenic effect (inhibition of 5α-reductase with consequent reduction in the production of dihydrotestosterone [DHT]), various studies in the literature have shown the antioxidant and anti-inflammatory properties of SR.74
In a 1997 study, Paubert-Braquet et al75 proved that SR has anti-inflammatory effects as it is able to inhibit the production of 5-lipoxygenase metabolites and leukotrienes.
Colado-Velázquez et al76 demonstrated the following effects of SR: antioxidant effect and significant reduction in the gene expression of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 and growth factors FGFs and VEGFs.
Latil et al77 proved that SR is able to inhibit gene expression of the pro-inflammatory cytokine macrophage migration inhibitory factor (MIF) and the two chemotactic agents MCP-1 and IFN-γ-induced protein 10 (IP-10).
Other studies have found significant positive effects of SR on inflammation biomarkers in biological samples of patients with prostatic inflammation.78,79
Furthermore, several in vitro studies have described the antioxidant and anti-inflammatory activity of most of its components.
Currently, available commercial SR extracts contain fatty acids and phytosterols.80–82
Capric acid is a saturated fatty acid (SFA), which has antioxidant and anti-inflammatory activities, inhibits the production of iNOS and nitric oxide radical, transcriptional activity of NF-κB, and COX-2 activity, and prevents the gene expression of chemotactic factor MCP-1.83–85
Even caprylic acid (SFA) has antioxidant action and also inhibits the production of chemokine IL-8.85,86
Lauric acid (SFA) also has antioxidant and anti-inflammatory action and is able to inhibit the COX-2 enzyme.85
Myristic acid (SFA) is a powerful scavenger of nitric oxide, superoxide, hydroxyl, and lipid peroxide.85,87
Palmitic acid (SFA) is capable of inhibiting the production of pro-inflammatory cytokine TNF-α.88
Oleic acid is an unsaturated fatty acid (UFA), and it is an antioxidant as it is able to inhibit the activation of transcription factor NF-κB, expression of the iNOS enzyme, and production of radical NO and ROS.86 Oleic acid also has anti-inflammatory action as it reduces the expression of COX-2 and prostaglandins E-2.89
Linoleic acid and linolenic acid are UFAs; they have antioxidant and anti-inflammatory activities, since they are able to reduce the gene expression of factor NF-κB and the production of iNOS, ROS, and RNS. In particular, linoleic acid also has anti-COX2 anti-inflammatory activity.90–92
Phytosterols campesterol, stigmasterol, and β-sitosterol are all antioxidants; in particular, β-sitosterol increases the activity of antioxidants SOD and glutathione peroxidase.93,94
Previous studies have also proven that other antioxidant substances are present in SR, including ferulic acid, vanillic acid, triterpenes, gallic acid, caffeic acid esters, flavonoids isoquercetin, avicularin, astragalin, rutin, manghaslin, and kaempferol.82,95
Quercetin
Quercetin is a flavonoid present in high concentrations in capers, red onion, lovage, and dill. It has been used successfully in the treatment of prostatitis, due to its antioxidant and anti-inflammatory properties; besides being an excellent scavenger of superoxide anion and nitric oxide radical, quercetin inhibits the production of ROS, IL-6, IL-8, TNF-α, and MCP-1 and blocks the activation of factor NF-κB.96–98
In a randomized, double-blind, placebo-controlled study, Shoskes et al99 proved that quercetin (500 mg orally twice daily for 4 weeks) was able to significantly improve clinical symptoms in patients with chronic prostatitis.
Carnitine
Carnitine is a molecule synthesized in the human body mainly in the liver and kidneys, whereas external natural sources are meat, milk, and codfish. Carnitine is a very powerful scavenger of superoxide anion, hydrogen peroxide, and peroxynitrite and also suppresses nitric oxide radical production and iNOS gene expression.100,101 It also has anti-inflammatory property as it impairs the production of C-reactive protein (CRP), IL-1, IL-6, TNF-α, and TGF-β1.102,103 Carnitine has been successfully used in the treatment of nonbacterial prostatovesiculoepididymitis with leukocytospermia.104
Bee pollen extract
Several studies have reported the successful use of pollen extract (PE) in the treatment of prostatitis.105–107
PE is a powerful scavenger of hydroxyl radicals, hydrogen peroxide, and superoxide anion.108,109
The main constituents of PE are fatty acids, phenols, and flavonoids. In particular, linolenic and linoleic acids are present in high amounts, palmitic acid and oleic acid are present in slightly lower quantities; capric acid, eicosenoic acid, and arachidic acid are present in limited quantities; gallic acid and quercetin are also present.108,109 Its fatty acid, quercetin, and gallic acid content give bee pollen antioxidant and anti-inflammatory properties.89–92,96–98,110
Curcumin
A few positive experiences have been reported in the literature on the therapeutic effects of curcumin in chronic prostatitis.111,112 Curcumin is a phenolic compound that is the main component of Curcuma longa, a plant widely used in the East as a spice, particularly in India as the main ingredient of curry. Curcumin has antioxidant scavenging activity against both ROS (superoxide anion and hydrogen peroxide) and RNS.113–115 Since curcumin has antioxidant activity against peroxy radicals, it impairs lipid peroxidation and DNA fragmentation.116
Curcumin also has anti-inflammatory action, since it suppresses the activation of transcription factor NF-κB and is capable of inhibiting the production of cytokines TNF-α, IL-1, IL-2, IL-8, IL-12, and MCP-1; curcumin, furthermore, causes a downregulation of the enzymatic activity of COX-2, lipoxygenase, and iNOS.117,118
Moreover, curcumin has antifibrotic action, inhibiting the action of growth factors TGF-β1 and basic fibroblast growth factor (bFGF).119–121
Furthermore, curcumin has been shown to have a chemopreventive effect on the onset of prostate cancer, because it interferes with the proliferation of prostate cancer and the spreading of metastasis through downregulation of androgen receptors (ARs) and epidermal growth factor receptors (EGFRs) and promotion of cell cycle arrest.122 Due to its properties, curcumin can play a very important role, especially in consideration of newly acquired knowledge indicating that chronic prostatitis favors the development of prostate cancer.18–25,27
Resveratrol
Resveratrol is a natural phenol present in high concentrations in red grapes and grape-derived products (eg, red wine), blueberries, raspberries, apples, peanuts, plums, mulberry, pine trees (Pinus spp), legumes (Cassia spp, Pterolobium hexapetalum), etc. Resveratrol has antioxidant and anti-inflammatory properties; it has also been shown to have antifibrotic activity, since it can hinder the progression of chronic prostatitis by contrasting the effects of TGF-β and converting fibroblasts back into myofibroblasts within the prostate.123 A number of experimental studies have proven the efficacy of this substance in the treatment of chronic prostatitis.124–126
Monoterpenes
Monoterpenes are natural essential oils from plants that have antioxidant activities. A blend of these substances (Rowatinex® capsules; ROWA WAGNER GmbH & Co. KG, Bergisch Gladbach, Germany) was used to prove their efficacy in the treatment of chronic prostatitis patients.127 Rowatinex contains the following: alpha-pinene, beta-pinene, camphene, borneol, fenchone, anethole, and cineol. These substances have antioxidant and anti-inflammatory properties; in particular, they are able to inhibit enzymes iNOS and myeloperoxidase (MPO), transcription factor NF-κB, production of 8-isoprostane, enzyme COX-2, and pro-inflammatory cytokines TNF-α, IL-1β, IL-2, and IL-6.128–133
The formula containing the monoterpenes (Rowatinex capsules) was studied in a randomized controlled trial, and its therapeutic effects on 25 patients suffering from chronic prostatitis were compared with those obtained in another group of 25 patients who were treated with ibuprofen (a non-steroidal anti-inflammatory drug).127 Therapeutic efficacy was assessed in the two treatment groups by evaluating the variations in the score of a specific questionnaire (NIH-CPSI) after 6 weeks of treatment. NIH-CPSI is a validated questionnaire that is used to assess the intensity of prostatitis symptoms (pain, urinary symptoms, and quality of life). After treatment, symptom improvement was significantly higher (P = 0.04) in the group of patients treated with monoterpenes (Rowatinex) compared with those treated with ibuprofen.127
Epilobium
Epilobium is a perennial herbaceous plant that includes various species; the species studied in relation to prostatitis are the following: Epilobium parviflorum, Epilobium angustifolium, and Epilobium hirsutum.
The properties of Epilobium are due to its high antioxidant content: myricetin, quercetin, and kaempferol (flavonoids), and oenothein B (a macrocyclic tannin). In particular, the extract of Epilobium perviflorum has been shown to have strong antioxidant activity (inhibition of enzyme MPO), anti-inflammatory activity (inhibition of enzymes cyclooxygenase-1 [COX-1] and COX-2), and antibacterial activity.134–136 Moreover, oenothein B, which is present in Epilobium in high concentrations (20%–35%), was shown to powerfully inhibit cellular proliferation.136–138 In particular, oenothein B hinders the progression of prostate cancer by inducing the production of neutral endopeptidase (NEP), an enzyme that inactivates the neuropeptides that stimulate neoplastic cellular proliferation.138,139
N-acetylcysteine (NAC)
NAC has also been observed in experimental studies that have proven its antioxidant and anti-inflammatory efficacy in prostatitis.140 Its mechanism of actions are believed to consist in the inhibition of COX-2 and suppression of transcription factor NF-κB.140,141
Furthermore, NAC has been shown to have chemopreventive activity for prostate cancer, because it strongly suppresses the proliferation, migration, invasion, and adhesion of human prostate cancer cells.142,143
Discussion and conclusion
It is evident that oxidative stress plays an important role at various levels in both acute and chronic inflammations. If inflammatory cells were not to release ROS, one of the key elements of the immune response could not take place, ie, neutralization and killing of the bacteria responsible for the infection. In the absence of reactive species (ROS and RNS), activation of factor NF-κB would not take place, along with the consequent production of pro-inflammatory cytokines, growth factors FGF and TGF-β, and other inflammatory mediators such as COX-2 and lipoxygenase. Furthermore, by regulating the functions of T cells, ROS play an important role in the modulation of the immune response.144 Oxidative stress is the most important cause of tissue damage in chronic prostatitis. As chronic prostatitis progresses, fibrotic and calcified areas are inevitably formed, which cause dysuria and reinfections.60–62
Confirming the role played by oxidative stress in chronic prostatic inflammation, several studies have proven the presence of oxidative stress markers in genital secretions or urine of patients suffering from the disease.12–14,17 In conclusion, antioxidants can play an essential role in the treatment of chronic prostatitis. To date, a very few studies have analyzed the therapeutic effect of antioxidants in chronic prostatitis patients, although numerous studies have demonstrated the properties of the various antioxidants and pointed out their potential efficacy.79,99,104–107,111,112,127
Further randomized controlled studies on this topic are therefore needed, especially considering the enormous growth in interest in chronic prostatitis over the past few years, since excessive oxidative stress can favor the progression of inflammation and the development of prostate cancer.
Disclosure
The author reports no conflicts of interest in this work.
References
Collins MM, Stafford RS, O’Leary MP, Barry MJ. How common is prostatitis? A national survey of physician visits. J Urol. 1998;159(4):1224–1228. | ||
Suskind AM, Berry SH, Ewing BA, Elliott MN, Suttorp MJ, Clemens JQ. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND Interstitial Cystitis Epidemiology Male Study. J Urol. 2013;189(1):141–145. | ||
Krieger JN, Lee SWH, Jeon J, Cheah PY, Liong ML, Riley DE. Epidemiology of prostatitis. Int J Antimicrob Agents. 2008;31(Suppl. 1):85–90. | ||
Britton JJ, Carson CC. Prostatitis. AUA Update Series. 1998;17:154–159. | ||
Riley DE, Krieger JN. X Chromosomal short tandem repeat polymorphisms near the phosphoglycerate kinase gene in men with chronic prostatitis. Biochim Biophys Acta. 2002;1586(1):99–107. | ||
Dohle GR. Inflammatory-associated obstructions of the male reproductive tract. Andrologia. 2003;35(5):321–324. | ||
Nickel JC. Prostatitis and related conditions, orchitis, and epididymitis. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 10th ed., Vol. 1. Philadelphia: Elsevier Saunders; 2011:327–356. | ||
Mändar R, Raukas E, Türk S, Korrovits P, Punab M. Mycoplasmas in semen of chronic prostatitis patients. Scand J Urol Nephrol. 2005;39(6):479–482. | ||
Maskell R. Gardnerella vaginalis and prostatitis. The Lancet. 1981;318(8246):581–582. | ||
Krieger JN, Nyberg L Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282(3):236–237. | ||
Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L. The role of oxidative stress during inflammatory processes. Biol Chem. 2014;395(2):203–230. | ||
Pasqualotto FF, Sharma RK, Potts JM, Nelson DR, Thomas AJ, Agarwal A. Seminal oxidative stress in patients with chronic prostatitis. Urology. 2000;55(6):881–885. | ||
Potts JM, Pasqualotto FF. Seminal oxidative stress in patients with chronic prostatitis. Andrologia. 2003;35(5):304–308. | ||
Shahed AR, Shoskes DA. Oxidative stress in prostatic fluid of patients with chronic pelvic pain syndrome: correlation with gram positive bacterial growth and treatment response. J Androl. 2000;21(5):669–675. | ||
Zhou J-F, Xiao W-Q, Zheng Y-C, Dong J, Zhang S-M. Increased oxidative stress and oxidative damage associated with chronic bacterial prostatitis. Asian J Androl. 2006;8(3):317–323. | ||
Lou JG, Dong J, Zheng YC, Zhang SM, Xiao WQ, Zhou JF. Increased oxidative stress and damage in patients with chronic bacterial prostatitis. Biomed Environ Sci. 2006;19(6):481–486. | ||
Kullisaar T, Türk S, Punab M, Mändar R. Oxidative stress – cause or consequence of male genital tract disorders? Prostate. 2012;72(9):977–983. | ||
Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60(1):78–83. | ||
Jiang J, Li J, Yunxia Z, Zhu H, Liu J, Pumill C. The role of prostatitis in prostate cancer: meta-analysis. PLoS One. 2013;8(12):e85179. | ||
Cheng I, Witte JS, Jacobsen SJ, et al. Prostatitis, sexually transmitted diseases, and prostate cancer: the California Men’s Health Study. PLoS One. 2010;5(1):e8736. | ||
Gupta-Elera G, Garrett AR, Robison RA, O’Neill KL. The role of oxidative stress in prostate cancer. Eur J Cancer Prev. 2012;21(2):155–162. | ||
Sandhu JS. Prostate cancer and chronic prostatitis. Curr Urol Rep. 2008;9(4):328–332. | ||
Paschos A, Pandya R, Duivenvoorden WCM, Pinthus JH. Oxidative stress in prostate cancer: changing research concepts towards a novel paradigm for prevention and therapeutics. Prostate Cancer Prostatic Dis. 2013;16(3):217–225. | ||
Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett. 2009;282(2):125–136. | ||
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. | ||
Minelli A, Bellezza I, Conte C, Culig Z. Oxidative stress-related aging: a role for prostate cancer? Biochim Biophys Acta. 2009;1795(2):83–91. | ||
Kaya E, Ozgok Yasar, Zor M, et al. Oxidative stress parameters in patients with prostate cancer, benign prostatic hyperplasia and asymptomatic inflammatory prostatitis: a prospective controlled study. Adv Clin Exp Med. 2017;26(7):1095–1099. | ||
Kennett EC, Chuang CY, Degendorfer G, Whitelock JM, Davies MJ. Mechanisms and consequences of oxidative damage to extracellular matrix. Biochem Soc Trans. 2011;39(5):1279–1287. | ||
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. | ||
Gatti G, Rivero V, Motrich RD, Maccioni M. Prostate epithelial cells can act as early sensors of infection by up-regulating TLR4 expression and proinflammatory mediators upon LPS stimulation. J Leukoc Biol. 2006;79(5):989–998. | ||
Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240–273. | ||
Paulis G, Conti E, Voliani S, et al. Evaluation of the cytokines in genital secretions of patients with chronic prostatitis. Arch Ital Urol Androl. 2003;75(4):179–186. | ||
Hochreiter WW, Nadler RB, Koch AE, et al. Evaluation of the cytokines interleukin 8 and epithelial neutrophil activating peptide 78 as indicators of inflammation in prostatic secretions. Urology. 2000;56(6):1025–1029. | ||
Nadler RB, Koch AE, Calhoun EA, et al. IL-1β and Tnf-α in prostatic secretions are indicators in the evaluation of men with chronic prostatitis. J Urol. 2000;164(1):214–218. | ||
Desireddi NV, Campbell PL, Stern JA, et al. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-1α as possible biomarkers for the chronic pelvic pain syndrome. J Urol. 2008;179(5):18571861–18621862; discussion. | ||
Khalili M, Mutton LN, Gurel B, et al. Loss of Nkx3.1 expression in bacterial prostatitis: a potential link between inflammation and neoplasia. Am J Pathol. 2010;176(5):2259–2268. | ||
Us H, Kim ME, Kim CS, et al. Acute bacterial prostatitis in Korea: clinical outcome, including symptoms, management, microbiology and course of disease. Int J Antimicrob Agents. 2008;31(Suppl 1):S96–101. | ||
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid Redox Signal. 2014;20(7):1126–1167. | ||
Paiva CN, Bozza MT. Are reactive oxygen species always detrimental to pathogens? Antioxid Redox Signal. 2014;20(6):1000–1037. | ||
Fang FC. Antimicrobial actions of reactive oxygen species. MBio. 2011;2(5):e00141-11–11. | ||
Kullisaar T, Türk S, Kilk K, Ausmees K, Punab M, Mändar R. Increased levels of hydrogen peroxide and nitric oxide in male partners of infertile couples. Andrology. 2013;1(6):850–858. | ||
Nossuli TO, Hayward R, Jensen D, Scalia R, Lefer AM. Mechanisms of cardioprotection by peroxynitrite in myocardial ischemia and reperfusion injury. Am J Physiol Heart Circ Physiol. 1998;275(2):H509–H519. | ||
Sehrawat S, Rouse BT. Interplay of regulatory T cell and Th17 cells during infectious diseases in humans and animals. Front Immunol. 2017;8(1723–42):341. | ||
Lawrence T. The nuclear factor NF- B pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. | ||
Tarcan T, Siroky MB, Krane RJ, Azadzoi KM. Isoprostane 8-epi PGF2alpha, a product of oxidative stress, is synthesized in the bladder and causes detrusor smooth muscle contraction. Neurourol Urodyn. 2000;19(1):43–51. | ||
Nickel JC, True LD, Krieger JN, et al. Consensus development of a histopathological classification system for chronic prostatic inflammation. BJU Int. 2001;87(9):797–805. | ||
Gandaglia G, Briganti A, Gontero P, et al. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int. 2013;112(4):432–441. | ||
Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5(11):491. | ||
Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–326. | ||
Alexander RB, Ponniah S, Hasday J, Hebel JR. Elevated levels of proinflammatory cytokines in the semen of patients with chronic prostatitis/chronic pelvic pain syndrome. Urology. 1998;52(5):744–749. | ||
Ding XG, Sw L, Zheng XM, Lq H. IFN-gamma and TGF-beta1, levels in the expressed prostatic secretions of patients with chronic abacterial prostatitis. Zhonghua Nan Ke Xue. 2006;12(11):982–984. | ||
Motrich R, Maccioni M, Molina R, et al. Presence of INFγ-secreting lymphocytes specific to prostate antigens in a group of chronic prostatitis patients. Clin Immunol. 2005;116(2):149–157. | ||
Volchegorskiıˇ IA, Tarasov NI, Seregin SP. The role of free-radical lipid oxidation in the pathogenesis of chronic prostatitis. Urol Nefrol. 1997;5:24–25. | ||
Lotti F, Tamburrino L, Marchiani S, et al. DNA fragmentation in two cytometric sperm populations: relationship with clinical and ultrasound characteristics of the male genital tract. Asian J Androl. 2017;19(3):272–279. | ||
Yy H, Cao SS, Lü JQ. Impact of chronic prostatitis/chronic pelvic pain syndrome on sperm DNA fragmentation and nucleoprotein transition. Zhonghua Nan Ke Xue. 2013;19(10):907–911. | ||
Alshahrani S, Mcgill J, Agarwal A. Prostatitis and male infertility. J Reprod Immunol. 2013;100(1):30–36. | ||
Aitken R, Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reproduction. 2001;122(4):497–506. | ||
Okin D, Medzhitov R. Evolution of Inflammatory Diseases. Curr Biol. 2012;22(17):R733–R740. | ||
Wong L, Hutson PR, Bushman W. Prostatic inflammation induces fibrosis in a mouse model of chronic bacterial infection. PLoS One. 2014;9(6):e100770. | ||
Cantiello F, Cicione A, Salonia A, et al. Periurethral fibrosis secondary to prostatic inflammation causing lower urinary tract symptoms: a Prospective Cohort Study. Urology. 2013;81(5):1018–1024. | ||
Bushman WA, Jerde TJ. The role of prostate inflammation and fibrosis in lower urinary tract symptoms. Am J Physiol Renal Physiol. 2016;311(4):F817–F821. | ||
Soric T, Selimovic M, Bakovic L, Šimurina T, Selthofer R, Dumic J. Clinical and biochemical influence of prostatic stones. Urol Int. 2017;98(4):449–455. | ||
Mazzoli S. Biofilms in chronic bacterial prostatitis (NIH-II) and in prostatic calcifications. FEMS Immunol Med Microbiol. 2010;59(3):337–344. | ||
Engelhardt PF, Seklehner S, Brustmann H, Riedl C, Lusuardi L. Tumor necrosis factor-α expression in patients with obstructive benign prostatic hyperplasia is associated with a higher incidence of asymptomatic inflammatory prostatitis NIH category IV and prostatic calcification. Scand J Urol. 2015;49(6):472–478. | ||
Murphy SF, Schaeffer AJ, Thumbikat P. Immune mediators of chronic pelvic pain syndrome. Nat Rev Urol. 2014;11(5):259–269. | ||
Motrich RD, Maccioni M, Riera CM, Rivero VE. Autoimmune prostatitis: state of the art. Scand J Immunol. 2007;66(2-3):217–227. | ||
Alexander RB, Brady F, Ponniah S. Autoimmune prostatitis: evidence of T cell reactivity with normal prostatic proteins. Urology. 1997;50(6):893–899. | ||
John H, Maake C, Barghorn A, Zbinden R, Hauri D, Joller-Jemelka HI. Immunological alterations in the ejaculate of chronic prostatitis patients: clues for autoimmunity. Andrologia. 2003;35(5):294–299. | ||
Stamatiou K, Pierris N. Mounting resistance of uropathogens to antimicrobial agents: a retrospective study in patients with chronic bacterial prostatitis relapse. Investig Clin Urol. 2017;58(4):271–280. | ||
Cai T, Mazzoli S, Meacci F, et al. Epidemiological features and resistance pattern in uropathogens isolated from chronic bacterial prostatitis. J Microbiol. 2011;49(3):448–454. | ||
Stevermer JJ, Easley SK. Treatment of prostatitis. Am Fam Phys. 2000;61(10):3015–3022. | ||
Rees J, Abrahams M, Doble A, Cooper A; the Prostatitis Expert Reference Group (PERG). Diagnosis and treatment of chronic bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: a consensus guideline. BJU Int. 2015;116(4):509–525. | ||
Meares EM. Chronic bacterial prostatitis and the problem of antibiotic diffusion. Calif Med. 1971;114(2):29. | ||
McNicholas TA, Kirby RS, Lepor H. Evaluation and nonsurgical management of benign prostatic hyperplasia. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 10th ed, Vol. 3. Philadelphia: Elsevier Saunders; 2011:2650–2651. | ||
Paubert-Braquet M, Mencia Huerta J-M, Cousse H, Braquet P. Effect of the lipidic lipidosterolic extract of Serenoa repens (Permixon®) on the ionophore A23187-stimulated production of leukotriene B4 (LTB4) from human polymorphonuclear neutrophils. Prostaglandins Leukot Essent Fatty Acids. 1997;57(3):299–304. | ||
Colado-Velázquez J, Mailloux-Salinas P, Medina-Contreras JML, Cruz-Robles D, Bravo G. Effect of Serenoa repens on oxidative stress, inflammatory and growth factors in obese wistar rats with benign prostatic hyperplasia. Phytother Res. 2015;29(10):1525–1531. | ||
Latil A, Pétrissans M-T, Rouquet J, Robert G, de La Taille A. Effects of hexanic extract of Serenoa repens (permixon® 160 mg) on inflammation biomarkers in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Prostate. 2015;75(16):1857–1867. | ||
Vela Navarrete R, Garcia Cardoso JV, Barat A, Manzarbeitia F, López Farré A. BPH and inflammation: pharmacological effects of permixon on histological and molecular inflammatory markers. Results of a double blind pilot clinical assay. Eur Urol. 2003;44(5):549–555. | ||
Morgia G, Cimino S, Favilla V, et al. Effects of Serenoa repens, selenium and lycopene (Profluss®) on chronic inflammation associated with benign prostatic hyperplasia: results of FLOG (Flogosis and Profluss in Prostatic and Genital Disease), a Multicentre Italian Study. Int Braz J Urol. 2013;39(2):214–221. | ||
Penugonda K, Lindshield B. Fatty acid and phytosterol content of commercial saw palmetto supplements. Nutrients. 2013;5(9):3617–3633. | ||
Schantz MM, Bedner M, Long SE, et al. Development of saw palmetto (Serenoa repens) fruit and extract standard reference materials. Anal Bioanal Chem. 2008;392(3):427–438. | ||
Booker A, Suter A, Krnjic A, et al. A phytochemical comparison of saw palmetto products using gas chromatography and (1) H nuclear magnetic resonance spectroscopy metabolomic profiling. J Pharm Pharmacol. 2014;66(6):811–822. | ||
Park E-J, Kim SA, Choi Y-M, et al. Capric acid inhibits NO production and STAT3 activation during LPS-induced osteoclastogenesis. PLoS One. 2011;6(11):e27739. | ||
Kim H-J, Yoon H-J, Kim S-Y, Yoon Y-R. A medium-chain fatty acid, capric acid, inhibits RANKL-induced osteoclast differentiation via the suppression of NF-κB signaling and blocks cytoskeletal organization and survival in mature osteoclasts. Mol Cells. 2014;37(8):598–604. | ||
Henry GE, Momin RA, Nair MG, Dewitt DL. Antioxidant and cyclooxygenase activities of fatty acids found in food. J Agric Food Chem. 2002;50(8):2231–2234. | ||
Hoshimoto A, Suzuki Y, Katsuno T, Nakajima H, Saito Y. Caprylic acid and medium-chain triglycerides inhibit IL-8 gene transcription in Caco-2 cells: comparison with the potent histone deacetylase inhibitor trichostatin A. Br J Pharmacol. 2002;136(2):280–286. | ||
Srivastava A, Rao LJM, Shivanandappa T. 14-Aminotetradecanoic acid exhibits antioxidant activity and ameliorates xenobiotics-induced cytotoxicity. Mol Cell Biochem. 2012;364(1-2):1–9. | ||
Karsten S, Schäfer G, Schauder P. Cytokine production and DNA synthesis by human peripheral lymphocytes in response to Palmitic, Stearic, Oleic, and Linoleic acid. J Cell Physiol. 1994;161(1):15–22. | ||
Yt O, Lee JY, Lee J, et al. Oleic acid reduces lipopolysaccharide-induced expression of iNOS and COX-2 in BV2 murine microglial cells: possible involvement of reactive oxygen species, p38 MAPK, and IKK/NF-kappaB signaling pathways. Neurosci Lett. 2009;464(2):93–97. | ||
Ambrozova G, Pekarova M, Lojek A. Effect of polyunsaturated fatty acids on the reactive oxygen and nitrogen species production by raw 264.7 macrophages. Eur J Nutr. 2010;49(3):133–139. | ||
Ren J, Chung SH. Anti-inflammatory effect of α-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-κB and mitogen-activated protein kinase pathways. J Agric Food Chem. 2007;55(13):5073–5080. | ||
Sato I, Kofujita H, Tsuda S. Identification of COX inhibitors in the hexane extract of Japanese horse chestnut (Aesculus turbinata) seeds. J Vet Med Sci. 2007;69(7):709–712. | ||
Yoshida Y, Niki E. Antioxidant effects of phytosterol and its components. J Nutr Sci Vitaminol. 2003;49(4):277–280. | ||
Vivancos M, Moreno J. β-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radical Biol Med. 2005;39(1):91–97. | ||
Olennikov DN, Zilfikarov IN, Khodakova SE. Phenolic compounds from Serenoa repens fruit. Chem Nat Compd. 2013;49(3):526–529. | ||
López-López G, Moreno L, Cogolludo A, et al. Nitric oxide (NO) scavenging and NO protecting effects of quercetin and their biological significance in vascular smooth muscle. Mol Pharmacol. 2004;65(4):851–859. | ||
Sato M, Miyazaki T, Kambe F, Maeda K, Seo H. Quercetin, a bioflavonoid, inhibits the induction of interleukin 8 and monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha in cultured human synovial cells. J Rheumatol. 1997;24(9):1680–1684. | ||
Shoskes DA, Nickel JC. Quercetin for chronic prostatitis/chronic pelvic pain syndrome. Urol Clin North Am. 2011;38(3):279–284. | ||
Shoskes DA, Zeitlin SI, Shahed A, Rajfer J. Quercetin in men with category III chronic prostatitis: a preliminary prospective, double-blind, placebo-controlled trial. Urology. 1999;54(6):960–963. | ||
Gülçin I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006;78(8):803–811. | ||
Koc A, Ozkan T, Karabay AZ, Sunguroglu A, Aktan F. Effect of L-carnitine on the synthesis of nitric oxide in RAW 264·7 murine macrophage cell line. Cell Biochem Funct. 2011;29(8):679–685. | ||
Lee B-J, Lin J-S, Lin Y-C, Lin P-T. Antiinflammatory effects of L-carnitine supplementation (1000 mg/d) in coronary artery disease patients. Nutrition. 2015;31(3):475–479. | ||
Jiang F, Zhang Z, Zhang YI, Wu J, Yu LI, Liu SU. L-carnitine ameliorates the liver inflammatory response by regulating carnitine palmitoyltransferase I-dependent PPARγ signaling. Mol Med Rep. 2016;13(2):1320–1328. | ||
Vicari E, La Vignera S, Calogero AE. Antioxidant treatment with carnitines is effective in infertile patients with prostatovesiculoepididymitis and elevated seminal leukocyte concentrations after treatment with nonsteroidal anti-inflammatory compounds. Fertil Steril. 2002;78(6):1203–1208. | ||
Wagenlehner FM, Schneider H, Ludwig M, Schnitker J, Brähler E, Weidner W. A pollen extract (Cernilton) in patients with inflammatory chronic prostatitis-chronic pelvic pain syndrome: a multicentre, randomised, prospective, double-blind, placebo-controlled phase 3 study. Eur Urol. 2009;56(3):544–551. | ||
Elist J. Effects of pollen extract preparation prostat/poltit on lower urinary tract symptoms in patients with chronic nonbacterial prostatitis/chronic pelvic pain syndrome: a randomized, double-blind, placebo-controlled study. Urology. 2006;67(1):60–63. | ||
Cai T, Verze P, La Rocca R, Anceschi U, de Nunzio C, Mirone V. The role of flower pollen extract in managing patients affected by chronic prostatitis/chronic pelvic pain syndrome: a comprehensive analysis of all published clinical trials. BMC Urol. 2017;17(1):32. | ||
Nakajima Y, Tsuruma K, Shimazawa M, Mishima S, Hara H. Comparison of bee products based on assays of antioxidant capacities. BMC Complement Altern Med. 2009;9:4. | ||
Araújo JS, Chambó ED, Costa M, Cavalcante da Silva SMP, Lopes de Carvalho CA, M Estevinho L. Chemical composition and biological activities of mono- and heterofloral bee pollen of different geographical origins. Int J Mol Sci. 2017;18(5):921. | ||
Badhani B, Sharma N, Kakkar R. Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015;5(35):27540–27557. | ||
Morgia G, Russo GI, Urzì D, et al. A phase II, randomized, single-blinded, placebo-controlled clinical trial on the efficacy of Curcumina and Calendula suppositories for the treatment of patients with chronic prostatitis/chronic pelvic pain syndrome type III. Arch Ital Urol Androl. 2017;89(2):110–113. | ||
Cai T, Mazzoli S, Bechi A, et al. Serenoa repens associated with Urtica dioica (ProstaMEV) and curcumin and quercitin (FlogMEV) extracts are able to improve the efficacy of prulifloxacin in bacterial prostatitis patients: results from a prospective randomised study. Int J Antimicrob Agents. 2009;33(6):549–553. | ||
Ak T, Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174(1):27–37. | ||
Naksuriya O, Okonogi S. Comparison and combination effects on antioxidant power of curcumin with gallic acid, ascorbic acid, and xanthone. Drug Discov Ther. 2015;9(2):136–141. | ||
Fujisawa S, Atsumi T, Ishihara M, Kadoma Y, Cytotoxicity KY. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer Res. 2004;24(2B):563–569. | ||
Zhang QY, Mo ZN, Liu XD. Reducing effect of curcumin on expressions of TNF-alpha, IL-6 and IL-8 in rats with chronic nonbacterial prostatitis. Zhonghua Nan Ke Xue. 2010;16(1):84–88. | ||
Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14(2):141–153. | ||
Olivera A, Moore TW, Hu F, et al. Inhibition of the NF-κB signaling pathway by the curcumin analog, 3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31): anti-inflammatory and anti-cancer properties. Int Immunopharmacol. 2012;12(2):368–377. | ||
Song K, Peng S, Sun Z, Li H, Yang R. Curcumin suppresses TGF-β signaling by inhibition of TGIF degradation in scleroderma fibroblasts. Biochem Biophys Res Commun. 2011;411(4):821–825. | ||
Kumari A, Dash D, Singh R. Curcumin inhibits lipopolysaccharide (LPS)-induced endotoxemia and airway inflammation through modulation of sequential release of inflammatory mediators (TNF-α and TGF-β1) in murine model. Inflammopharmacology. 2017;25(3):329–341. | ||
Kumar P, Kadakol A, Shasthrula PK, et al. Curcumin as an adjuvant to breast cancer treatment. Anticancer Agents Med Chem. 2015;15(5):647–656. | ||
Teiten MH, Gaascht F, Eifes S, Dicato M, Diederich M. Chemopreventive potential of curcumin in prostate cancer. Genes Nutr. 2010;5(1):61–74. | ||
Gharaee-Kermani M, Moore BB, Macoska JA. Resveratrol-mediated repression and reversion of prostatic myofibroblast phenoconversion. PLoS One. 2016;11(7):e0158357. | ||
He Y, Zeng H, Yu Y, et al. Resveratrol improved the progression of chronic prostatitis via the downregulation of c-kit/SCF by activating Sirt1. J Agric Food Chem. 2017;65(28):5668–5673. | ||
He Y, Zeng HZ, Yu Y, et al. Resveratrol improves prostate fibrosis during progression of urinary dysfunction in chronic prostatitis. Environ Toxicol Pharmacol. 2017;54:120–124. | ||
Zeng H, He Y, Yu Y, et al. Resveratrol improves prostate fibrosis during progression of urinary dysfunction in chronic prostatitis by mast cell suppression. Mol Med Rep. 2018;17(1):918–924. | ||
Lee CB, Ha US, Lee SJ, Kim SW, Cho YH. Preliminary experience with a terpene mixture versus ibuprofen for treatment of category III chronic prostatitis/chronic pelvic pain syndrome. World J Urol. 2006;24(1):55–60. | ||
Kim DS, Lee HJ, Jeon YD, et al. Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am J Chin Med. 2015;43(4):731–742. | ||
Zhou JY, Tang FD, Mao GG, Bian RL. Effect of alpha-pinene on nuclear translocation of NF-kappa B in THP-1 cells. Acta Pharmacol Sin. 2004;25(4):480–484. | ||
Juergens UR. Anti-inflammatory properties of the monoterpene 1.8-cineole: current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014;64(12):638–646. | ||
de Cássia da Silveira E Sá R, Andrade LN, de Sousa DP. A review on anti-inflammatory activity of monoterpenes. Molecules. 2013;18(1):1227–1254. | ||
Ocaña A, Reglero G. Effects of thyme extract oils (from Thymus vulgaris, Thymus zygis, and Thymus hyemalis) on cytokine production and gene expression of oxLDL-stimulated THP-1-macrophages. J Obes. 2012;2012:104706–11. | ||
Kang P, Kim KY, Lee HS, Min SS, Seol GH. Anti-inflammatory effects of anethole in lipopolysaccharide-induced acute lung injury in mice. Life Sci. 2013;93(24):955–961. | ||
Steenkamp V, Gouws MC, Gulumian M, Elgorashi EE, van Staden J. Studies on antibacterial, anti-inflammatory and antioxidant activity of herbal remedies used in the treatment of benign prostatic hyperplasia and prostatitis. J Ethnopharmacol. 2006;103(1):71–75. | ||
Hevesi Tóth B, Blazics B, Kéry A. Polyphenol composition and antioxidant capacity of Epilobium species. J Pharm Biomed Anal. 2009;49(1):26–31. | ||
Kiss AK, Bazylko A, Filipek A, et al. Oenothein B’s contribution to the anti-inflammatory and antioxidant activity of Epilobium sp. Phytomedicine. 2011;18(7):557–560. | ||
Stolarczyk M, Piwowarski JP, Granica S, Stefan´ska J, Naruszewicz M, Kiss AK. Extracts from Epilobium sp. herbs, their components and gut microbiota metabolites of Epilobium ellagitannins, urolithins, inhibit hormone-dependent prostate cancer cells-(LNCaP) proliferation and PSA secretion. Phytother Res. 2013;27(12):1842–1848. | ||
Yoshida T, Yoshimura M, Amakura Y. Chemical and biological significance of oenothein B and related ellagitannin oligomers with macrocyclic structure. Molecules. 2018;23(3):552. | ||
Kiss A, Kowalski J, Melzig MF. Effect of Epilobium angustifolium L. extracts and polyphenols on cell proliferation and neutral endopeptidase activity in selected cell lines. Pharmazie. 2006;61(1):66–69. | ||
Wang LL, Huang YH, Yan CY, et al. N-acetylcysteine ameliorates prostatitis via miR-141 regulating Keap1/Nrf2 signaling. Inflammation. 2016;39(2):938–947. | ||
Pan X, Wu X, Yan D, Peng C, Rao C, Yan H. Acrylamide-induced oxidative stress and inflammatory response are alleviated by N-acetylcysteine in PC12 cells: involvement of the crosstalk between Nrf2 and NF-κB pathways regulated by MAPKs. Toxicol Lett. 2018;288:55–64. | ||
Supabphol A, Supabphol R. Antimetastatic potential of N-acetylcysteine on human prostate cancer cells. J Med Assoc Thai. 2012;95(Suppl 12):S56–S62. | ||
Lee YJ, Lee DM, Lee CH, et al. Suppression of human prostate cancer PC-3 cell growth by N-acetylcysteine involves over-expression of Cyr61. Toxicol In Vitro. 2011;25(1):199–205. | ||
Belikov AV, Schraven B, Simeoni L. T cells and reactive oxygen species. J Biomed Sci. 2015;22:85. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.