Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 14
Interleukin-16 rs4072111 Polymorphism is Associated with the Risk of Peri-Implantitis in the Chinese Population
Received 30 August 2021
Accepted for publication 25 November 2021
Published 15 December 2021 Volume 2021:14 Pages 1629—1635
DOI https://doi.org/10.2147/PGPM.S336857
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Martin H Bluth
Zongfei Chen,1 Guanhua Chen2
1Fujian Key Laboratory of Oral Diseases & Fujian Provincial Engineering Research Center of Oral Biomaterial & Stomatological Key Laboratory of Fujian College and University, School and Hospital of Stomatology, Fujian Medical University, Fuzhou, Fujian, People’s Republic of China; 2Institute of Stomatology & Research Center of Dental and Craniofacial Implants, School and Hospital of Stomatology, Fujian Medical University, Fuzhou, Fujian, People’s Republic of China
Correspondence: Zongfei Chen
Fujian Key Laboratory of Oral Diseases & Fujian Provincial Engineering Research Center of Oral Biomaterial & Stomatological Key Laboratory of Fujian College and University, School and Hospital of Stomatology, Fujian Medical University, 246 Yangqiao Zhong Road, Fuzhou, Fujian, 350002, People’s Republic of China
Tel/Fax +86-0591-83700838
Email [email protected]
Purpose: Peri-implantitis (PI) is a major contributor to dental implant failure. Genetic predisposition plays an essential role in the development of PI. The purpose of this study was to investigate the correlation of IL-16 gene single nucleotide polymorphisms (SNPs), rs11556218 and rs4072111, with PI at the gene level.
Patients and Methods: A total of 162 patients with PI and 162 cases with healthy implants were recruited as the case and control groups, respectively. The genotypes were analysed using direct sequencing. The genotype and allele proportion between the case and control groups were compared using the chi-square test. The periodontal status of patients carrying different genotypes was analysed, including gingival index, plaque index, calculus index, peri-implant pocket depth (PPD), and clinical attachment level (CAL).
Results: The case and control groups were age- and gender-matched. In the case group, the rs4072111 CT genotype was majorly observed, and the T allele carriers showed a high PI risk. Patients with the rs4072111 CT genotype had worse periodontal status, which was reflected by the higher levels of the gingival index, plaque index, calculus index, PPD and CAL. The distribution of the rs11556218 genotype and T allele showed no significant difference between the case and control groups (P > 0.05).
Conclusion: The CT genotype of IL-16 gene rs4072111 SNP can be used as a factor assessing PI risk. Therefore, IL-16 genetic variation may be related to PI susceptibility in the Chinese Han population.
Keywords: peri-implantitis, IL-16, rs4072111, rs11556218, susceptibility
Introduction
Peri-implantitis (PI) is the reversible inflammation of the soft tissue surrounding oral implants.1 Bacterial growth on oral implants is the root cause PI, which causes the accumulation of plaque around the implant and stimulates the inflammatory response of the body.2 The clinical manifestations of PI are gingival redness, swelling, bleeding on probing, and radiographic signs of bone loss.3 In severe cases, a fistula around the dental implant may sometimes overflow with pus, causing alveolar bone absorption around the implant.4 The occurrence of PI negatively affects the success of dental implantation. Various factors have been reported to influence the occurrence of PI, such as microbial growth, dental plaque and oral hygiene.5 In addition, recent studies have demonstrated that genetic polymorphism is a major contributor to PI.6
Single nucleotide polymorphisms (SNPs) are DNA sequence polymorphisms caused by single nucleotide variation at the genome level. They are common heritable variants in humans and are widely present in the human genome, accounting for more than 90% of all known polymorphisms.7 Regarding PI, multiple SNPs in different genes have been identified to be associated with the genetic predisposition for PI. In the Chinese Han population, osteoprotegerin gene (OPG) rs2073618 SNP has been reported to be associated with the risk of PI. In addition, the rs2073618 CC genotype carriers are susceptible to PI.8 Recently, the genetic association of TNF-α, IL-1A, and IL-1B polymorphisms with PI has been studied in the Chinese non-smoking population.9 Activated T cells primarily produce IL-16 that can bind to CD4+ molecules and can activate monocytes to produce inflammatory factors, such as IL-6, TNF-α, IL-1β and IL-15.10 IL-16 is located on chromosome 15q26.3, comprises seven exons and six introns. Rs11556218 and rs4072111 are two common SNPs located in the exon region of IL-16 gene. As previously reported, rs11556218 and rs4072111 are associated with the susceptibility to several human diseases, such as osteoporosis and coronary artery disease.11,12 The two SNPs have been reported to be related to the genetic susceptibility of periodontitis.13 In light of the important role of periodontitis in PI, the genetic correlation between IL-16 gene and PI should be investigated. Therefore, this study was designed to explore the genetic association of IL-16 gene rs11556218 and rs4072111 SNPs with PI at the gene level.
Materials and Methods
Study Objects
A total of 162 patients with PI who visited the School and Hospital of Stomatology, Fujian Medical University from August 2019 to March 2021 were selected as the case group. In addition, 162 cases with healthy implants were included as the control group. All the participants were Chinese Han population. The inclusion criteria were as follows: (1) Patients have signed the informed consent form; (2) Patients have at least one implant in the mouth; (3) The insertion of the superstructure has been completed for at least 1 year. The exclusion criteria were as follows: (1) Patients who have systemic diseases; (2) Patients who have been administered NSaids and adrenocorticosteroids within the past 3 months; (3) Patients with other oral infections, jawbone cysts and other local lesions; (4) Women who are pregnant or breastfeeding; (5) Patients caused by iatrogenic factors such as unreasonable design, type of restoration, use of adhesive and removal of residual adhesive, etc. The diagnostic criteria for healthy implants were as follows: (1) No soft tissue redness and swelling; (2) No probing bleeding; (3) No excessive concentration; (4) Unexplored depth increased; (5) Bone resorption at the top level of the alveolar ridge after bone remodeling. The diagnostic criteria for PI were as follows: (1) Excessive concentration or probing bleeding; (2) The probing depth increased compared with the baseline; (3) Bone resorption at the top level of alveolar ridge was observed after bone remodeling. In the absence of baseline data, the following criteria were used for diagnosis: (1) Probing depth ≥6 mm; (2) Excessive concentration or probing bleeding; (3) A distance of ≥3 mm from the crest of the bone around the implant to the crown of the bone within the implant.14 The clinical information of the patients was collected subsequently after the completion of the dental implant. This study was conducted in accordance with the Declaration of Helsinki. The current study design was approved by the ethics committee of School and Hospital of Stomatology, Fujian Medical University (ethical code: 2019–028-02; Approval date: 10th January, 2019).
Template DNA Extraction
For DNA extraction, the patients were asked to abstain from food and water for 30 minutes before the extraction, followed by water gargling. Using sterile cotton swabs, the buccal mucosa of patients was taken and placed in sterile 15×103 μL Eppendorf tubes. The genomic DNA was extracted using phenol/chloroform/isoamyl alcohol followed by isopropanol precipitation and cleaned using an oral swab genomic DNA extraction kit (Tian Gen, DP322-03, Beijing, China). DNA concentration was determined by NanoDrop 1000 (Thermo Fisher Scientific, Waltham, MA USA) via measuring the A260/A280 ratios.
PCR Assay
Polymerase chain reaction (PCR) was performed using the genomic DNA as the template. The primer sequences were as follows: rs11556218, forward primer: 5′-GCTCAGGTTCACAGAGTGTTTCCATA-3′, reverse primer: 5′-TGTGACAATCACAGCTTGCCTG-3′; rs4072111, forward primer: 5′- CACTGTGATCCCGGTCCAGTC-3′, reverse primer: 5′- TTCAGGTACAAACCCAGCCAGC −3′. 25 μL PCR reaction system was prepared, including 5 μL DNA template, 12.5 μL Go Tag mixed enzyme (including Taq DNA polymerase, dNTP, 10 × PCR buffer), 0.25 μL forward and 0.25 μL reverse primes, and 7 μL sterile deionized water. The PCR cycle conditions were as follows: 94°C for 5 min, denaturation for 1 min, renaturation for 30 s at 72°C, extension for 1 min at 61°C, and 30 cycles were run. Finally, the temperature was maintained at 72°C for 5 min.
Genotyping
After purification, the PCR products were outsourced to Sangon Biotech Company (Beijing, China) for sequencing using an ABI 3130xL Genetic Analyzer (Applied Biosystems, Foster City, California, USA) according to the manufacturer’s recommendations.15
Statistical Approach
SPSS 17.0 software (SPSS, Chicago IL, USA) was applied for the data analysis. The genotype and allele frequency was directly counted. The hardy-Weinberg equilibrium was calculated for the control group. Differences between two groups were calculated using the Mann–Whitney U-test for non-normally distributed continuous variables, Student’s t test for normally distributed continuous variables, chi-squared test for categorical variables. Differences between multiple groups were compared using one-way analysis of variance (ANOVA) analysis. A P value less than 0.05 was defined as statistically significant.
Results
Demographic and Clinical Information of the Study Population
Chi-square test results revealed no significant difference between the case and control groups regarding age and gender distribution, demonstrating that the two groups were age (P = 0.249) and gender (P = 0.578) matched (Table 1). The comparison of clinical information including periodontitis history (P = 0.059), tooth loss reason (P = 0.369), platform type (P = 0.317), position (P = 0.055) and peri-implant phenotype (P = 0.149) showed no significant difference between the two groups (Table 1). In addition, no significant difference was observed in the lifestyle information of the study population, including history of alcohol and smoking, frequencies of brushing, dental floss and mouth washing (P > 0.05, Table 1). Therefore, the two groups were comparable.
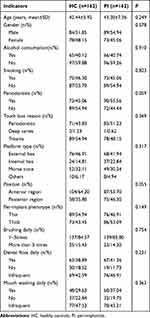 |
Table 1 Clinical Information of the Subjects |
Patient’s Periodontal Condition
Patient’s periodontal condition was assessed by detecting gingival index, plaque index, calculus index, peri-implant pocket depth (PPD), clinical attachment level (CAL), and the periodontal condition was compared between the case and control groups. As shown in Table 2, patients who suffer from PI had higher levels of gingival index, plaque index, calculus index, PPD and CAL (P < 0.001) than those in the control group. The findings indicated that patients with poor periodontal conditions were at high risk of PI.
 |
Table 2 Periodontal Status of Subjects |
Genotype Distribution Frequencies of IL-16 Gene rs11556218 and Rs4072111 Polymorphisms
As shown in Table 3, the three genotypes of IL-16 gene rs11556218 SNP were detected, including TT, TG and GG. The three genotypes showed minor differences in distribution frequency between the two groups (P = 0.795; P = 0.378, respectively). In addition, the T and G allele frequency between the two groups was not statistically significant (P = 0.521). For rs4072111 polymorphism, the three genotypes of CC, CT and TT were observed in the study subjects. The comparison of differences between groups suggested that the frequency of CT genotypes was significantly increased in the PI group compared with CC genotypes (P = 0.007, Table 3). Moreover, the frequency of T allele in the PI group was significantly higher than that in the control group (P = 0.030, Table 3). These results suggested that rs4072111 CT genotype and T allele carriers were at higher risk of PI.
 |
Table 3 Frequency Distribution of IL-16 Gene Rs11556218 and Rs4072111 Genotype and Allele in Healthy Controls and Peri-Implantitis Groups |
The Periodontal Status of Patients Carrying Different Genotypes
According to the rs4072111 genotype information, all the patients were divided into three groups, and the periodontal status index of the three groups was compared. ANOVA analysis results revealed that patients with the CC genotype had lower scores of the gingival index, plaque index, calculus index, PPD and CAL, whereas patients with the CT genotype had significantly higher scores (Table 4). The periodontal status of individuals with different rs11556218 genotypes was also analysed. As shown in Table 5, the levels of gingival index (P = 0.578), plaque index (P = 0.407), calculus index (P = 0.551), PPD (P = 0.482) and CAL (P = 0.784) showed no significant difference among the three groups (P > 0.05). These findings indicated that individuals carrying rs4072111 CT genotype had poor periodontal status.
 |
Table 4 Periodontal Status in Patients with Rs4072111 Gene Polymorphism |
 |
Table 5 Periodontal Status in Patients with Rs11556218 Gene Polymorphism |
Discussion
IL-16 is a pro-inflammatory cytokine that was originally designated as a lymphocyte chemoattractant factor. IL-16 can inhibit the growth of periodontal membrane cells such as fibroblasts, collagen cells and stromal cells, and reduce the adhesion of fibroblasts. This affects the repair and metabolic functions of periodontal membrane cells and leads to the damage of periodontal tissue, which is not conducive to the success of implants.16 Early studies have reported that patients with periodontal disease secrete more TNF-α, IL-6 and IL-16 factors than healthy people.17 In addition, various studies have reported the association between IL-16 gene polymorphism and inflammatory diseases susceptibility. Gu et al have studied the effect of IL-16 polymorphism in Graves’ disease and identified that the rs4072111 locus was associated with Graves’ disease and corresponding eye diseases. Moreover, individuals carrying the TT genotype and T allele had a high risk to suffer from Graves’ disease.18 In addition, in the Chinese Han population, the G allele of rs11556218, C allele of rs4778889, and T allele of rs4072111 were frequently detected in patients with systemic lupus erythematosus (SLE), indicating the genetic association of IL-16 gene polymorphism with systemic lupus erythematosus (SLE) susceptibility.19 Notably, IL-16 gene SNPs have been identified to be related to the development of periodontitis in several study populations.13,20,21 In the present study, two common SNPs in IL-16 gene, including rs11556218 and rs4072111, were selected, and genotype and allele distribution in PI cases were analyzed in 162 healthy implants and 162 PI cases. The case and control groups were age and gender matched. The distribution of gene frequency of the two SNPs in the two groups conforms to Hardy–Weinberg equilibrium, indicating that the selected samples are representative and comparable. Moreover, IL-16 gene rs4072111 polymorphism showed a close association with the PI onset.
Rs4072111 polymorphism is located in exon 6 of IL-16 gene and carries the C/T mutation, resulting in a serine to alanine substitution.12 In the present study, the genotype and allele distributions of rs4072111 polymorphism showed statistical significance between the case and control groups. It implied that IL-16 gene rs4072111 polymorphism plays an important role in the genetic predisposition of PI. It was found that more PI cases carried rs4072111 CT genotype, whereas the CC genotype carriers showed higher frequencies in the control group. The allele distribution analysis results demonstrated that there were more T-allele carriers in PI patients than in the control group, indicating that the T allele might be a risk factor for the hereditary susceptibility of PI. Moreover, individuals carrying rs4072111 CT genotype had worse periodontal status. It was concluded that the CT genotype and T allele of rs4072111 might be a pathogenic site of PI, which may lead to altered cytokine expression or biological activity and further modulate the individual’s risk for PI. Consistently, IL-16 gene rs4072111 SNP exists in a variety of inflammatory diseases. In SLE cases, the rs4072111 T allele was predominant.19 In the Brazilians population, more rs4072111 CT genotype carriers are detected in individuals with periodontitis than that in the healthy control group.13 These results are consistent with our findings in patients with PI. It was concluded that the PI susceptibility in patients with rs4072111 T allele may be related to the relationship between this SNP and inflammation.
Rs11556218 is also located in exon 6 of the IL-16 gene, and brings the change of alanine to lysine. The SNP has been reported to be associated with the genetic susceptibility of several cancers, including nasopharyngeal carcinoma and colorectal cancer.22 In China, it is also suggested to have a regulatory effect on the pathogenesis of coronary artery disease, osteoporosis, type 2 diabetes mellitus and so on.11,23,24 In the current study, the three genotypes of rs11556218 were detected in patients with PI. However, the genotype and allele distributions showed no difference between the PI and control groups. It was concluded that IL-16 gene rs11556218 might lack a genetic association with PI susceptibility. As genetic association studies are susceptible to false positive results owing to genetic population diversity, population structure and multiple testing, this study requires further validation in a larger study population. Moreover, other clinical samples from the gingival sulcus or the gingival tissue around implants are also necessary for further validation.
Conclusion
In the Chinese Han population, IL-16 rs4072111 genetic variation can be used as a genetic marker for PI susceptibility. The findings may be useful in public health genomics and future advanced clinical practice. Because detection of gene polymorphism can be completed in advance, it is of great benefit for early intervention and prognosis in the early disease stage.
Ethics Statement
The current study design was approved by the ethics committee of School and Hospital of Stomatology, Fujian Medical University and written informed content was obtained from each participant.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kim S, Kang SR, Park HJ, Kim B, Kim TI, Yi WJ. Quantitative measurement of peri-implant bone defects using optical coherence tomography. J Periodontal Implant Sci. 2018;48(2):84–91. doi:10.5051/jpis.2018.48.2.84
2. Nojiri T, Chen CY, Kim DM, et al. Establishment of perpendicular protrusion of type I collagen on TiO2 nanotube surface as a priming site of peri-implant connective fibers. J Nanobiotechnology. 2019;17(1):34. doi:10.1186/s12951-019-0467-1
3. Gleiznys D, Gleiznys A, Abraskeviciute L, Vitkauskiene A, Saferis V, Sakalauskiene J. Interleukin-10 and interleukin-1beta cytokines expression in leukocytes of patients with chronic peri-mucositis. Med Sci Monit. 2019;25:7471–7479. doi:10.12659/MSM.915464
4. Li H, Chen Z, Zhong X, Li J, Li W. Mangiferin alleviates experimental peri-implantitis via suppressing interleukin-6 production and Toll-like receptor 2 signaling pathway. J Orthop Surg Res. 2019;14(1):325. doi:10.1186/s13018-019-1387-3
5. Diachkova E, Corbella S, Taschieri S, Tarasenko S. Nonsurgical Treatment of Peri-Implantitis: case Series. Dent J. 2020;8(3). doi:10.3390/dj8030078
6. Kadkhodazadeh M, Tabari ZA, Pourseyediyan T, Najafi K, Amid R. Relationship between genetic polymorphisms with periodontitis and peri-implantitis in the Iranian population: a literature review. J Long Term Eff Med Implants. 2016;26(2):183–190. doi:10.1615/JLongTermEffMedImplants.2016015197
7. Ying HJ, Fang M, Chen M. Progress in the mechanism of radiation-induced lung injury. Chin Med J. 2021;134(2):161–163. doi:10.1097/CM9.0000000000001032
8. Zhou J, Zhao Y. Osteoprotegerin Gene (OPG) polymorphisms associated with peri-implantitis susceptibility in a Chinese han population. Med Sci Monit. 2016;22:4271–4276. doi:10.12659/msm.897592
9. He K, Jian F, He T, Tang H, Huang B, Wei N. Analysis of the association of TNF-alpha, IL-1A, and IL-1B polymorphisms with peri-implantitis in a Chinese non-smoking population. Clin Oral Investig. 2020;24(2):693–699. doi:10.1007/s00784-019-02968-z
10. Chang Y, Hsiao YM, Hu CC, et al. Synovial Fluid Interleukin-16 Contributes to Osteoclast Activation and Bone Loss through the JNK/NFATc1 Signaling Cascade in Patients with Periprosthetic Joint Infection. Int J Mol Sci. 2020;21(8). doi:10.3390/ijms21082904
11. Ma X, Chen Y, Zhang Q, et al. Interleukin-16 rs11556218 is associated with a risk of osteoporosis in Chinese postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;210:90–93. doi:10.1016/j.ejogrb.2016.10.009
12. Luo SX, Li S, Zhang XH, et al. Genetic polymorphisms of interleukin-16 and risk of knee osteoarthritis. PLoS One. 2015;10(5):e0123442. doi:10.1371/journal.pone.0123442
13. Souza VH, Visentainer JEL, Zacarias JMV, et al. Association of IL16 polymorphisms with periodontitis in Brazilians: a case- control study. PLoS One. 2020;15(9):e0239101. doi:10.1371/journal.pone.0239101
14. Renvert S, Persson GR, Pirih FQ, Camargo PM. Peri-implant health, peri-implant mucositis, and peri-implantitis: case definitions and diagnostic considerations. J Periodontol. 2018;89(Suppl 1):S304–S312. doi:10.1002/JPER.17-0588
15. Matyushenko V, Isakova-Sivak I, Smolonogina T, Dubrovina I, Tretiak T, Rudenko L. Genotyping assay for differentiation of wild-type and vaccine viruses in subjects immunized with live attenuated influenza vaccine. PLoS One. 2017;12(7):e0180497. doi:10.1371/journal.pone.0180497
16. Pawlowski P, Reszec J, Eckstein A, et al. Markers of inflammation and fibrosis in the orbital fat/connective tissue of patients with Graves’ orbitopathy: clinical implications. Mediators Inflamm. 2014;2014:412158. doi:10.1155/2014/412158
17. Kawamoto D, Amado PPL, Albuquerque-Souza E, et al. Chemokines and cytokines profile in whole saliva of patients with periodontitis. Cytokine. 2020;135:155197. doi:10.1016/j.cyto.2020.155197
18. Gu XJ, Cui B, Zhao ZF, et al. Association of the interleukin (IL)-16 gene polymorphisms with Graves’ disease. Clin Immunol. 2008;127(3):298–302. doi:10.1016/j.clim.2008.01.017
19. Xue H, Gao L, Wu Y, et al. The IL-16 gene polymorphisms and the risk of the systemic lupus erythematosus. Clin Chim Acta. 2009;403(1–2):223–225. doi:10.1016/j.cca.2009.03.016
20. Folwaczny M, Glas J, Torok HP, et al. Prevalence of the −295 T-to-C promoter polymorphism of the interleukin (IL)-16 gene in periodontitis. Clin Exp Immunol. 2005;142(1):188–192. doi:10.1111/j.1365-2249.2005.02902.x
21. Tsai IS, Tsai CC, Ho YP, Ho KY, Wu YM, Hung CC. Interleukin-12 and interleukin-16 in periodontal disease. Cytokine. 2005;31(1):34–40. doi:10.1016/j.cyto.2005.02.007
22. Mo CJ, Peng QL, He Y, et al. Positive association between IL-16 rs11556218 T/G polymorphism and cancer risk: a meta-analysis. Asian Pac J Cancer Prev. 2014;15(11):4697–4703. doi:10.7314/apjcp.2014.15.11.4697
23. Chen Y, Huang H, Liu S, et al. IL-16 rs11556218 gene polymorphism is associated with coronary artery disease in the Chinese Han population. Clin Biochem. 2011;44(13):1041–1044. doi:10.1016/j.clinbiochem.2011.06.010
24. Cheng F, Liu L, Zhang H, Zhu Y, Li X, Li H. Association of IL-16 gene polymorphisms with the risk of developing type 2 diabetes mellitus in the Chinese Han population. Biosci Rep. 2019;39(8). doi:10.1042/BSR20190821
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
