Back to Journals » Journal of Blood Medicine » Volume 13
Influence of α2-Macroglobulin, Anti-Parasite IgM and ABO Blood Group on Rosetting in Plasmodium falciparum Clinical Isolates and Their Associations with Disease Severity in a Ghanaian Population
Authors Bandoh B, Kyei-Baafour E , Aculley B, van der Puije W , Tornyigah B, Akyea-Mensah K, Hviid L, Ngala RA , Frempong MT, Ofori MF
Received 24 August 2021
Accepted for publication 7 March 2022
Published 18 March 2022 Volume 2022:13 Pages 151—164
DOI https://doi.org/10.2147/JBM.S329177
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Martin H Bluth
Betty Bandoh,1,2 Eric Kyei-Baafour,2 Belinda Aculley,2 William van der Puije,2 Bernard Tornyigah,2 Kwadwo Akyea-Mensah,2 Lars Hviid,3,4 Robert A Ngala,1 Margaret T Frempong,1 Michael F Ofori2
1Department of Molecular Medicine, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana; 2Department of Immunology, Noguchi Memorial Institute for Medical Research, College of Health Sciences, University of Ghana, Accra, Ghana; 3Department of Immunology and Microbiology, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; 4Department of Infectious Diseases, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark
Correspondence: Michael F Ofori, Department of Immunology, Noguchi Memorial Institute for Medical Research, College of Health Sciences, University of Ghana, Post Office Box LG581, Legon, Accra, Ghana, Tel +233 244 715975, Fax +233 302 502182, Email [email protected]
Purpose: The severity of Plasmodium falciparum infections is associated with the ability of the infected red blood cells to cytoadhere to host vascular endothelial surfaces and to uninfected RBCs. Host blood group antigens and two serum proteins α2-macroglobulin (α2M) and IgM have been implicated in rosette formation in laboratory-adapted P. falciparum. However, there is only limited information about these phenotypes in clinical isolates.
Methods: This was a hospital-based study involving children under 12 years-of-age reporting to the Hohoe Municipal Hospital with different clinical presentations of malaria. Parasite isolates were grown and rosette capabilities and characteristics were investigated by fluorescence microscopy. α2M and IgM were detected by ELISA.
Results: Rosette formation was observed in 46.8% (75/160) of the parasite isolates from all the blood groups tested. Rosettes were more prevalent (55%) among isolates from patients with severe malaria compared to isolates from patients with uncomplicated malaria (45%). Rosette prevalence was highest (30%) among patients with blood group O (30%) and B (29%), while the mean rosette frequency was higher in isolates from patients with blood group A (28.7). Rosette formation correlated negatively with age (r = − 0.09, P= 0.008). Participants with severe malaria had a lower IgM concentration (3.683± 3.553) than those with uncomplicated malaria (5.256± 4.294) and the difference was significant (P= 0.0228). The mean concentrations of anti-parasite IgM measured among the clinical isolates which formed rosettes was lower (4.2 ± 3.930 mg/mL), than that in the non rosetting clinical isolates (4.604 ± 4.159 mg/mL) but the difference was not significant (P=0.2733). There was no significant difference in plasma α2M concentration between rosetting and non rosetting isolates (P=0.442).
Conclusion: P. falciparum parasite rosette formation was affected by blood group type and plasma concentration of IgM. A lower IgM concentration was associated with severe malaria whilst a higher α2M concentration was associated with uncomplicated malaria.
Keywords: Plasmodium falciparum, severe malaria, uncomplicated malaria, rosettes, blood group, alpha-2 macroglobulin, immunoglobulin M
Introduction
Despite the recent decline in P. falciparum infections globally, malaria continues to affect the African continent with most of the disease burden and mortalities occurring in this region.1 Case incidence of malaria among children under 5 years-of-age was estimated to be 225/1000 individuals at risk in 2019, which was a slight decline from the year 2000 estimate. Malaria deaths in children less than 5 years declined between year 2000 and 2019 by 17% worldwide.1 Plasmodium falciparum continues to be the dominant species in the sub-region, where the climate favors the Anopheles vector.2 P. falciparum is virulent and is responsible for the majority of malaria mortality and severe morbidity.1,3
The pathogenesis of P. falciparum malaria is directly linked to the ability of the parasite to express variant surface antigens (VSA) on the surface of IRBCs, which allows the parasites to establish long-lasting chronic infection.4 Since the publication of the full genome of P. falciparum three major families of variant genes have been studied extensively.5,6 The proteins expressed by these variant genes include Erythrocyte Membrane Protein 1 (PfEMP1); RIFIN (Repetitive Interspersed family of polypeptides) proteins; STEVOR (Sub telomeric variable open reading frame) proteins.7 In P. falciparum infection, the VSA expressed is mainly for evading the host immune responses by allowing IRBCs to sequester in tissues, thereby avoiding their removal by the spleen.8,9 These parasite ligands (RIFINs, STEVOR, PfEMP1) have all been studies and known to be released onto IRBC at specific times in the parasite life cycle. PfEMP1 is transcribed at the ring stage and exported to the surface of the IRBC at the late trophozoite stage whilst RIFINs and STEVORs are transcribed and expressed later in the pigmented trophozoite and schizont stages.10–12
The ability of the IRBC to attach to uninfected RBCs is another binding process, known as rosetting, which has been found to be associated with severe malaria in African children.13–15 Some host factors including complement receptor 1 (CR1), heparan sulphate (HS), blood group antigens and recently host serum proteins such immunoglobulin M (IgM) IgM and α2M have been identified to be associated with rosette formation.15–17 For instance, polymorphism in CR1, thalassemia and red blood cells such as the AS trait has been shown to confer protection against severe malaria.18–20 Another important biological role of PfEMP1 is their affinity for binding to blood group A and B antigens, which has also been linked to severe malaria.21 Various studies have shown the involvement of IgM as one of the serum proteins involved in the attachment of rosette formation.22,23 Whilst IgM remains the largest and first immunoglobulin to appear in response to malaria antigens, the structure of IgM and its pentameric nature make it an effective immune effector molecule.16,24 Previous studies have implicated serum IgM in rosette formation in children and their contribution to the development of severe malaria.15,25
Another serum protein identified to be involved in rosette formation is α2M, which is a non-immunoglobulin molecule and one of the most abundant proteins found in peripheral blood circulation in humans.16,25 α2M is a protease inhibitor, which is produced by the liver and locally synthesised by macrophages, adrenocortical cells and fibroblast and part of the innate immune system.26 In humans, α2M has been demonstrated to play an important role in maintaining homeostasis of cytokines and growth factors.27 Increased α2M plasma levels have consistently been seen during periods of life characterized by growth, development, and differentiation such as embryogenesis, pregnancy, and childhood.26,28
Laboratory-adapted parasites strains16 and patient parasite isolates25 have previously been used to show that serum α2M works in synergy with IgM to increase avidity of binding multiple PfEMP1 molecules on the IRBC surface to mediate rosetting. This study seeks to investigate further the associations of anti-parasite IgM and α2M plasma concentrations, as well as the effect of ABO blood group types on rosette formation among field isolates and their association with severity of malaria infection.
Materials and Methods
Ethical Statement
Ethical clearance for this study was obtained from by the Institutional Review Board of the Noguchi Memorial Institute for Medical Research, Ghana (Study number 026/13-14) and the Ethical Review Committee of the Ghana Health Service (MOH; file GHS-ERC 08/05/14).
The study complied with the Helsinki declaration for conducting research involving human subjects. Written informed consent was obtained from each parent/guardian of all the study participants.
Study Design and Population
The study was cross-sectional and involved 206 children less than 12 years of age who were admitted to or attended the outpatient department (OPD) of the Hohoe Municipal Hospital with different malaria pathologies (uncomplicated malaria and severe malaria). In this study, severe malaria was defined as a child presenting with signs and symptoms of malaria and in the presence of P. falciparum asexual parasitaemia with one or more of either impaired consciousness (Blantyre coma score of <3), acidosis, hypoglycaemia, hyperparasitaemia, renal impairment (acute kidney injury), severe malarial anaemia (haemoglobin concentration of <5 g/dl), Jaundice and others as defined by the Physician in-Charge. Uncomplicated malaria was also defined as children presented with both microscopy and RDT positive for malaria but with no features of severe malaria.
Rosetting is defined as the binding of uninfected red blood cells to a centrally Plasmodium falciparum infected red blood cell. Different categories of resetting include small rosette defined as the binding of an infected RBC by 2 to 3 uninfected RBCs. A medium rosette is a Plasmodium falciparum infected RBC bound to 4 or 5 uninfected RBCs. A large rosette is an infected RBC bound by more than 5 uninfected RBCs. In this study, rosette frequency is defined as the percentage of total mature-parasite-infected cells forming rosettes against two hundred cells infected with matured parasites whilst rosette prevalence referred to proportion of the total parasite isolates collected throughout the study period that formed rosettes.
Sample Collection
In brief, the study was explained to the parents/guardians after which informed consent was sought from them. Approximately 3 mL of whole blood was collected after rapid diagnostic test (RDT) gave a positive result. In addition, the presence of parasites when viewed under a light microscopy confirmed malaria infection and a PCR test was used to confirm the Plasmodium falciparum malaria cases and differentiation of species of mixed infection cases. Haemoglobin levels were measured using automated haematology analyser (Sysmex KX-21N) and blood groups determined using standard protocols as described elsewhere.29 The blood samples were centrifuged and separated into infected red blood cells and plasma. The plasma was stored in a −80°C freezer until ready to be used for the measurement of IgM and α2M levels by ELISA. Approximately, 200uL of the patients’ pellet RBC was e used for short term in vitro cultures in the patients’ own blood and the rest stored in liquid nitrogen for long term storage.
ABO and Rhesus Blood Grouping
Approximately three drops (20 µL) of whole blood were put on a clean white tile. The blood was individually mixed with an equal volume of anti-A, anti-B or anti-D (Fortress Diagnostic Limited, UK.) after which, the tile was rocked gently for 5–10 minutes. The blood drops were observed for agglutination. The observation of agglutination in the blood drops indicated the presence of rhesus (Rh) antigen corresponding to the anti-sera used. Lack of agglutination for both A and B indicated the blood group O.
Parasitemia Estimation by Light Microscopy
Thick and thin blood smears were prepared on microscopic glass slide for all RDT confirmed malaria positive samples. The thick blood smear was prepared by placing a drop of blood on the slide and spreading it out evenly in a circular motion. On the same slide, a thin blood smear was also prepared by placing a drop of blood on the slide and using another slide held at 45 degrees to the drop on the first slide. The thin film side was immersed in methanol (VWR, USA) to fix the cells and dried whilst the thick film was only air dried. The dried slides were stained with 10% Giemsa stain (Merck, Germany) for 15 minutes. Slides were washed under running water and air dried. A drop of immersion oil (Sigma Aldrich, USA) was put on the slide and observed under a light microscope at 100X objective lens. The parasitaemia was calculated as the number of parasites divided by the number of white blood cells all multiplied by 8000 and the species differentiation was done to confirm that sample was positive for P. falciparum.
Short Term in vitro Cultivation of Fresh Plasmodium falciparum Clinical Isolates
The Plasmodium parasite isolates were maintained in short term culture (one cycle) in the erythrocytes of the participants with RPMI-1640 medium supplemented with 1% L-glutamine, (Sigma Aldrich, USA, Cat no. R8758) (50 µg/m gentamycin (Thermo Fisher, Cat no. 15750060), 50 mg/L hypoxanthine (Sigma Aldrich, UK, H9377), and 10% heat-inactivated type AB+ human serum (Sigma Aldrich, USA, Cat no. H6914). Cultures were incubated at 37 °C with a gas mixture of 5% O2, 2% CO2, balanced with N2 (Air Liquide, UK, Cat No. 4687). The parasites were maintained until the late trophozoite and schizont stage when the rosette capability was assessed using a fluorescent microscope.
Rosette Formation Analysis by Fluorescence Microscopy
Rosetting was assessed in the first cycle of the in vitro growth when parasites reached late trophozoite stages. In brief, aliquot of 100 ul of the parasite culture was picked from the 5mL culture flask into a 1.5mL tube followed by the addition of 2ul of 25ug/mL ethidium bromide and incubated for 15 minutes at 37°C. About 10uL of the ethidium bromide-stained parasites were pipetted onto a fluorescence microscope slide and covered with a coverslip and read under fluorescence and at X40 magnification using Olympus BX 43 (Olympus, Japan). Rosettes were counted per 200 infected red blood cells and images recorded. Isolates rosettes were then categorized into small, medium and large based on the number of uninfected RBCs bound to a centrally IRBC. In isolates with different sized rosettes, the predominant rosette type is used to categorise it.
Measurement of Alpha-2-Macroglobulin Concentration Using ELISA
The plasma concentration of α2M was measured using a commercial ELISA kit (RAB 0600: Sigma Aldrich) following the manufacturer’s instructions and protocols. In brief, samples (diluted at 1:2000), controls, and standards were added to pre-coated plates at 200 µL/well. Plates were incubated for two and half hours at room temperature with gentle shaking at 200rmp. Plates were washed with 300uL of wash buffer and detection antibody was added at 100 µL/well and incubated for one hour at RT. Streptavidin solution was added at 100µL/well and incubated for 45 minutes. The plates were developed with 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate reagent at 100 µL/well for 30 minutes in the dark. Reaction was stopped with 50 µL of Stop solution and plate read immediately at 450 nm.
Measurement of Plasma Anti-Parasite IgM Concentrations Using ELISA
The plasma concentration of anti-parasite IgM was measured using indirect ELISA technique. High binding 96 - well ELISA plates (Nunc Maxisorp, Thermo Fisher Scientific) were coated with soluble crude malaria antigen (0.5 X 106 schizonts/ well) at 50ul/well and incubated at 4°C overnight. Plates were retrieved and washed 3X with 200ul/well of washing buffer (PBST; 1X PBS supplemented with 0.05% Tween 20 at pH 7.2) and blocked with 200ul per well blocking buffer (5% non-fat dry milk (Marvel, UK) in PBS) and incubated for 1 hour at room temperature on a shaker (150rpm). Plasma and controls were added at 50ul/well diluted 100X in assay buffer (1% milk/PBS). Positive control (pool of hyperimmune Ghanaian adults) and negative controls (pool of unexposed Europeans) were added alongside the samples in their respective wells for each plate. The plates were incubated for 1 hour at room temperature. Anti-parasite IgM was detected using 100ul/well of goat anti-human IgM conjugated Horseradish Peroxidase (HRP-IgM) (Invitrogen, USA) at a dilution of 1:1000 for 1 hour. Plates were developed with 50L/well TMB for 10 minutes and stopped with 50ul/well of 0.2M H2SO4. The optical density was read at 450nm Biotek plate reader (VT, USA) and converted to concentrations using ADAMSEL (version b040; Ed Remarque).
Statistical Analysis
Spearman rank correlation was performed to analyse the associations between the clinical variables and the concentration of anti-parasite IgM or α2M. Clinical isolates were stratified as rosetting and non rosetting and differences between the clinical variables were compared by students t-test and Mann Whitney test where appropriate. A linear regression analysis was used to determine the relationship between host variables and rosette formation. P<0.05 were considered statistically significant. All the data collected was analysed using Graph pad prism 5.0 (Graph pad Software, Inc).
Results
Clinical Characteristics of Study Population
A total of 230 children were enrolled into the study over a two-year period. In the first year, 116 participants were recruited whilst the second year had 114. At the end of recruitment, the data base was cleaned to exclude all participants who were positive by RDT but had no parasites present in their sample after light microscopy. Most of them had taken medication before hospital visit and their in vitro cultures were not successful. Based on our criteria for participant enrollment, malaria cases were carefully classified into either severe or uncomplicated. There were more males (110) than females (96) in the study and the mean age of the children was 5.0 years (7 months −11 years). The mean haemoglobin level was 9.3g/dL (3.6–13.9g/dL) for the general population. When this was stratified, anaemia was seen in 90 (43.6%) whilst severe anaemia was seen in 4 (1.9%) of the study participant. In 52 (25%) of the participants, parasite density (parasitemia) was above 100,000 parasites /ul. Most of the participants were of blood group O type (94/206). The clinical characteristics of participants with severe cases was compared to those with uncomplicated cases. The mean age for the uncomplicated malaria was 5 years whilst for the severe cases it was 3.8 years. Haemoglobin level was lower in the severe cases (8.4g/dL) than the uncomplicated (10.3g/dl). There was no significant difference in the mean parasitemia between the uncomplicated and the severe cases (P = 0.31). Patients with severe disease had a lower body weight than those with uncomplicated malaria (Table 1).
 |
Table 1 Clinical Characteristics of Study Participants |
Rosette Capabilities Among P. falciparum Clinical Isolates
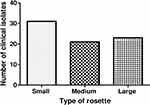
Rosetting assays were run on parasite isolates when they were at the trophozoite stage at a parasitemia of >2%. Infected RBC are shown as bright spots not bound by uninfected RBCs (Figure 1A). Rosettes are shown as IRBCs (bright spot) bound by two or more uninfected RBCs as observed under the fluorescence microscope (Figure 1B). Rosettes were categorized as small (Figure 1C), medium (Figure 1D) or large, defined by the number of uninfected RBCs bound to the IRBC. Out of the 206 parasite isolates put into short term culture, 160 were successful and were used for the rosetting assay. Rosette formation was observed in 75 (46.8%) of the clinical isolates comprising 42 from the severe malaria category and 33 from the uncomplicated malaria category but the difference in rosette formation capabilities was not statistically significant (P=0.3). Rosetting rate of 56% (42/75) among parasites from severe disease cases was higher than those found among uncomplicated malaria cases (44%, 33/75). Majority (30) of the samples had large rosettes followed by those with small rosettes (24) with 21 of them forming medium rosettes (Figure 2).
Interestingly, when the rosettes were categorized according to the source of the parasite isolate, whether from severe malaria or uncomplicated malaria, it was observed that small rosettes were from parasites collected from severe malaria patients and the difference was statistically significant (P=0.015) (Table 2).
 |
Table 2 Rosette Categorization in Severe and Uncomplicated Malaria Isolates |
Rosette Formation Was Observed in All Blood Group Types in Different Proportions
Rosette formation was observed in all blood groups with rosettes frequently seen in isolates from blood group O followed by blood group B type. Rosetting frequency was highest in parasite isolates from blood group A. There was no statistical difference in mean rosetting frequency among the blood groups (P=0.5). Rosette characteristics were different among the blood groups.
Large rosettes (bound by >5 uninfected RBC) were seen in blood group types A and B whilst group O showed smaller rosettes (Figure 3).
Rosetting Capabilities of P. falciparum Clinical Isolates from Different Disease Categories in Different Blood Group Types
Rosette formation was seen in all four blood group types in different proportions, but the number of rosettes did not differ significantly between disease categories (Figure 4). The chance of finding a rosette (rosette prevalence) among the clinical isolate was higher in blood group O type (30.1%) and blood group B type (29.8%). On average, blood group A had the highest mean frequency (rosettes per 200 IRBC) of 28.7 rosettes per isolate as compared to 16.7 rosettes per isolate observed among blood group O type isolates. The highest number of rosettes per isolate was recorded in blood group B followed by A with the least in blood group AB. Generally, there was rosette formation among parasites from all disease categories grown in all blood group types with the only exception being among blood group AB where resetting was not observed in the severe malaria group (Figure 4).
Host Factors That Affect Rosette Formation
When the measured clinical variables were analysed between rosetting and non rosetting clinical isolates, it was observed that parasitemia was higher among rosetting isolates compare to non rosetting isolates (P=0.002) (Table 3). The age of the participant was not associated with rosetting although resetting was commonly found among parasite isolates from younger children than parasites from older children (p<0.9) (Table 4). The relationship between rosette formation and measured parameters were assessed using a linear regression analysis.
 |
Table 3 Effect of Patient Variables on Rosetting and Non Rosetting Isolates |
 |
Table 4 Association Between Patients’ Parameters and Plasma Levels of α2M Concentration |
Parasitemia and Rosette Formation
When the measured clinical variables were analysed between rosetting and non rosetting clinical isolates, it was observed that parasitemia was higher among rosetting isolates as compare to non rosetting isolates (P=0.002) (Table 3).
Association of Plasma Concentrations of α2M on Rosette Formation and Malaria Severity
The α2M concentration weakly correlated positively with rosette frequencies (r=0.080 but the difference was not significant (p=0.52) (Figure 5A). Although α2M concentration was found to be higher in uncomplicated malaria (mean = 2.27 mg/mL) than in severe malaria (mean=1.92 mg/mL), (P = 0.2), the difference was not statistically significant. A significantly higher α2M concentration was observed in patients with a higher parasitemia (P= 0.02) (Table 4).
Also, in correlation analysis of non resetting parasite isolates, only parasitemia was found to correlate with α2M concentration (P= 0.04) whilst age and hemoglobin levels did not show any significant association (Table 5).
 |
Table 5 Association of α2M Concentration on Non Rosetting Isolates |
To determine the effect of α2M concentration on rosette formation, the mean plasma concentration of α2M was compared between rosette formation by isolates from severe and uncomplicated malaria patients. It was observed that the mean concentration of rosetting isolates [2.08 mg/mL (0.3mg/mL - 5.4mg/mL)] was similar to that of non rosetting isolates [2.02mg/mL (0.3mg/mL - 4.9mg/mL)] (P=0.3534) (Figure S1).
Association of Plasma Concentration of Anti-Parasite IgM on Rosette Formation and Disease Severity
The anti-parasite IgM concentrations did not correlate with rosette frequencies among all the isolates studied (Figure 5B) although a weak negative correlation was observed it was not statistically significant (r=−0.06, p=0.61). Also, the mean plasma concentration of anti-parasite IgM among the study population was 4.5 mg/mL (±4.0). Males had higher mean anti-parasite IgM levels (4.6±4.4 mg/mL) compared to the females (4.2±3.7 mg/mL), P<0.05. Participants with severe malaria had a lower mean anti-parasite IgM concentration (3.7±3.6mg/mL) as compared to those with uncomplicated malaria (5.3±4.3) and the difference was statistically significant (P=0.02) (Figure S2).
The mean plasma concentration of anti-parasite IgM measured among patients with rosetting forming isolates was lower at 4.2 (±3.930) as compared to the mean anti-parasite IgM concentration of (4.604 (±4.159) from patients whose parasite isolates did not form rosettes.
In correlation analysis, among the rosetting clinical isolates, anti-parasite IgM concentration was affected by only haemoglobin levels (P=0.02) whilst all the other variables were not significantly affected by the anti-parasite IgM concentration (Table 6).
 |
Table 6 Association of Anti-Parasite IgM Concentration on Clinical Variables Among Rosetting Parasite Isolates |
Discussion
Studies have shown that among patients, rosetting rate is dependent on the disease category of either severe or uncomplicated and that among severe malaria, typically therefore, rosette formation is more pronounced in severe malaria than in uncomplicated malaria cases. In this study, rosette formation was observed in 46.8% (75/160) of the parasite isolates tested. This level of rosetting is lower as compared to higher rosetting rates found in other studies,13 but not unexpected as this could be as a result of the relatively fewer number of isolates obtained from the participants with severe malaria in the study. Even so, rosetting rate was higher (56%) among the severe malaria cases compared to the uncomplicated (44%). The difference was however not statistically significant. This agrees with other studies which showed that among severe malaria cases, there was higher rate of rosetting compared to uncomplicated malaria, though rosette formation was found in all categories of disease in different proportions.13,25,30,31
A number of reasons could account for the lower rosetting rate observed among the parasite isolates. Barragan et al showed that among children from Kenya and Gabon, repeated malaria attacks in high malaria transmission areas resulted in exposure to antigens which prompted an antibody mediated response that recognises many rosetting antigens.32 These anti-rosetting antibodies produced in response to repeated exposure to parasite ligand has been linked to a reduced rosetting rates and gradual acquisition of immunity in children.33 In support of this, others found high levels of antibodies to a parasite ligand (NTS-DBL1α) known to be associated with rosetting in children in malaria endemic areas34,35 which resulted in a reduced level of rosetting in the clinical isolates from that endemic community.
Parasite ligand PfEMP1 being expressed by var genes have the ability to produce clonally different phenotypes of the genes (antigenic variation) with different binding affinities that protect the parasite from immune clearance.36 Other factors such as host immunity, genetics and polymorphisms in certain host factors may be involved in the development of severe malaria aside rosetting.37
Rosette formation was enhanced by blood group A antigens and the age of the child. This is consistent with a study done elsewhere which supports the high frequency of rosetting found in isolates from blood group A type.21 Though rosette formation was prevalent in groups O and B, it was blood group A isolates that produced the largest rosettes supporting similar findings in other studies.21 Remarkably, small rosettes were associated with severe disease compared to large ones but this is contrary to other studies where larger rosettes were found to be associated with severe malaria.14,38 The dominant blood group in our study area was blood group O and this blood group has been associated with formation of small sized rosette and in essence protect against severe disease.39
Parasitemia also affected rosette formation and there is conflicting data on the effect of parasitemia on rosetting among African children. There is conflicting data on the effect of parasitemia on rosetting among African children. Whilst some studies found positive correlation between parasitemia and rosetting,14,40,41 other studies show no association of rosette formation with high parasitemia.42,43 Our findings agree with studies by Marsh et al, as well as the Heddini and later Rowe which showed a positive correlation between parasitemia (parasite densities) and rosetting in African isolates.13,41,42 A previous study conducted in India which investigated DBL-α (parasite ligand that support rosetting) diversity in severe disease in Odisha population showed significantly higher parasitemia among rosetting isolates.44 These findings implicate rosette formation to contribute to elevated parasite densities that favor invasion of the host cells from bursting schizont.14 Therefore, the elevated parasitemia seen among the rosetting isolate is probably advantageous to ensuring the survival and replication of the parasite.12,45
Our study showed a lower anti-parasite IgM concentration among rosetting isolates and this was positively correlated with rosette frequency consistent with findings by others that showed a positive association between rosette frequency and IgM concentrations in Kenyan children.15,46 Conversely, higher anti-parasite IgM concentrations were found among patients whose parasite isolate did not form rosettes and this supports recent findings that show that high IgM concentrations among children in endemic areas is protective against severe malaria.16,47,48
A higher mean α2M serum concentration was found among uncomplicated malaria patients than severe malaria patients, who had a lower mean α2M concentration. Similar observations were made in a study in Thailand where higher α2M concentrations were found in women with malaria parasites compared to women who did not have parasites.49 Increased α2M concentration has also been shown in children suffering from “chagasis” (Trypanosoma cruzi infection) in Bolivia.50
A high α2M concentration among the non rosetting isolates contradicts findings elsewhere which showed otherwise.16 However, α2M concentration was positively associated with parasitemia among non rosetting isolates only, a phenomenon not observed among the rosetting isolates studied. During malaria infections, merozoites use proteolytic enzymes in re-invading red blood cells whilst schizont degradation of hemoglobin involves the use of these same proteolytic enzymes. Therefore, α2M is produced by the body in response to high proteolytic enzymes to binds irreversibly to these proteolytic enzymes and protecting the tissues from tissue damage.51 The findings also shows that α2M may elicit high parasitemia in non rosetting isolates and therefore α2M may be used as a biological marker for high parasitemia in malaria infected individuals.
Parasite isolates from patients with high α2M concentrations had more rosettes than those from patients with low α2M concentration. This confirms the role of α2M in enhancing rosette formation by increasing the avidity of binding of PfEMP1 molecules on the surface of the IRBC consistent with others studies.16,25
This may confirm the role of this serum protein in supporting high parasite growth in lieu of severe disease. Increases in α2M concentration has been documented in diseases that result in high plasma and tissue proteinase levels such as cystic fibrosis rheumatoid arthritis and pancreatitis.50 During Plasmodium infections, are used in the rapturing and re-invading of red blood cells and this process generates high amounts of proteinases that effectively trigger high production of α2M to remove the excess proteinases.51
Conclusion
This study has shown that rosette formation in P. falciparum clinical isolates is affected by the blood group type of the patient with isolates from blood group A type producing the highest number of rosettes per isolates.
A low IgM concentration was associated with severe disease whilst a high α2M concentration was associated with uncomplicated malaria. In relation to disease outcome, blood group A type and low anti-parasite IgM concentration increased the risk of severe malaria among participants.
Abbreviations
α2M, alpha 2 macroglobulin; CR1, complement receptor 1; ELISA, enzyme linked immunosorbent assay; ERC, ethical review committee; GHS, Ghana health service; HRP, horse radish peroxidase; HS, heparan sulphate; IgM, immunoglobulin M; IRBC, infected red blood cells; MOH, ministry of health; NHS, normal human serum; OD, optical density; OPD, out patient department; PBS, phosphate buffered saline; PBST, phosphate buffered saline 0.1% Tween 20 detergent; PfEMP1, plasmodium falciparum erythrocyte membrane protein 1; RBC, red blood cells; RDT, rapid diagnostic test; RIFIN, P. falciparum-encoded repetitive interspersed families of polypeptides; RPMI, Roswell Parks Memorial Institute; SM, severe malaria; STEVOR, subtelomeric variable open reading frame; TMB, 3,3′,5,5′-tetramethylbenzidine; UM, uncomplicated malaria; VSA, variant surface antigens.
Data Sharing Statement
The datasets analysed in this study are available from the corresponding author on reasonable request.
Acknowledgments
The authors are grateful to the parents and guardians of the children involved in the study. Special thanks also go to the management and staff of the Hohoe Municipal Hospital as well as the staff of the Immunology Department, NMIMR. Mr Alex Danso-Coffie is thanked for his logistic and technical support.
Author Contributions
All authors made significant contribution to the work, whether in the conception, study design, implementation, acquisition of data, analysis or interpretation. All the authors were involved in the drafting, revising and critical reviewing of the article. They all agreed on the journal to which the article has been submitted and agreed to be accountable for all aspects of the work.
Funding
Michael F. Ofori and Lars Hviid are supported by the Danish Research Council for Development Research (Grant No. 17-02-KU). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global trends in the burden of Malaria; 2020.
2. Molina-Cruz A, Zilversmit MM, Neafsey DE, Hartl DL, Barillas-Mury C. Mosquito vectors and the globalization of Plasmodium falciparum Malaria. Annu Rev Genet. 2016;50(1):447–465. doi:10.1146/annurev-genet-120215-035211
3. Rasti N, Wahlgren M, Chen Q. Molecular aspects of malaria pathogenesis. FEMS Immunol Med Microbiol. 2004;41(1):9–26. doi:10.1016/j.femsim.2004.01.010
4. Kirchgatter K, Del Portillo HA. Clinical and molecular aspects of severe malaria. An Acad Bras Cienc. 2005;77(3):455–475. doi:10.1590/s0001-37652005000300008
5. Gardner MJ, Hall N, Fung E, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419(6906):498–511. doi:10.1038/nature01097
6. Petter M, Haeggström M, Khattab A, et al. Variant proteins of the Plasmodium falciparum RIFIN family show distinct subcellular localization and developmental expression patterns. Mol Biochem Parasitol. 2007;156(1):51–61. doi:10.1016/j.molbiopara.2007.07.011
7. Wahlgren M, Goel S, Akhouri RR. Variant surface antigens of Plasmodium falciparum and their roles in severe malaria. Nat Rev Microbiol. 2017;15(8):479–491. doi:10.1038/nrmicro.2017.47
8. Belachew EB. Immune response and evasion mechanisms of Plasmodium falciparum parasites. J Immunol Res. 2018;2018:1–6. doi:10.1155/2018/6529681
9. Hviid L, Jensen ATR. PfEMP1 - a Parasite Protein Family of Key Importance in Plasmodium Falciparum Malaria Immunity and Pathogenesis. Vol. 88. Elsevier Ltd; 2015. doi:10.1016/bs.apar.2015.02.004
10. Blomqvist K, Normark J, Nilsson D, et al. var gene transcription dynamics in Plasmodium falciparum patient isolates. Mol Biochem Parasitol. 2010;170(2):74–83. doi:10.1016/j.molbiopara.2009.12.002
11. Chan JA, Fowkes FJI, Beeson JG. Surface antigens of Plasmodium falciparum-infected erythrocytes as immune targets and malaria vaccine candidates. Cell Mol Life Sci. 2014;71(19):3633–3657. doi:10.1007/s00018-014-1614-3
12. Yam XY, Niang M, Madnani KG, Preiser PR. Three is a crowd – new insights into rosetting in Plasmodium falciparum. Trends Parasitol. 2017;33(4):309–320. doi:10.1016/j.pt.2016.12.012
13. Doumbo OK, Thera MA, Koné AK, et al. High levels of Plasmodium falciparum rosetting in all clinical forms of severe malaria in African children. Am J Trop Med Hyg. 2009;81(6):987–993. doi:10.4269/ajtmh.2009.09-0406
14. Mercereau-Puijalon O, Guillotte M, Vigan-Womas I. Rosetting in Plasmodium falciparum: a cytoadherence phenotype with multiple actors. Transfus Clin Biol. 2008;15(1–2):62–71. doi:10.1016/j.tracli.2008.04.003
15. Rowe JA, Shafi J, Kai OK, Marsh K, Raza A. Nonimmune IgM but not IgG binds to the surface of Plasmodium falciparum-infected erythrocytes and correlates with rosetting and severe malaria. Am J Trop Med Hyg. 2002;66(6):692–699. doi:10.4269/ajtmh.2002.66.692
16. Stevenson L, Laursen E, Cowan GJ, et al. α2-macroglobulin can crosslink multiple plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) molecules and may facilitate adhesion of parasitized erythrocytes. PLoS Pathog. 2015;11(7):1–19. doi:10.1371/journal.ppat.1005022
17. Rout R, Dhangadamajhi G, Mohapatra BN, Kar SK, Ranjit M. High CR1 level and related polymorphic variants are associated with cerebral malaria in eastern-India. Infect Genet Evol. 2011;11(1):139–144. doi:10.1016/j.meegid.2010.09.009
18. Panda AK, Panda M, Tripathy R, Pattanaik SS, Ravindran B, Das BK. Complement Receptor 1 variants confer protection from severe Malaria in Odisha, India. PLoS One. 2012;7(11):e49420. doi:10.1371/journal.pone.0049420
19. Zimmerman PA, Ferreira MU, Howes RE, Mercereau-Puijalon O. Red Blood Cell Polymorphism and Susceptibility to Plasmodium Vivax. Vol. 81. Elsevier; 2013. doi:10.1016/B978-0-12-407826-0.00002-3
20. Stevenson L, Huda P, Jeppesen A, et al. Investigating the function of Fc-specific binding of IgM to Plasmodium falciparum erythrocyte membrane protein 1 mediating erythrocyte rosetting. Cell Microbiol. 2015;17(6):819–831. doi:10.1111/cmi.12403
21. Moll K, Palmkvist M, Ch’ng J, Kiwuwa MS, Wahlgren M. Evasion of immunity to Plasmodium falciparum: rosettes of blood group a impair recognition of PfEMP1. PLoS One. 2015;10(12):1–18. doi:10.1371/journal.pone.0145120
22. Heddini A, Treutiger CJ, Wahlgren M. Enrichment of immunoglobulin binding Plasmodium falciparum-infected erythrocytes using anti-immunoglobulin-coated magnetic beads. Am J Trop Med Hyg. 1998;59(5):663–666. doi:10.4269/ajtmh.1998.59.663
23. Somner EA, Black J, Pasvol G. Multiple human serum components act as bridging molecules in rosette formation by Plasmodium falciparum-infected erythrocytes. Blood. 2000;95(2):674–682. doi:10.1182/blood.v95.2.674
24. Akhouri RR, Goel S, Furusho H, Skoglund U, Wahlgren M. Architecture of human IgM in complex with P. falciparum erythrocyte membrane Protein 1. Cell Rep. 2016;14(4):723–736. doi:10.1016/j.celrep.2015.12.067
25. Perez L, Perez ML, Van Der Puije W, Castberg FC, Ofori MF, Hviid L. Binding of human serum proteins to Plasmodium falciparum ‑ infected erythrocytes and its association with malaria clinical presentation. Malar J. 2020;19:1–9. doi:10.1186/s12936-020-03438-8
26. Atanasova E, Martinova F, Jelev D, et al. Alpha-2 macroglobulin is the simplest serum biomarker for liver fibrosis and fibrogenesis in chronic Hepatitis C. J Med Dent Pract. 2015;2(2):153–164. doi:10.18044/medinform.201522.153
27. Rehman AA, Ahsan H, Khan FH. Alpha-2-macroglobulin: a physiological guardian. J Cell Physiol. 2013;228(8):1665–1675. doi:10.1002/jcp.24266
28. Lindner I, Hemdan NYA, Buchold M, et al. Α2-macroglobulin inhibits the malignant properties of astrocytoma cells by impeding Β-Catenin signaling. Cancer Res. 2010;70(1):277–287. doi:10.1158/0008-5472.CAN-09-1462
29. Mujahid A, Dickert FL. Blood group typing: from classical strategies to the application of synthetic antibodies generated by molecular imprinting. Sensors. 2016;16(1). doi:10.3390/s16010051
30. Chotivanich KT, Udomsangpetch R, Pipitaporn B, et al. Rosetting characteristics of uninfected erythrocytes from healthy individuals and malaria patients. Ann Trop Med Parasitol. 1998;92(1):45–56. doi:10.1080/00034989860166
31. Kaul DK, Roth EF, Nagel RL, Howard RJ, Handunnetti SM. Rosetting of Plasmodium falciparum-infected red blood cells with uninfected red blood cells enhances microvascular obstruction under flow conditions. Blood. 1991;78(3):812–819. doi:10.1182/blood.v78.3.812.bloodjournal783812
32. Barragan A, Kremsner PG, Weiss W, Wahlgren M, Carlson J. Age-related buildup of humoral immunity against epitopes for rosette formation and agglutination in African areas of malaria endemicity. Infect Immun. 1998;66(10):4783–4787. doi:10.1128/iai.66.10.4783-4787.1998
33. Adams Y, Rowe JA, Craig AG. The effect of anti-rosetting agents against malaria parasites under physiological flow conditions. PLoS One. 2013;8(9):e73999. doi:10.1371/journal.pone.0073999
34. Quintana P, Ch J, Moll K, Zandian A, Nilsson P. Antibodies in children with malaria to PfEMP1, RIFIN and SURFIN expressed at the Plasmodium falciparum parasitized red blood cell surface. Sci Rep. 2018:1–14. DOI:10.1038/s41598-018-21026-4
35. Horata N, Kalambaheti T, Craig A, Khusmith S. Sequence variation of PfEMP1-DBL α in association with rosette formation in Plasmodium falciparum isolates causing severe and uncomplicated malaria. Malar J. 2009;8(1):1–11. doi:10.1186/1475-2875-8-184
36. Bachmann A, Scholz J, Janßen M, et al. Evidence of promiscuous endothelial binding by Plasmodium falciparum-infected erythrocytes. Cell Microbiol. 2014;11(3):1–14. doi:10.1016/j.molbiopara.2014.07.006.The
37. Mcquaid F, Rowe JA, Rowe JA, Mcquaid F, Rowe JA. Rosetting revisited: a critical look at the evidence for host erythrocyte receptors in Plasmodium falciparum rosetting. Parasitology. 2020;147(1):1–11. doi:10.1017/S0031182019001288
38. Jötten AM, Moll K, Wahlgren M, Wixforth A, Westerhausen C. Blood group and size dependent stability of P. falciparum infected red blood cell aggregates in capillaries. Biomicrofluidics. 2020;14(2):1–10. doi:10.1063/1.5125038
39. Vigan-Womas I, Guillotte M, Juillerat A, et al. Structural basis for the ABO blood-group dependence of plasmodium falciparum rosetting. PLoS Pathog. 2012;8(7):33. doi:10.1371/journal.ppat.1002781
40. Heddini A, Pettersson F, Kai O, et al. Fresh isolates from children with severe plasmodium falciparum malaria bind to multiple receptors. Infect Immunity. 2001;69(9):5849–5856. doi:10.1128/IAI.69.9.5849
41. Marsh K, Rowe JA, Obiero J, Marsh K, Raza A. Short report: positive correlation between rosetting and parasitemia in Plasmodium falciparum clinical isolates. Am J Trop Med Hyg. 2002;66:3–6. doi:10.4269/ajtmh.2002.66.458
42. Treutiger CJ, Scholander C, Carlson J, et al. Rouleaux-forming serum proteins are involved in the rosetting of Plasmodium falciparum-infected erythrocytes. Exp Parasitol. 1999;93(4):215–224. doi:10.1006/expr.1999.4454
43. Lehman LG, Vu-Quoc B, Carlson J, Kremsner PG. Plasmodium falciparum: inhibition of erythrocyte rosette formation and detachment of rosettes by pentoxifylline. Trans R Soc Trop Med Hyg. 1997;91(1):74–75. doi:10.1016/S0035-9203(97)90402-8
44. Rout R, Dhangadamajhi G, Mohapatra BN, Kar SK, Ranjit M. Genetic diversity of PfEMP1-DBL 1-α and its association with severe malaria in a hyperendemic state of India. Asian Pac J Trop Med. 2010;3(7):505–509. doi:10.1016/S1995-7645(10)60122-8
45. Chen Q, Barragan A, Fernandez V, et al. Identification of Plasmodium falciparum erythrocyte membrane protein I (PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum. J Exp Med. 1998;187(1):15–23. doi:10.1084/jem.187.1.15
46. Rovira-Vallbona E, Moncunill G, Bassat Q, et al. Low antibodies against Plasmodium falciparum and imbalanced pro-inflammatory cytokines are associated with severe malaria in Mozambican children: a case-control study. Malar J. 2012;11:1–11. doi:10.1186/1475-2875-11-181
47. Couper KN, Phillips RS, Brombacher F, Alexander J. Parasite-specific IgM plays a significant role in the protective immune response to asexual erythrocytic stage Plasmodium chabaudi AS infection. Parasite Immunol. 2005;27(5):171–180. doi:10.1111/j.1365-3024.2005.00760.x
48. Czajkowsky DM, Salanti A, Ditlev SB, et al. IgM, FcμRs, and malarial immune evasion. J Immunol. 2010;184(9):4597–4603. doi:10.4049/jimmunol.1000203
49. Pied S, Nosten F, Nosten F, Nosten F, Nosten F, Mazier D. Materials and methods. Parasite. 1995;2(3):263–268. doi:10.1051/parasite/1995023263
50. Medrano NM, Luz MRMP, Cabello PH, Tapia GT, Van Leuven F, Araújo-Jorge TC. Acute Chagas’ disease: plasma levels of alpha-2-macroglobulin and C-reactive protein in children under 13 years in a high endemic area of Bolivia. J Trop Pediatr. 1996;42(2):68–74. doi:10.1093/tropej/42.2.68
51. Bapat PR, Satav AR, Husain AA, et al. Differential levels of Alpha-2-macroglobulin, haptoglobin and sero-transferrin as adjunct markers for TB diagnosis and disease progression in the malnourished tribal population of Melghat, India. PLoS One. 2015;10:1–17. doi:10.1371/journal.pone.0133928
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.