Back to Journals » Journal of Pain Research » Volume 17
Is the Capsaicin 179 mg (8% w/w) Cutaneous Patch an Appropriate Treatment Option for Older Patients with Peripheral Neuropathic Pain?
Authors Pickering G, Engelen S, Stupar M, Ganry H, Eerdekens M
Received 17 August 2023
Accepted for publication 26 February 2024
Published 27 March 2024 Volume 2024:17 Pages 1327—1344
DOI https://doi.org/10.2147/JPR.S435809
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Gisèle Pickering,1 Sylvia Engelen,2 Maria Stupar,2 Hervé Ganry,3 Mariëlle Eerdekens2
1Faculty of Medicine Inserm 1107, Clinical Pharmacology Centre, CPC/CIC Inserm 1405 University Hospital, Clermont-Ferrand, France; 2Grünenthal GmbH, Aachen, Germany; 3Hergan Consulting 4U, Amiens, 80000, France
Correspondence: Mariëlle Eerdekens, Grünenthal GmbH, Zieglerstrasse 6, Aachen, 52078, Germany, Tel +492415 691090, Email [email protected]
Introduction: Capsaicin 179 mg (8% weight per weight) cutaneous patch (“capsaicin patch”) is a recommended topical treatment for peripheral neuropathic pain (PNP). In older patients, topical treatments may be preferred over systemic treatments, but data specific to the older population are scarce.
Methods: We conducted pooled analyses of multiple clinical trials to evaluate efficacy and safety of capsaicin patch in older patients. The analysis of efficacy included four randomized, double-blind, 12-week studies with similar trial design comparing a single treatment of capsaicin 179 mg cutaneous patch vs low-dose control patch in post-herpetic neuralgia. For the safety evaluation, data were pooled from 18 interventional studies in which capsaicin patch was used in PNP with varying etiologies.
Results: Capsaicin patch had similar analgesic efficacy in elderly (n=582) and non-elderly patients (n=545) in terms of change from baseline to 2– 12 weeks in the 11-point numeric pain rating scale (NPRS) score for average pain over the previous 24 hours. In both age groups, decrease in NPRS score was significantly greater with capsaicin patch vs control. Older patients treated with capsaicin patch were significantly more likely than those in the control group to achieve responder status (ie mean decrease in NPRS score from baseline to week 2– 12 of at least 30% or ≥ 2 points): 36.1% vs 27.1% (odds ratio [OR] [95% CI] 1.52 [1.06, 2.18]; P=0.0231) and 33.1% vs 20.9% (OR [95% CI] 1.90 [1.30, 2.78]; P=0.0009) for active treatment vs control group, respectively. Similar proportions of non-elderly patients (n=2,311) and elderly patients (n=537) treated with capsaicin patch experienced treatment-emergent adverse events (TEAEs) (81.6% and 78.1%, respectively) and serious TEAEs (8.2% and 7.2%), with application-site reactions the most common TEAEs in both groups.
Conclusion: The capsaicin patch was equally efficacious and well tolerated in older patients as in younger patients.
Plain Language Summary: Peripheral neuropathic pain is a common challenge among the elderly, yet effective treatments for this age group remain underexplored. This research focuses on the use of a high-concentration capsaicin patch, a specialized treatment for this type of pain. The patch, which is applied directly to the affected skin area, has been shown to reduce pain significantly for up to 12 weeks. This analysis of multiple clinical trials showed that the high-concentration capsaicin patch significantly reduced pain intensity and was well tolerated in older patients with peripheral neuropathic pain.
Keywords: capsaicin patch, elderly, peripheral neuropathic pain, pooled analysis, topical treatment
Introduction
Neuropathic pain, a type of chronic pain that arises as a direct consequence of a lesion or disease affecting the somatosensory system,1 may affect ~30% of older adults (aged >60 years).2 This compares to a prevalence of 3–18% in the general population in Western countries.3 Most commonly, localized neuropathic pain has a peripheral presentation characterized by a consistent and circumscribed area of maximum pain.4 Neuropathic pain impairs quality of life and can exacerbate functional decline with age.5 Persistent pain (of any type) in older adults has also been associated with an increased risk of frailty.5
Pharmacotherapy is the recommended first-line treatment for localized neuropathic pain.6 However, neuropathic pain can be difficult to treat, and oral systemic treatment options (such as antidepressants, antiepileptic drugs, and/or opioids) often provide incomplete pain relief, with side effects and drug─drug interactions that can limit long-term use.6–8 Locally applied treatments that target the allodynic or hyperalgesic area with very limited systemic effects may be preferred, particularly in older patients with multiple comorbidities and polypharmacy.6–8
The 5% lidocaine plaster and capsaicin 179 mg (8% weight per weight) cutaneous patch (hereafter referred to as the capsaicin patch) are both recommended as first-line treatment options for localized neuropathic pain,6,9 with some guidelines positioning the capsaicin patch as second line.10 Of these, the capsaicin patch typically has the broader indication: for peripheral neuropathic pain (PNP) in Europe, and for pain associated with post-herpetic neuralgia (PHN) and painful diabetic peripheral neuropathy (pDPN) in the US.11,12 Up to four patches are applied by a physician (or a healthcare professional under physician supervision) to the most painful skin areas for a duration of 30 or 60 minutes, depending on the PNP condition and/or pain site. Treatment may be repeated every 3 months.
In a head-to-head trial, the capsaicin patch provided more rapid onset of pain relief and greater satisfaction than pregabalin in patients with PNP,13 while a network meta-analysis suggested similar efficacy and fewer side effects when compared with oral systemic agents (pregabalin, duloxetine, and gabapentin) in pDPN.14 Other topical or locally acting therapies have been evaluated or are in development for neuropathic pain, including phenytoin cream,15 botulinum toxin,16 topical cannabinoids,17 ketamine, muscle relaxants and α2-adrenergic agents, and locally applied μ-opioid peptide (MOP) and nociceptin/orphanin FQ peptide (NOP) agonists. Currently, there is insufficient evidence to recommend these.6–8
Although clinical trials of topical cutaneous therapies (including the capsaicin patch) have included older patients, efficacy and safety data in this population have up to now not been reported separately.7 In the authors’ experience, clinicians’ concerns over the benefit–risk profile of the capsaicin 179 mg patch in the elderly (particularly regarding local tolerability) have sometimes limited its clinical use in this population. To address these concerns, analyses were conducted on pooled data from clinical trials of the capsaicin patch to evaluate its efficacy and safety in pooled subpopulations of older and other adult patients.
Methods
Selection of Studies for Pooled Analysis
The studies included in this analysis were identified from the clinical development program for the capsaicin patch. This program comprised a series of interventional Phase 2, 3, and 4 trials, specifically aimed at assessing the efficacy and safety of the capsaicin 179 mg patch in treating various neuropathic pain conditions. All trials were supported by companies with direct involvement in the development of the capsaicin patch. An additional search of the literature resulted in the identification of clinical trials that included adult and older patients but lacked specific information regarding older patients. Therefore these trials were not considered in this analysis. For the pooled analysis of efficacy according to participant age, only randomized, double-blind, 12-week efficacy studies with similar trial design, and that included patients older than the median age in the pooled dataset (ie aged ≥73 years), were selected. Four Phase 2/3 studies (C108, C110, C116, and C117) were identified (pooled n=1,272), each of which compared the efficacy of a single treatment with the capsaicin patch with that of a low-concentration capsaicin (0.04%) control patch in patients with moderate-to-severe neuropathic pain resulting from PHN (Table 1). Excluded single-treatment randomized controlled trials included STEP (comparator, placebo) and trials C107, C109, C112, and C119 that did not include patients ≥73 years old. Duration of patch application was 60 minutes, except for trial C108 in which some patients were treated for 30 minutes or 60 minutes. Only patients who were treated for 60 minutes in the double-blind phase were included in the pooled capsaicin patch treatment group, whereas the pooled control group contained all patients treated with the control patch for any duration across the four studies. All treated patients received pretreatment of their painful areas with a topical local anesthetic cream (lidocaine 4%) before study patch application, to offset potential treatment-related discomfort or pain resulting from capsaicin. Patients used a diary to record numeric pain rating scale (NPRS) scores (from 0 to 10, with 0 indicating no pain and 10 indicating worst possible pain) from the evening of the treatment day (day 0) through to the evening before the week 12 visit. Baseline NPRS scores were recorded during the screening period (starting 7 days before randomization for trials C108 and C110, and 14 days before randomization for C116 and C117, and ending prior to randomization). The primary efficacy endpoint for each individual trial was the mean percent change from baseline to weeks 2–8 for “average pain for the past 24 hours” using the NPRS. However, as the trials were of 12 weeks’ duration, the predefined analysis of the mean percent change from baseline to weeks 2–12 for “average pain for the past 24 hours” was considered for the current analyses, to ensure that the full duration of the trial was reflected.
 |
Table 1 Overview of Phase 2, 3, and 4 Trials (N=18) in Peripheral Neuropathic Pain Conditions in the Capsaicin Patch Database |
For analysis of safety and tolerability, data were pooled from all 18 interventional Phase 2, 3, and 4 studies, regardless of PNP indication (N=4,099 treated patients) (Table 1). Overall, 1,924 patients had received a single application of the capsaicin patch, and 924 patients had received multiple (up to nine) capsaicin patch treatments. Of the 1,251 patients overall who received only control or standard of care (SoC) treatment, 819 patients received a control (low-dose capsaicin or placebo) patch, and 432 received SoC treatment. Capsaicin and control patches were applied for 30, 60, or 90 minutes. Adverse event (AE) data for the individual studies have been previously reported.13,18,20–33
All trials had been approved by an institutional review board and conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice guidelines, and applicable regulatory requirements. All patients enrolled in the trials provided their written informed consent.
Pooled Dataset Analyses
Efficacy in the pooled efficacy population was evaluated in two patient subpopulations defined by median age at baseline: <73 years and ≥73 years. The cut-off of 73 years was chosen as it represents the median age of participants in the included trials, providing a balanced perspective on the treatment’s impact across the study population. The following outcomes, all based on the NPRS score for patients’ average pain intensity experienced in the previous 24 hours, were evaluated in the capsaicin patch and low-dose capsaicin control patch groups: mean change and mean percent change from baseline to week 2–12; and responder status defined as mean decrease in NPRS score from baseline to week 2–12 of i) ≥30%; ii) ≥50%; and iii) ≥2 points. The cut-off values for the definition of responders have been based on the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) recommendations.34
In the pooled safety population, treatment-emergent adverse events (TEAEs) were also evaluated in two patient subpopulations, <75 years and ≥75 years, according to treatment received (capsaicin patch or control/SoC). The age threshold was set at 75 years in line with regulatory guidelines that emphasize the importance of evaluating treatment effects in patients aged 75 years and above, a demographic often underrepresented in clinical research.35 Overall, 1,924 patients had received a single application of the capsaicin patch, and 924 patients had received multiple (up to nine) capsaicin patch treatments. Some patients were offered the option to enter long-term open-label extension trials and receive capsaicin patch treatments after having completed a randomized single-application trial. As a result, of the 924 patients with multiple capsaicin patch treatments, 153 patients had already received a prior control treatment.
The cut-off of 75 years for the elderly subpopulation was selected to encompass the upper two of the three life stages that the older adult population is commonly divided into (“young-old” [65–74 years], “middle-old” [75–84 years], and “old-old” [≥85 years]).36 AEs were considered treatment-emergent if onset was on or after the first topical local anesthetic application or if a pre-existing medical condition worsened on or after the first day of treatment. All AEs for the pooled analysis were coded to the Medical Dictionary for Regulatory Activities version 13.1.
Statistical Methods
No formal sample size calculation was conducted. The analyses for efficacy and safety were individual participant data pooled analyses. Efficacy analyses were performed in the pooled intent-to-treat population. Baseline pain scores were calculated as the mean of the NPRS scores for “average pain for the past 24 hours” obtained during the screening period. In the treatment period, NPRS scores for “average pain for the past 24 hours” were again collected on a daily basis, with missing scores imputed using a modified last observation carried forward (LOCF) approach. If NPRS scores were missing on any day, the previous non-missing score was used for imputation; if all post-treatment NPRS scores were missing, then the baseline score was used for imputation (baseline observation carried forward [BOCF]). Mean and least squares (LS) mean were calculated for average NPRS scores over weeks 2–12, and for absolute change and percent change in NPRS scores from baseline to week 2–12, for the active treatment and control group within each subpopulation (aged <73 and ≥73 years). Treatment differences were calculated as the difference of the LS mean between the active treatment and control groups using gender-stratified analysis of covariance (ANCOVA) with baseline pain as the covariate. The P-value was also computed using gender-stratified ANCOVA to test for the difference between the active treatment and pooled control groups, with baseline pain as covariate. For responder rates, the odds ratio (OR) was estimated by logistic regression with treatment as main effect, and gender and baseline pain as covariates. The P-value was also computed using logistic regression, with gender and baseline pain as covariates.
For the safety analyses, patient data were summarized based on actual treatment received. Analyses of TEAEs for the treatment and pooled control/SoC groups are presented using descriptive statistics.
Results
Analysis Populations
The clinical development program for the capsaicin patch included 18 interventional Phase 2, 3, and 4 trials comprising controlled double-blind trials, open-label repeated- and single-application trials in patients with PHN, pDPN, human immunodeficiency virus infection-related peripheral neuropathy (HIV-PN), and other PNP conditions (Table 1). These trials included 4,099 treated patients (2,848 who received capsaicin patch [QUTENZA®] and 1,251 subjects who received only low-dose capsaicin control patches, placebo patches, or SoC treatment). Patients randomized to SoC received pharmacologic or other treatment deemed optimal for managing their pain in accordance with routine best medical practice. Methods and findings for the individual studies have been reported previously.13,18–33,37
Demographic and baseline pain characteristics for both the pooled efficacy and pooled safety populations were similar across the treatment groups (Table 2); no subpopulation analyses by age were conducted. Mean age in the pooled efficacy population (70 years) was greater than in the pooled safety population (60–62 years), reflecting the older age profile of patients with PHN compared with other common PNP conditions, including patients with pDPN and HIV-PN.
 |
Table 2 Demographic and Baseline Characteristics of the Pooled Analysis Populations |
The pooled efficacy population encompassed 292 patients aged <73 years and 305 aged ≥73 years who had been randomized to the capsaicin patch, and 253 and 277 patients aged <73 and ≥73 years, respectively, who were randomized to the low-dose control patch; all patients had PHN (Table 3).
 |
Table 3 NPRS Score for “Average Pain for the Past 24 Hours” – Change from Baseline and Responder Frequencies at Weeks 2–12 for the Capsaicin Vs Low-Dose Control Patch, by Age Category (ITT Analysis)a |
The pooled safety population included 2,311 patients aged <75 years and 537 aged ≥75 years who received the capsaicin patch (Table 4). Of the 1,404 patients overall who received a control patch or SoC treatment at any time (ie as their only treatment or before receiving the capsaicin patch), 1,110 were aged <75 years, and 294 were ≥75 years. Of the 1,251 patients who received only control patch or SoC treatment (ie who never received the capsaicin patch), 991 were aged <75 years, and 260 were ≥75 years. Overall, in the pooled safety population, most patients had PHN (1,636 patients), pDPN (928 patients), or HIV-PN (899 patients). A total of 636 patients had other neuropathic pain indications. Among the 831 patients aged ≥75 years, 669 had PHN, 97 had pDPN, and 1 patient had HIV-PN.
 |
Table 4 Overview of TEAEs by Age Category and for Selected Indications (as-Treated Analysis)a |
Pooled Efficacy Analysis
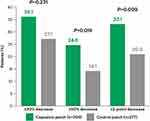
Among patients aged ≥73 years, baseline NPRS scores for “average pain in the past 24 hours” were similar for the active treatment and control groups (mean [standard error (SE)] 5.8 [0.1] in each group) (Table 3). Average NPRS scores at weeks 2–12 had improved from baseline in both the capsaicin and the control patch groups. However, improvement in the capsaicin patch group was significantly greater than for control, with a treatment difference for LS mean absolute change from baseline (95% confidence interval [CI]) of ‒0.4 (‒0.7, ‒0.1) (P=0.003), and for LS mean percent change from baseline of ‒8.3 (‒13.2, ‒3.5) (P=0.0009), in the ≥73 years age group (Table 3). Patients aged ≥73 years in the capsaicin patch group were significantly more likely than those in the control group to achieve responder status defined by mean decrease in NPRS score from baseline to week 2–12: 36.1% vs 27.1% of patients achieved a mean decrease of ≥30% (OR [95% CI] for active treatment vs control group: 1.52 [1.06, 2.18]; P=0.0231); 24.6% vs 14.1% achieved a mean decrease of ≥50% (OR [95% CI] 1.99 [1.29, 3.08]; P=0.0019); and 33.1% vs 20.9% achieved a mean decrease of ≥2 points (OR [95% CI] 1.90 [1.30, 2.78]; P=0.0009) (Table 3; Figure 1).
Baseline pain scores in the <73 years age group (mean [SE] 5.6 [0.1] and 5.5 [0.1] in the active treatment and control group, respectively) were slightly lower than in the older age group. However, the treatment differences (for the active treatment minus control group) for absolute change and relative change from baseline in NPRS scores for “average pain in the past 24 hours” (‒0.4 [‒0.7, ‒0.1] and ‒8.8% [‒14.3%, ‒3.2%], respectively) were similar to those for the ≥73 years subpopulation. The magnitude of response and, consequently, responder rates were higher in patients aged <73 years than those aged ≥73 years. As this was the case for both the treatment and control groups, the resulting odds ratios were slightly lower for the ≥50% responder definition but still reaching statistically significant improvements for the ≥30% and ≥2-point responder definitions.
Pooled Safety and Tolerability Analysis
In the overall pooled safety population, TEAEs were reported for 78.7% of patients who received the capsaicin patch, and for 65.8% of patients who received a control patch or SoC, prior to the TEAE (Table 4; Figure 2).
Among patients ≥75 years old, 81.6% of patients after treatment with the capsaicin patch experienced TEAEs, compared with 72.4% in the control group. A similar proportion of patients in the active treatment group vs control group had serious TEAEs (8.2% vs 6.1%, respectively) and discontinued treatment due to a TEAE (0.9% vs 1.0%) (Table 4; Figure 2). The most frequently reported TEAEs in patients aged ≥75 years who received the capsaicin patch were application-site reactions (63.1% of patients vs 49.3% for control) (Table 4; Figure 2). In this age group, erythema was the most common application-site reaction with the capsaicin patch (42.3%), followed by pain (34.1%) (Table 5; Figure 2).
Other TEAEs that were reported in ≥5% of patients aged ≥75 years who received the capsaicin patch were nausea (reported for 6.0% and 3.7% of patients who received active treatment or control/SoC, respectively) and erythema reported separately from application-site reaction (5.0% and 1.0%, respectively) (Table 6).
 |
Table 5 Selected Application-Site Reactions by Age Category (as-Treated Analysis) |
 |
Table 6 Most Common TEAEs by System Organ Class and Age Category (as-Treated Analysis) |
Similar proportions of patients aged <75 and ≥75 years who were treated with the capsaicin patch experienced TEAEs (78.1% and 81.6%, respectively), serious TEAEs (7.2% and 8.2%), and TEAEs leading to discontinuation of study treatment (1.0% and 0.9%) (Table 4; Figure 2). Among patients who received control or SoC treatment, patients aged <75 years were less likely to experience TEAEs (64.1% vs 72.4%) and serious TEAEs (4.1% vs 6.1%), and more likely to experience TEAEs leading to discontinuation (2.3% vs 1.0%), than patients aged ≥75 years. There were 16 deaths in total: 13 in the <75 years age group and 3 in the ≥75 years age group. None of the deaths were considered by the investigators to be related to trial medication and were instead deemed to be consequences of underlying disease(s).
Application-site reactions, the most common TEAEs in each age group, occurred with similar frequency in patients aged ≥75 (63.1%) and <75 (62.0%) years who received the capsaicin patch (Table 5). Among patients in the control/SoC group, 49.3% and 30.7% of patients aged ≥75 and <75 years, respectively, experienced application-site reactions. Each type of application-site reaction was more common with active treatment than with a control patch in both age groups.
With the capsaicin patch, the pattern of TEAEs, TEAEs leading to discontinuation from treatment, and application-site reactions across application durations of 30, 60, or 90 minutes were similar in the ≥75 years and <75 years age groups (Figure 3).
Discussion
The pooled analysis of data from interventional clinical studies supports the efficacy and safety of the capsaicin patch in older patients with PNP. A statistically significant treatment effect compared with a control patch was observed; additionally, improvements were reported in pain scores relative to control that were similar in patients aged ≥73 and <73 years with PHN. The analyses also support the safety and tolerability of the capsaicin patch in older patients, and the safety profile did not differ between patients aged ≥75 and <75 years in a mixed PNP pooled population. Application-site reactions were the most commonly reported AE in both the ≥75 and <75 years age groups.
There is no scientific basis for a differential effect of a topical treatment such as the capsaicin 179 mg patch based on age as its effect is not determined by systemic exposure.38 Nevertheless, it is important to verify in a clinical setting that the effectiveness of the capsaicin 179 mg patch is not age-dependent. In patients aged ≥73 years, a single treatment with the capsaicin patch resulted in an absolute treatment difference vs control of ‒0.4 (95% CI ‒0.7, ‒0.1) in change in the NPRS score from baseline to week 2–12. This difference in pain relief between the capsaicin patch and control was statistically significant. Moreover, the treatment difference between the capsaicin patch and control in change in NPRS score from baseline to week 2–12 was the same as that observed in adult patients aged <73 years, namely ‒0.4 (95% CI ‒0.7, ‒0.1). The question as to whether statistically significant results in chronic pain studies are clinically relevant is still a matter of interpretation.39 The IMMPACT group recommended that statistical significance is necessary but that other factors should also be considered when evaluating the clinical relevance of the difference between treatments.34 Not all factors proposed by the IMMPACT group can be considered given the limitations of the current study; nevertheless, all evaluable factors support the clinical relevance of the statistical significant effect as follows: 1) the tolerability profile of the capsaicin patch is (except for a higher incidence of transient application-site reactions) similar to that of control/SoC; the treatment with capsaicin patch has 2) no requirement for titration, 3) no negative impact on pill burden, 4) ensured compliance as the application is performed by a healthcare professional, 5) a novel mechanism of action that only requires treatment of the affected areas locally, 6) no drug–drug interactions, and 7) a treatment effect that lasts for up to 12 weeks. Of note, responder rates for a 30% decrease in NPRS score from baseline in adult patients with PNP after administration of the capsaicin patch (55.7%) were non-inferior to those obtained with daily pregabalin (54.5%), and the median time to pain intensity reduction (where 50% of patients had a 30% reduction in NPRS scores over 3 consecutive days) was significantly shorter for the capsaicin patch (7.5 days [95% CI 6.0, 10.0] vs 36.0 days [95% CI 22.0, –50.0] for pregabalin) in a head-to-head trial of 8 weeks’ duration.13
In terms of tolerability, the most striking difference between patients aged <75 years and those aged ≥75 years is the more frequent occurrence in the latter of erythema. This may relate, in part, to the progressive decrease of thickness of the skin with increasing age.40 In our analysis, TEAEs leading to discontinuation of the capsaicin patch were reported for ≤1% of adults in each age group, supporting the tolerability of this topical treatment. In the head-to-head comparison with daily oral pregabalin, no patient receiving the capsaicin patch, and 8.5% of patients receiving pregabalin, discontinued study medication because of TEAEs.13
Strengths of these analyses include the large, pooled populations of older patients, mainly from controlled studies. Limitations of the efficacy analysis include potential for bias relating to differences in the number of patients included from each source trial in the pooled datasets, and minor differences in aspects of the trial design of the included studies. Additionally, as the pooled efficacy population included only patients with PHN, the findings may not reflect the breadth of PNP conditions experienced by older patients. Further, the efficacy analyses were all based on a single assessment tool, the NPRS, and were limited to a single 60-minute treatment with the capsaicin patch. The use of LOCF may no longer be considered as a justifiable imputation method;41 however, BOCF analysis was conducted as a sensitivity analysis with similar results (data not shown). Data on the longer-term efficacy of repeated treatment in older patients would be of benefit, as would data on the impact on patients’ quality of life, including in relation to sleep.
The availability of topical treatments with negligible systemic exposure may have particular advantages in the vulnerable population of older adults.6–8 Older patients taking oral, centrally acting treatments for neuropathic pain are at increased risk of systemic AEs as a result of age-related pharmacokinetic and pharmacodynamic changes and polypharmacy.6,42,43 As such, a reduced incidence of systemic AEs, and limited drug─drug interactions when taken concomitantly with other pain medications, is a benefit of topical patch treatments in this population.6,8 Somnolence and dizziness, common AEs associated with the use of oral pain medications, can be associated with falls, and have been shown to be more common in older (≥60 years) than in younger adults receiving pregabalin.44 Reduction or withdrawal of psychotropic medications is recommended by the World Health Organization as a preventive measure for falls, the second leading global cause of accidental injury-related death.45 In our analysis, somnolence and dizziness were reported for 0.6% and 3.0%, respectively, of older adults who received the capsaicin patch, and for 1.0% and 4.4%, respectively, of patients who received control/SoC.
Most of the trials included in our analyses were pivotal Phase 3 trials evaluating a single treatment of the capsaicin patch, in which the use of concomitant medications was kept stable over the trial duration. However, for one trial comparing multiple treatments with the capsaicin patch with SoC in a pDPN population, the use of concomitant neuropathic pain medication has been evaluated over 52 weeks. Whilst the percentage of patients using antidepressants, antiepileptics, and opioids remained fairly stable in the capsaicin patch 30-minute treatment group (10.9%, 28.2%, and 10.9% of patients at baseline, vs 11.0%, 29.5%, and 11.0% at end of trial, respectively), in the group treated with SoC the use of these medications increased considerably (from 7.7%, 32.3%, and 8.4% at baseline to 15.1%, 43.2%, and 11.6% at end of trial, respectively).24,37 Thus, it thus appears that patients treated with the capsaicin patch do not require additional concomitant oral neuropathic pain medication over time. This could be of particular benefit in older patients as polypharmacy and a high medication burden can become overwhelming, especially for those who cannot rely on help to take their medications, sometimes resulting in medication nonadherence and suboptimal management of chronic conditions.46
Clear instructions are available for healthcare professionals on how to administer the capsaicin patch. The treatment ensures local delivery of capsaicin to hyperactive nociceptor nerves and does not result in clinically relevant systemic exposure. Consequently, no dose adjustment is required for patients with hepatic or renal impairment.47,48
Of note, in our safety analysis, ≤1% of patients in each age group experienced AEs that led to discontinuation of study treatment. This may reflect findings from a recent discrete choice experiment conducted in German patients with PNP (almost half of them aged ≥60 years), showing that local skin-related side effects were more acceptable to patients than systemic side effects such as dizziness, fatigue, and nausea.49 Overall, the published literature in the wider adult population confirms the acceptable tolerability of the capsaicin patch, with transient application-site discomfort and pain as the main adverse effects.50–52 These are generally tolerable, usually resolve without sequelae within a short period after treatment, and in almost all cases can be well managed with local cooling and/or oral analgesics.52,53 Adherence to the full intended treatment duration of capsaicin indicated that patch application-related pain was not a barrier to use.
In conclusion, the data from these pooled analyses indicate that in older patients with PNP, the capsaicin patch is efficacious and has a tolerability profile similar to that observed in adults. The safety profile is characterized mainly by local application-site reactions, with low rates of AE-related treatment discontinuation across age populations. Therefore, this topical treatment provides a valuable alternative to systemic oral therapies in older patients, limiting the risk of systemic AEs and the risk of drug–drug interactions with concomitant medications.
Abbreviations
AE, adverse event; ANCOVA, analysis of covariance; BOCF, baseline observation carried forward; CI, confidence interval; HIV-PN, human immunodeficiency virus infection-related peripheral neuropathy; IMMPACT, Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials; LOCF, last observation carried forward; LS, least squares; MOP, μ-opioid peptide; NOP, nociceptin/orphanin FQ peptide; NPRS, numeric pain rating scale; OR, odds ratio; pDPN, painful diabetic peripheral neuropathy; PHN, post-herpetic neuralgia; PNP, peripheral neuropathic pain; SE, standard error; SoC, standard of care; TEAE, treatment-emergent adverse event.
Data Sharing Statement
Grünenthal will endeavor to share clinical information from applicable studies, ie clinical study reports and clinical data from interventional clinical trials, with suitably qualified scientific and medical researchers as necessary for conducting legitimate research. Access requests must be submitted through ClinicalStudyDataRequest.com. Further information is available at https://www.grunenthal.com/en/science/clinical-trials/data-sharing-clinical-trials.
Analysis Preregistration
The clinical trials included in the pooled analyses were preregistered in independent institutional registries. The pooled analyses were preplanned, but the analysis plan was not preregistered.
Acknowledgments
Medical writing support was provided by NexGen Healthcare Communications.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Grünenthal GmbH. Grünenthal GmbH was involved across all stages of the publication.
Disclosure
Gisèle Pickering has received consultancy fees from Grünenthal, Mylan, MundiPharma, and Sanofi. Sylvia Engelen, Maria Stupar, and Mariëlle Eerdekens are employees of Grünenthal GmbH. Hervé Ganry has received consultancy fees from Grünenthal GmbH.
References
1. Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi:10.1212/01.wnl.0000282763.29778.59
2. Stompór M, Grodzicki T, Stompór T, Wordliczek J, Dubiel M, Kurowska I. Prevalence of chronic pain, particularly with neuropathic component, and its effect on overall functioning of elderly patients. Med Sci Monit. 2019;25:2695–2701. doi:10.12659/MSM.911260
3. Smith BH, Torrance N. Epidemiology of neuropathic pain and its impact on quality of life. Curr Pain Headache Rep. 2012;16(3):191–198. doi:10.1007/s11916-012-0256-0
4. Allegri M, Baron R, Hans G, et al. A pharmacological treatment algorithm for localized neuropathic pain. Curr Med Res Opin. 2016;32(2):377–384. doi:10.1185/03007995.2015.1129321
5. Saraiva MD, Suzuki GS, Lin SM, de Andrade DC, Jacob-Filho W, Suemoto CK. Persistent pain is a risk factor for frailty: a systematic review and meta-analysis from prospective longitudinal studies. Age Ageing. 2018;47(6):785–793. doi:10.1093/ageing/afy104
6. Pickering G, Martin E, Tiberghien F, Delorme C, Mick G. Localized neuropathic pain: an expert consensus on local treatments. Drug Des Devel Ther. 2017;11:2709–2718. doi:10.2147/DDDT.S142630
7. Pickering G, Lucchini C. Topical treatment of localized neuropathic pain in the elderly. Drugs Aging. 2020;37(2):83–89. doi:10.1007/s40266-019-00739-9
8. Luchting B, Azad SC. Pain therapy for the elderly patient: is opioid-free an option? Curr Opin Anaesthesiol. 2019;32(1):86–91. doi:10.1097/ACO.0000000000000675
9. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
10. Moisset X, Bouhassira D, Avez Couturier J, et al. Pharmacological andnon-pharmacological treatments for neuropathic pain: systematic review and Frenchrecommendations. Rev Neurol. 2020;176(5):325–352. doi:10.1016/j.neurol.2020.01.361
11. Averitas Pharma Inc. Prescribing information. QUTENZA® (capsaicin) topical system. 2022. www.fda.gov/medwatch.
12. Grunenthal Ltd. Qutenza 179 mg cutaneous patch;2022. Available from: https://www.medicines.org.uk/emc/product/573/smpc.
13. Haanpää M, Cruccu G, Nurmikko TJ, et al. Capsaicin 8% patch versus oral pregabalin in patients with peripheral neuropathic pain. Eur J Pain. 2016;20(2):316–328. doi:10.1002/ejp.731
14. van Nooten F, Treur M, Pantiri K, Stoker M, Charokopou M. Capsaicin 8% patch versus oral neuropathic pain medications for the treatment of painful diabetic peripheral neuropathy: a systematic literature review and network meta-analysis. Clin Ther. 2017;39(4):787–803.e18. doi:10.1016/j.clinthera.2017.02.010
15. Kopsky DJ, van Eijk RPA, Warendorf JK, Keppel Hesselink JM, Notermans NC, Afje V. Enriched enrollment randomized double-blind placebo-controlled cross-over trial with phenytoin cream in painful chronic idiopathic axonal polyneuropathy (EPHENE): a study protocol. Trials. 2022;23(1):888. doi:10.1186/s13063-022-06806-8
16. Lippi L, de Sire A, Folli A, et al. Multidimensional effectiveness of botulinum toxin in neuropathic pain: a systematic review of randomized clinical trials. Toxins. 2022;14(5):308. doi:10.3390/toxins14050308
17. Quintero JM, Pulido G, Giraldo LF, Leon MX, Diaz LE, Bustos RH. A systematic review on cannabinoids for neuropathic pain administered by routes other than oral or inhalation. Plants. 2022;11(10):1357. doi:10.3390/plants11101357
18. Backonja M, Malan TP, Vanhove GF, Tobias JK. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomized, double-blind, controlled study with an open-label extension. Pain Med. 2010;11(4):600–608. doi:10.1111/j.1526-4637.2009.00793.x
19. Webster LR, Malan TP, Tuchman MM, Mollen MD, Tobias JK, Vanhove GF. A multicenter, randomized, double-blind, controlled dose finding study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. J Pain. 2010;11(10):972–982. doi:10.1016/j.jpain.2010.01.270
20. Webster LR, Tark M, Rauck R, Tobias JK, Vanhove GF. Effect of duration of postherpetic neuralgia on efficacy analyses in a multicenter, randomized, controlled study of NGX-4010, an 8% capsaicin patch evaluated for the treatment of postherpetic neuralgia. BMC Neurol. 2010;10(1):92.
21. Irving GA, Backonja MM, Dunteman E, et al. A multicenter, randomized, double-blind, controlled study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. Pain Med. 2011;12:99–109. doi:10.1111/j.1526-4637.2010.01004.x
22. Webster LR, Nunez M, Tark MD, et al. Tolerability of NGX-4010, a capsaicin 8% dermal patch, following pretreatment with lidocaine 2.5%/prilocaine 2.5% cream in patients with post-herpetic neuralgia. BMC Anesthesiol. 2011;11:25. doi:10.1186/1471-2253-11-25
23. Simpson DM, Robinson-Papp J, Van J, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. J Pain. 2017;18(1):42–53. doi:10.1016/j.jpain.2016.09.008
24. Vinik AI, Perrot S, Vinik EJ, et al. Capsaicin 8% patch repeat treatment plus standard of care (SOC) versus SOC alone in painful diabetic peripheral neuropathy: a randomised, 52-week, open-label, safety study. BMC Neurol. 2016;16(1):251. doi:10.1186/s12883-016-0752-7
25. Simpson DM, Brown S, Tobias JK, Vanhove GF. NGX-4010, a capsaicin 8% dermal patch, for the treatment of painful HIV-associated distal sensory polyneuropathy results of a 52-week open-label study. Clin J Pain. 2014;30(2):134–142. doi:10.1097/AJP.0b013e318287a32f
26. Simpson DM, Brown S, Tobias J. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology. 2008;70(24):2305–2313. doi:10.1212/01.wnl.0000314647.35825.9c
27. Simpson DM, Estanislao L, Brown SJ, Sampson J. An open-label pilot study of high-concentration capsaicin patch in painful HIV neuropathy. J Pain Symptom Manage. 2008;35(3):299–306. doi:10.1016/j.jpainsymman.2007.04.015
28. Clifford DB, Simpson DM, Brown S, et al. A randomized, double-blind, controlled study of NGX-4010, a capsaicin 8% dermal patch, for the treatment of painful HIV-associated distal sensory polyneuropathy. J Acquir Immune Defic Syndr. 2012;59(2):126–133. doi:10.1097/QAI.0b013e31823e31f7
29. Webster LR, Peppin JF, Murphy FT, Tobias JK, Vanhove GF. Tolerability of NGX-4010, a capsaicin 8% patch, in conjunction with three topical anesthetic formulations for the treatment of neuropathic pain. J Pain Res. 2012;5:7–13.
30. Simpson DM, Gazda S, Brown S, et al. Long-term safety of ngx-4010, a high-concentration capsaicin patch, in patients with peripheral neuropathic pain. J Pain Symptom Manage. 2010;39(6):1053–1064.
31. Gálvez R, Navez ML, Moyle G, et al. Capsaicin 8% patch repeat treatment in nondiabetic peripheral neuropathic pain. Clin J Pain. 2017;33(10):921–931.
32. Jensen TS, Høye K, Fricová J, et al. Tolerability of the capsaicin 8% patch following pretreatment with lidocaine or tramadol in patients with peripheral neuropathic pain: a multicentre, randomized, assessor-blinded study. Eur J Pain. 2014;18(9):1240–1247.
33. Backonja M, Wallace MS, Blonsky R, et al. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurol. 2008;7(12):1106–1112. doi:10.1016/S1474-4422(08)70228-X
34. Smith SM, Dworkin RH, Turk DC, et al. Interpretation of chronic pain clinical trial outcomes: IMMPACT recommended considerations. Pain. 2020;161(11):2446–2461. doi:10.1097/j.pain.0000000000001952
35. European Medicines Agency. ICH Topic E 7. Studies in support of special populations: geriatrics; 1994. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-7-studies-support-special-populations-geriatrics-step-5_en.pdf.
36. Little W, Vyain S, Cody-Rydzewski S, et al. Introduction to Sociology; 2014. Available from: https://opentextbc.ca/introductiontosociology/.
37. Vinik AI. Repeat treatment with capsaicin 8% patch (179mg capsaicin cutaneous patch): effects on pain, quality of life, and patient satisfaction in painful diabetic peripheral neuropathy: an open-label, randomized controlled clinical trial. J Curr Med Res Opinion. 2019;02(12):388–401. doi:10.15520/jcmro.v2i12.242
38. Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011;107(4):490–502. doi:10.1093/bja/aer260
39. Olsen MF, Bjerre E, Hansen MD, Tendal B, Hilden J, Hróbjartsson A. Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: systematic review of empirical studies. J Clin Epidemiol. 2018;101:87–106.e2. doi:10.1016/j.jclinepi.2018.05.007
40. Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (I): blood flow, pH, thickness, and ultrasound echogenicity. Skin Res Technol. 2005;11(4):221–235. doi:10.1111/j.0909-725X.2005.00151.x
41. Lachin JM. Fallacies of last observation carried forward analyses. Clin Trials. 2016;13(2):161–168. doi:10.1177/1740774515602688
42. Pickering G, Marcoux M, Chapiro S, et al. An algorithm for neuropathic pain management in older people. Drugs Aging. 2016;33(8):575–583. doi:10.1007/s40266-016-0389-7
43. Holt S, Schmiedl S, Thürmann PA. Potenziell inadäquate medikation für ältere menschen: die PRISCUS-liste. Dtsch Arztebl. 2010;107(31–32):1.
44. Mukai R, Hasegawa S, Umetsu R, et al. Evaluation of pregabalin-induced adverse events related to falls using the FDA adverse event reporting system and Japanese Adverse Drug Event Report databases. J Clin Pharm Ther. 2019;44(2):285–291. doi:10.1111/jcpt.12790
45. World Health Organization. Falls; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/falls.
46. Farrell B, French Merkley V, Ingar N. Reducing pill burden and helping with medication awareness to improve adherence. Can Pharm J. 2013;146(5):262–269. doi:10.1177/1715163513500208
47. Babbar S, Marier JF, Mouksassi MS, et al. Pharmacokinetic analysis of capsaicin after topical administration of a high-concentration capsaicin patch to patients with peripheral neuropathic pain. Ther Drug Monit. 2009;31(4):502–510. doi:10.1097/FTD.0b013e3181a8b200
48. Aitken E, McColl G, Kingsmore D. The role of Qutenza® (topical capsaicin 8%) in treating neuropathic pain from critical ischemia in patients with end-stage renal disease: an observational cohort study. Pain Med. 2017;18(2):330–340. doi:10.1093/pm/pnw139
49. Schubert T, Kern KU, Schneider S, Baron R. Oral or topical pain therapy—how would patients decide? a discrete choice experiment in patients with peripheral neuropathic pain. Pain Pract. 2021;21(5):536–546. doi:10.1111/papr.12989
50. Dupoiron D, Jubier-Hamon S, Seegers V, et al. Peripheral neuropathic pain following breast cancer: effectiveness and tolerability of high-concentration capsaicin patch. J Pain Res. 2022;15:241–255. doi:10.2147/JPR.S341378
51. Tenreiro Pinto J, Pereira FC, Loureiro MC, Gama R, Fernandes HL. Efficacy analysis of capsaicin 8% patch in neuropathic peripheral pain treatment. Pharmacology. 2018;101(5–6):290–297. doi:10.1159/000487444
52. Knolle E, Zadrazil M, Kovacs GG, Medwed S, Scharbert G, Schemper M. Comparison of cooling and EMLA to reduce the burning pain during capsaicin 8% patch application: a randomized, double-blind, placebo-controlled study. Pain. 2013;154(12):2729–2736. doi:10.1016/j.pain.2013.08.001
53. Peppin JF, Majors K, Webster LR, Simpson DM, Tobias JK, Vanhove GF. Tolerability of NGX-4010, a capsaicin 8% patch for peripheral neuropathic pain. J Pain Res. 2011;4:385–392. doi:10.2147/JPR.S22954
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.