Back to Journals » Clinical Interventions in Aging » Volume 19
Lung Ultrasound Score as a Predictor of Failure to Wean COVID-19 Elderly Patients off Mechanical Ventilation: A Prospective Observational Study
Authors Wang Y, Yi Y, Zhang F, Yao YY, Chen YX , Wu CM, Wang RY, Yan M
Received 9 October 2023
Accepted for publication 6 February 2024
Published 19 February 2024 Volume 2024:19 Pages 313—322
DOI https://doi.org/10.2147/CIA.S438714
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Ying Wang,1,* Yu Yi,1,* Fan Zhang,1 Yuan-Yuan Yao,2 Yue-Xiu Chen,2 Chao-Min Wu,2 Rui-Yu Wang,2 Min Yan1,2
1Jiangsu Province Key Laboratory of Anesthesiology, Xuzhou Medical University, Xuzhou, 221004, People’s Republic of China; 2Department of Anesthesiology, the Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, 310016, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Min Yan, Email [email protected]
Background: The lung ultrasound score was developed for rapidly assessing the extent of lung ventilation, and it can predict failure to wean various types of patients off mechanical ventilation. Whether it is also effective for COVID-19 patients is unclear.
Methods: This single-center, prospective, observational study was conducted to assess the ability of the 12-region lung ultrasound score to predict failure to wean COVID-19 patients off ventilation. In parallel, we assessed whether right hemidiaphragmatic excursion or previously published predictors of weaning failure can apply to these patients. Predictive ability was assessed in terms of the area under the receiver operating characteristic curve (AUC).
Results: The mean age of the 35 patients in the study was (75 ± 9) years and 12 patients (37%) could not be weaned off mechanical ventilation. The lung ultrasound score predicted these failures with an AUC of 0.885 (95% CI 0.770– 0.999, p < 0.001), and a threshold score of 10 provided specificity of 72.7% and sensitivity of 92.3%. AUCs were lower for previously published predictors of weaning failure, and right hemidiaphragmatic excursion did not differ significantly between the two groups.
Conclusion: The lung ultrasound score can accurately predict failure to wean critically ill COVID-19 patients off mechanical ventilation, whereas assessment of right hemidiaphragmatic excursion does not appear helpful in this regard.
Trial Registration: https://clinicaltrials.gov/ct2/show/NCT05706441.
Keywords: lung ultrasound score, diaphragmatic excursion, spontaneous breathing trial, weaning, COVID-19, critical care
Background
The COVID-19 pandemic substantially increased numbers of patients admitted to the intensive care unit for respiratory failure, especially in the elderly, where the mortality rate among such patients can exceed 30%.1,2 Many COVID-19 patients require mechanical ventilation longer than 2–3 weeks.3 During the intensive care of these and other types of patients, clinicians face the difficult decision of whether to halt or continue mechanical ventilation: clinicians may prefer to continue it until the patient’s condition improves, but prolonged respiratory support increases risk of complications such as bacterial pneumonia and barotrauma with alveolar rupture, while occupying limited intensive care resources.4
Weaning off mechanical ventilation may fail for up to 20% of patients without COVID-19 or up to 40% of patients with COVID-19.5,6 Reliable prediction of which patients can or cannot be weaned off mechanical ventilation would help clinicians provide effective care and optimize the use of limited medical resources. Several predictors of weaning failure have been proposed, and perhaps the most widely used is the rapid shallow respiratory index (RSBI).7 RSBI was calculated as described by dividing breathing frequency by tidal volume. Others include the lung ultrasound score, which assesses pulmonary ventilation based on ultrasound images; and diaphragmatic excursion, in which the diaphragm is visualized through subcostal ultrasound.8,9 These predictors were developed before the COVID-19 pandemic, and whether they apply to COVID-19 patients is unclear. The RSBI, in particular, becomes less reliable for patients on prolonged mechanical ventilation, which is the situation for many critically ill COVID-19 patients.10
Here, we assessed whether the lung ultrasound score can reliably predict failure to wean COVID-19 patients off mechanical ventilation. We focused on this score because the pneumonia lesions in such patients can easily be monitored using ultrasound.11 We also assessed the predictive ability of diaphragmatic excursion, respiratory rate, and shallow fast breathing index.
Methods
Patients
For this prospective observational trial, we enrolled patients who (1) were admitted between January 11, 2023, and March 30, 2023, to the intensive care unit at our university-affiliated tertiary care medical center; (2) tested positive, at the time of admission, for the causative SARS-CoV-2 virus based on PCR analysis of nasopharyngeal or bronchoalveolar samples, alongside observed abnormalities like ground-glass shadows on chest computed tomography (Supplementary Figure S1); (3) were older than 18 years; (4) required mechanical ventilation due to respiratory failure; and (5) were judged by the attending physician to be able to undergo a spontaneous breathing test because the causes of intubation had sufficiently improved or resolved. Patients were excluded if they had flail chest or rib fractures, neuromuscular disease, or stridor indicating upper airway involvement.
Spontaneous Breathing Test and Weaning
During the spontaneous breathing test, enrolled patients were subjected to a pressure support ventilation of 8 cmH2O and a small amount of applied PEEP (4 to 5 cmH2O), while maintaining the same fractional inspired oxygen as during mechanical ventilation, for a duration of 60 minutes. At 30 min after starting the test, ultrasound assessments were performed to enable determination of the lung ultrasound score and diaphragmatic excursion (see next section). All manipulations were done during spontaneous breathing of the patient. Blood gases were also analyzed.
Patients were considered to pass the spontaneous breathing test unless they showed one of the following: altered mental status, malaise, sweating, respiratory rate above 35 beats/minute, heart rate > 140 and/or systolic blood pressure > 180 or < 90 mmHg, or obvious signs of extreme labor during breathing.12 Weaning failure was defined as failure in the spontaneous breathing test, or the need for non-invasive or mechanical ventilation or death within 48 h after passing the test.13
Attending physicians who were not involved in the study and who were unaware of ultrasound findings decided whether patients failed the spontaneous breathing test or required ventilation after passing the test.
Ultrasonography
All ultrasonography was performed by a trained researcher using an M9 system (Mindray, Shenzhen, Guangdong, China). For ultrasound imaging of the lung, patients were in the supine position with the head of the bed raised 10–15° and 12-region imaging was conducted (Supplementary Figure S2). In each hemithorax, the parasternal, anterior axillary, and posterior axillary lines were used to identify anterior, lateral and posterior areas, each of which was subdivided into upper and lower halves.14,15 A convex 3–5 MHz probe was used to assess aeration loss in each intercostal space according to a four-point scale: 0 indicated normal aeration, defined as the presence of A lines and one or two isolated vertical B lines; 1 indicated moderate loss of lung ventilation, defined as multiple well-defined B1 lines; 2 indicated severe loss of lung ventilation, defined as multiple fused vertical B lines; and 3 indicated alveolar consolidation (Supplementary Figure S3). For each region of interest, the worst score for aeration loss among the images was used, and the scores for all 12 regions were summed to obtain a total score (maximal possible: 36). Lung ultrasound imaging and calculation of the total score took 10–15 min.
For ultrasound imaging of the diaphragm, patients were in a semi-recumbent position with the head of the bed raised 20–30°. The 3–5 MHz probe was placed on the mid-clavicular line below the right subcostal margin, and it was finely repositioned to optimize imaging of the posterior third of the right diaphragm. Imaging in M-mode was performed during tidal breathing such that diaphragmatic excursion could be visualized along a selected line perpendicular to the diaphragm. The excursion was defined as the distance from baseline on the vertical axis to the height at maximum inspiration during a breathing cycle (Supplementary Figure S4). The excursion was defined as the average from at least three measurements. Diaphragm imaging and calculation of excursion took 5–10 min.
Data Collection
After admission to the intensive care unit and before the spontaneous breathing test, each patient underwent a standard medical examination, including medical history, acute physiology and chronic health evaluation II, determination of the sequential organ failure assessment score and assays of procalcitonin, interleukin (IL)-6 and cardiac troponin in plasma. Either troponin I or high-sensitivity troponin T was assayed because both reflect myocardial damage, which was defined here as a level above the 99th percentile reference limit.16 Data were also collected on respiratory rate, tidal volume, and fractional inspired oxygen as determined by the ventilator. RSBI was calculated as described.17 Durations of mechanical ventilation were recorded.
Sample Size
We speculated a weaning failure rate of 35% based on previous studies, and we defined a minimal acceptable area under the receiver operator characteristic curve (AUC) to be 0.80 for predicting failure.18 Based on a type I error rate of 0.10 and power of 0.9, we calculated a minimal sample of 31 patients, which we increased to 35 to allow for up to 10% loss to follow-up.
Statistical Analysis
Continuous data were presented as mean ± standard deviation (SD) if normally distributed, or as median and interquartile range (IQR) if skewed. Categorical data were reported as absolute or relative frequencies (%). Intergroup differences in continuous data were assessed for significance using unpaired Student’s t, Mann–Whitney, or paired Wilcoxon tests. Intergroup differences in categorical data were assessed using chi-squared or Fisher’s exact tests.
The predictive accuracy of the lung ultrasound score, respiratory rate or RSBI was assessed in terms of AUCs. The optimal cut-off values were determined using Youden’s index, then the corresponding sensitivity, specificity and other indicators of diagnostic performance were calculated. In addition to assessing predictive performance using a point cut-off, we estimated likelihood ratios using inconclusive limits, with ratios >10 or <0.2 defined as clinically valuable.19
Factors associated with weaning failure were identified using Spearman’s rank correlation. Elements with p < 0.05 in the significance variables of univariate analysis were included in multivariate logistic regression analysis.
SPSS 20.0 (IBM, Armonk, NY, USA) software was used for statistical analysis, and GraphPad Prism 9 (San Diego, CA, USA) was used for graphing. Results with p < 0.05 were considered significant.
Results
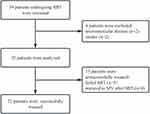
Of the 39 patients initially screened for enrollment, 35 were included in the study (Figure 1). All patients completed ultrasound imaging of the lung, but seven did not complete imaging of the diaphragm because they were unable to cooperate during the operation (2 patients) or they had abdominal distension (5 patients). The minimum age of the patients was 60 years, and the mean age was 75 ± 9 years (Table 1). Median duration of mechanical ventilation until the spontaneous breathing test was 14 days (IQR 9, 21 days) (Table 2).
 |
Table 1 Clinicodemographic Characteristics of Study Participants, Stratified by Whether They Were Weaned off Mechanical Ventilation |
 |
Table 2 Clinical and Spirometric Characteristics of the Overall Population and of Successfully and Unsuccessfully Weaned Patients |
Among the 35 patients, 13 could not be weaned off mechanical ventilation: five of those patients failed the spontaneous breathing test because of tachypnea or evident dyspnea, and the remaining eight passed the breathing test but had to resume mechanical ventilation within 48 h due to hemodynamic instability (2 patients) or progressive dyspnea (6 patients).
Compared to patients who could be weaned off mechanical ventilation, those who failed showed the following significant differences: higher procalcitonin level, higher lung ultrasound score, higher respiratory rate, and higher RSBI (Table 2). In contrast, diaphragmatic excursion did not differ significantly between the two groups.
Lung ultrasound score gave the best AUC for predicting weaning failure AUC 0.885, 95% CI 0.770–0.999), followed by RSBI (AUC 0.787, 95% CI 0.623, 0.950) and finally respiratory rate (AUC 0.715, 95% CI 0.514, 0.916) (Figure 2A, Table 3). A cut-off lung ultrasound score of 10 gave sensitivity of 92.3% and specificity of 72.7%. To assess predictive ability more comprehensively than with a point cut-off, we estimated inconclusive limits as described.19 Lung ultrasound scores >14 emerged as highly specific for predicting weaning failure, while scores <10 were highly sensitive for excluding weaning failure (Table 4, Figure 2B). The corresponding analysis of respiratory rate and RSBI led to likelihood ratios that were not clinically valuable (Supplementary Table S1).
 |
Figure 2 Area under the curve of the predictive index and inconclusive limits. (A) Receiver operating characteristic curves to assess the ability of lung ultrasound score (LUS), respiratory rate (RR) or rapid shallow breathing index (RSBI) to predict weaning failure. The curves were generated by Prism 9 software and used to determine the AUC (area under the curve) for each predictor. (B) Inconclusive limits on the ability of lung ultrasound score to predict weaning failure. Limits were calculated as described in Ray et al.19 Map was created using Prism 9 software. |
 |
Table 3 Performance of Different Indicators in Predicting Weaning Failure |
 |
Table 4 Likelihood Ratios Describing the Ability of Different Ranges of the Lung Ultrasound Score to Predict Weaning Failure |
As a potential alternative to the 12-region lung ultrasound score, we explored the predictive ability of an 8-region score calculated by summing the subscores for the anterior chest and lateral thoracic regions. We obtained an AUC of 0.832 (p < 0.001, Supplementary Figure S5).
Univariate analysis identified lung ultrasound score, respiratory rate and RSBI as significantly associated with weaning failure (Table 2), but multivariate regression identified lung ultrasound score as the only independent predictor (Table 5). The same result was obtained whether we included RSBI or respiratory rate in the multivariate model. We did not include the two together in one model because they correlated with each other (r = 0.79, p < 0.001; Supplementary Figure S6).
 |
Table 5 Multiple Logistic Regression to Identify Independent Predictors of Weaning Failure |
Discussion
In this prospective study focusing on critically ill mechanically ventilated patients afflicted with COVID-19, a striking demographic trend emerged – all our study participants were aged 60 years or older. This unforeseen but notable skew towards an older demographic was accompanied by a high prevalence of comorbidities, particularly hypertension, which was observed in approximately half of our patients (48.5%). This observation is consistent with a large body of literature that agrees that advanced age is an important risk factor for adverse COVID-19 outcomes.3,20 Older adults are known to undergo age-related changes in immune function, as well as a higher prevalence of comorbidities, which makes them more susceptible to the severe effects of the virus.21
Consistent with previous literature, our study reports a very high weaning failure rate of 37%.6,18 Our study suggests that calculating the lung ultrasound score after 30 min of spontaneous breathing can accurately predict whether a critically ill COVID-19 patient is ready to be weaned or not off mechanical ventilation. Two other previously published predictors of weaning failure, respiratory rate and RSBI, did not perform as well as lung ultrasound score. Diaphragmatic excursion was not useful for predicting weaning failure in our cohort, though this result should be interpreted carefully given that we were able to measure it in only 28 patients.
In our sample, the best cut-off value for lung ultrasound scores to predict unsuccessful weaning was ≥10, which is comparable to previous studies in elderly intensive care patients without COVID-19.22,23 Nevertheless, the cut-off of 10 in our study is lower than that of 13 in one of those studies,23 perhaps reflecting the greater mean age in our sample (75 vs 60 years) and, therefore, age-related reductions in rib mobility and volume per breath,24 lung elasticity and alveolar surface area,25 angiogenesis and vascular elasticity,26,27 as well as ATP production and energy reserves.28 It may also reflect pulmonary damage due to SARS-CoV-2.29,30 Furthermore, our study revealed that a lung ultrasound score of less than 10 indicates a significantly low risk of weaning failure, whereas scores exceeding 14 suggest a considerably high risk. Thus, we recommend that patients with a lung ultrasound score greater than 14 receive extended mechanical ventilation. Thille et al31 demonstrated the efficacy of prophylactic noninvasive mechanical ventilation post-extubation in reducing failure rates among high-risk non-COVID-19 patients. This finding has been recently corroborated by a network meta-analysis.32 Considering the heightened risk of weaning failure indicated by high LUS in COVID-19 patients, exploring the prophylactic use of noninvasive mechanical ventilation may be beneficial.
Transthoracic lung ultrasonography is reliable, accurate and non-invasive, and it can easily be performed at the bedside, giving it numerous advantages over traditional radiological methods for assessing lung ventilation.33 However, ultrasound has its inherent limitations. Assessing obese patients presents challenges due to the thickness of subcutaneous tissue in the rib cage. The presence of subcutaneous emphysema or extensive chest dressings can preclude the transmission of ultrasound beams to the lungs. Although the accuracy of ultrasonography depends on the operator’s proficiency and experience, lung ultrasonography can be performed accurately even by residents after approximately 25 supervised measurements.34 In addition, our analysis suggests that the 8-region lung ultrasound score may provide a reasonable alternative to the 12-region score for patients who are difficult to move.
In contrast to lung ultrasonography, diaphragmatic ultrasound using the anterior subcostal approach did not reveal useful differences that could help predict weaning failure in our critically ill COVID-19 patients. In addition, the necessary imaging could not be performed in several of our patients because of abdominal distention. This may reflect that all patients received enteral nutrition, consistent with international guidelines,35 and such nutrition can cause abdominal distension in two-thirds of critically ill COVID-19 patients.36 This distention may reflect direct gastrointestinal effects of SARS-CoV-2 and gastrointestinal dyskinesia due to heavy use of sedatives and prolonged mechanical ventilation.37,38
Two patients in our study developed diaphragmatic paralysis, which has been associated with longer weaning times and higher rates of weaning failure.8 The two patients in our study had 12-region lung ultrasound scores of 15 and 17, and both failed to be weaned during the study.
Our study found RSBI to exhibit inadequate predictive performance and therefore it should not be used as a stand-alone test in COVID-19 patients. Rather surprisingly, in our patients with failed weaning, the RSBI (55 breaths/min/L) was significantly lower than the threshold of 105 reported by Yang and Tobin in their original study predicting weaning failure.39 However, this is not an isolated occurrence, as one meta-analysis reported diverse predictive values for RSBI, likely attributed to differences in study methods, outcome classifications, and patient populations.7
In our cohort, the inflammatory parameter procalcitonin was significantly higher among patients who failed to be weaned than among those who were weaned, consistent with a previous study linking higher procalcitonin to more severe COVID-19.40 However, procalcitonin level did not predict weaning failure in our multivariate analysis. IL-6, whose elevation has been linked to more severe COVID-19 and worse outcomes,41 did not differ significantly between our patients who failed to be weaned and those who were weaned. Thus, our findings are not entirely consistent with previous reports that markers of infection and inflammation are also associated with weaning success.42,43 This may reflect our small sample. Further research is needed to clarify the association of inflammatory markers with weaning outcomes in COVID-19 patients. For example, studies should clarify whether elevated procalcitonin is part of an inflammatory syndrome associated with COVID-19, or it indicates concurrent bacterial infection requiring antibiotic therapy.44
Our results should be generalized carefully in light of the characteristics of our cohort. Just over one-third of patients underwent tracheostomy, which is recommended for patients requiring long-term mechanical ventilation.45 Rate of weaning failure did not differ significantly between patients who underwent tracheostomy or not (Table 1). Furthermore, the inclusion of patients with tracheal intubation and tracheotomy enhances the generalizability and external validity of our study findings. As these interventions are integral to the management of severe COVID-19 cases, omitting them from the study could limit the applicability of our conclusions to the broader patient population. Whether our findings are also applicable to younger COVID-19 critically ill patients remains needs to be explored. Indeed, our findings should be validated on a larger scale, preferably in a multisite population.
Future work should also assess cardiac function during the spontaneous breathing test. Since some weaning failures among COVID-19 patients are due to acute heart failure rather than respiratory failure, studies should explore whether combining cardiac function with lung ultrasonography improves prediction of weaning failure.
Conclusion
Our study not only advances our understanding of age-related vulnerabilities in the face of COVID-19 but also underscores the potential of lung ultrasound as a pivotal tool in guiding the weaning process for critically ill elderly patients. In our cohort, scores <10 predicted successful weaning, while scores >14 predicted failure. Our data suggest that measurement of diaphragmatic excursion is of limited usefulness for predicting weaning failure among these patients.
Abbreviations
AUC, Area under the curves; COVID-19, Coronavirus disease 2019; IL-6, Interleukin-6; RSBI, Rapid shallow breathing index.
Data Sharing Statement
The datasets in this study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The trial was approved by the Institutional Review Board of the Second Affiliated Hospital of the Medical College of Zhejiang University (IR2023-0020) and conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. Informed consent was obtained from the legal guardians of all study participants.
Acknowledgments
Ying Wang and Yu Yi are co-first authors for this study. We sincerely thank the intensive care staff for their hard work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Clinical Key Specialty Construction Project of China (2021-LCZDZK-01); Leading Health Talents of Zhejiang Province, Zhejiang Health Office No. 18(2020); and Key Laboratory of The Diagnosis and Treatment of Severe Trauma and Burn of Zhejiang Province.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Tyrrell CSB, Mytton OT, Gentry SV, et al. Managing intensive care admissions when there are not enough beds during the COVID-19 pandemic: a systematic review. Thorax. 2021;76(3):302–312. doi:10.1136/thoraxjnl-2020-215518
2. Auld SC, Caridi-Scheible M, Blum JM, et al. ICU and ventilator mortality among critically ill Adults with Coronavirus Disease 2019. Crit Care Med. 2020;48(9):e799–e804. doi:10.1097/CCM.0000000000004457
3. African C-CCOSI. Patient care and clinical outcomes for patients with COVID-19 infection admitted to African high-care or intensive care units (ACCCOS): a multicentre, prospective, observational cohort study. Lancet. 2021;397(10288):1885–1894. doi:10.1016/S0140-6736(21)00441-4
4. Brandi N, Ciccarese F, Rimondi MR, et al. An imaging overview of COVID-19 ARDS in ICU patients and its complications: a pictorial review. Diagnostics. 2022;12(4):846.
5. Thille AW, Richard JC, Brochard L. The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187(12):1294–1302. doi:10.1164/rccm.201208-1523CI
6. Vetrugno L, Orso D, Corradi F, et al. Diaphragm ultrasound evaluation during weaning from mechanical ventilation in COVID-19 patients: a pragmatic, cross-section, multicenter study. Respir Res. 2022;23(1):210. doi:10.1186/s12931-022-02138-y
7. Trivedi V, Chaudhuri D, Jinah R, et al. The usefulness of the rapid shallow breathing index in predicting successful extubation: a systematic review and meta-analysis. Chest. 2022;161(1):97–111. doi:10.1016/j.chest.2021.06.030
8. Kim WY, Suh HJ, Hong SB, Koh Y, Lim CM. Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med. 2011;39(12):2627–2630. doi:10.1097/CCM.0b013e3182266408
9. Mongodi S, De Luca D, Colombo A, et al. Quantitative lung ultrasound: technical aspects and clinical applications. Anesthesiology. 2021;134(6):949–965. doi:10.1097/ALN.0000000000003757
10. Verceles AC, Diaz-Abad M, Geiger-Brown J, Scharf SM. Testing the prognostic value of the rapid shallow breathing index in predicting successful weaning in patients requiring prolonged mechanical ventilation. Heart Lung. 2012;41(6):546–552. doi:10.1016/j.hrtlng.2012.06.003
11. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi:10.1016/S1473-3099(20)30086-4
12. Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033–1056. doi:10.1183/09031936.00010206
13. Schmidt GA, Girard TD, Kress JP, et al. Official Executive Summary of an American Thoracic Society/American College of Chest Physicians Clinical Practice Guideline: liberation from mechanical ventilation in critically ill adults. Am J Respir Crit Care Med. 2017;195(1):115–119. doi:10.1164/rccm.201610-2076ST
14. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577–591. doi:10.1007/s00134-012-2513-4
15. Hansell L, Milross M, Delaney A, Koo CM, Tian DH, Ntoumenopoulos G. Quantification of changes in lung aeration associated with physiotherapy using lung ultrasound in mechanically ventilated patients: a prospective cohort study. Physiotherapy. 2022;119:26–33. doi:10.1016/j.physio.2022.11.003
16. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40(3):237–269. doi:10.1093/eurheartj/ehy462
17. Song J, Qian Z, Zhang H, et al. Diaphragmatic ultrasonography-based rapid shallow breathing index for predicting weaning outcome during a pressure support ventilation spontaneous breathing trial. BMC Pulm Med. 2022;22(1):337. doi:10.1186/s12890-022-02133-5
18. Corradi F, Vetrugno L, Orso D, et al. Diaphragmatic thickening fraction as a potential predictor of response to continuous positive airway pressure ventilation in Covid-19 pneumonia: a single-center pilot study. Respir Physiol Neurobiol. 2021;284:103585. doi:10.1016/j.resp.2020.103585
19. Ray P, Le Manach Y, Riou B, Houle TT. Statistical evaluation of a biomarker. Anesthesiology. 2010;112(4):1023–1040. doi:10.1097/ALN.0b013e3181d47604
20. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. doi:10.1016/j.jinf.2020.04.021
21. Su K, Jin K. Aging during the pandemic: untangling the complexities of COVID-19 and geriatric care. Aging Dis. 2023;14(3):572–576. doi:10.14336/AD.2023.0405
22. Li S, Chen Z, Yan W. Application of bedside ultrasound in predicting the outcome of weaning from mechanical ventilation in elderly patients. BMC Pulm Med. 2021;21(1):217. doi:10.1186/s12890-021-01605-4
23. Soummer A, Perbet S, Brisson H, et al. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress*. Crit Care Med. 2012;40(7):2064–2072. doi:10.1097/CCM.0b013e31824e68ae
24. Skloot GS. The effects of aging on lung structure and function. Clin Geriatr Med. 2017;33(4):447–457. doi:10.1016/j.cger.2017.06.001
25. Schulte H, Muhlfeld C, Brandenberger C. Age-related structural and functional changes in the mouse lung. Front Physiol. 2019;10:1466. doi:10.3389/fphys.2019.01466
26. Jane-Wit D, Chun HJ. Mechanisms of dysfunction in senescent pulmonary endothelium. J Gerontol a Biol Sci Med Sci. 2012;67(3):236–241. doi:10.1093/gerona/glr248
27. Schneider JL, Rowe JH, Garcia-de-Alba C, Kim CF, Sharpe AH, Haigis MC. The aging lung: physiology, disease, and immunity. Cell. 2021;184(8):1990–2019. doi:10.1016/j.cell.2021.03.005
28. Desler C, Hansen TL, Frederiksen JB, Marcker ML, Singh KK, Juel Rasmussen L. Is there a link between mitochondrial reserve respiratory capacity and aging? J Aging Res. 2012;2012:192503. doi:10.1155/2012/192503
29. D’Agnillo F, Walters KA, Xiao Y, et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci Transl Med. 2021;13(620):eabj7790. doi:10.1126/scitranslmed.abj7790
30. Doglioni C, Ravaglia C, Chilosi M, et al. Covid-19 interstitial pneumonia: histological and immunohistochemical features on cryobiopsies. Respiration. 2021;100(6):488–498. doi:10.1159/000514822
31. Thille AW, Muller G, Gacouin A, et al. Effect of postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: a randomized clinical trial. JAMA. 2019;322(15):1465–1475. doi:10.1001/jama.2019.14901
32. Boscolo A, Pettenuzzo T, Sella N, et al. Noninvasive respiratory support after extubation: a systematic review and network meta-analysis. Eur Respir Rev. 2023;32(168):220196.
33. Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung Ultrasound for Critically Ill Patients. Am J Respir Crit Care Med. 2019;199(6):701–714. doi:10.1164/rccm.201802-0236CI
34. Rouby JJ, Arbelot C, Gao Y, et al. Training for lung ultrasound score measurement in critically ill patients. Am J Respir Crit Care Med. 2018;198(3):398–401. doi:10.1164/rccm.201802-0227LE
35. Warren M, McCarthy MS, Roberts PR. Practical application of the revised guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: a case study approach. Nutr Clin Pract. 2016;31(3):334–341. doi:10.1177/0884533616640451
36. Liu R, Paz M, Siraj L, et al. Feeding intolerance in critically ill patients with COVID-19. Clin Nutr. 2022;41(12):3069–3076. doi:10.1016/j.clnu.2021.03.033
37. Lamers MM, Beumer J, van der Vaart J, et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369(6499):50–54. doi:10.1126/science.abc1669
38. Reintam Blaser A, Preiser JC, Fruhwald S, et al. Gastrointestinal dysfunction in the critically ill: a systematic scoping review and research agenda proposed by the section of metabolism, endocrinology and nutrition of the European Society of Intensive Care Medicine. Crit Care. 2020;24(1):224. doi:10.1186/s13054-020-02889-4
39. Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324(21):1445–1450. doi:10.1056/NEJM199105233242101
40. Jackson I, Jaradeh H, Aurit S, et al. Role of procalcitonin as a predictor of clinical outcomes in hospitalized patients with COVID-19. Int J Infect Dis. 2022;119:47–52. doi:10.1016/j.ijid.2022.03.044
41. Burke H, Freeman A, Cellura DC, et al. Inflammatory phenotyping predicts clinical outcome in COVID-19. Respir Res. 2020;21(1):245. doi:10.1186/s12931-020-01511-z
42. Alladina JW, Levy SD, Hibbert KA, et al. Plasma concentrations of soluble suppression of tumorigenicity-2 and interleukin-6 are predictive of successful liberation from mechanical ventilation in patients with the acute respiratory distress syndrome. Crit Care Med. 2016;44(9):1735–1743. doi:10.1097/CCM.0000000000001814
43. Fleuren LM, Dam TA, Tonutti M, et al. Predictors for extubation failure in COVID-19 patients using a machine learning approach. Crit Care. 2021;25(1):448. doi:10.1186/s13054-021-03864-3
44. Vanhomwegen C, Veliziotis I, Malinverni S, et al. Procalcitonin accurately predicts mortality but not bacterial infection in COVID-19 patients admitted to intensive care unit. Ir J Med Sci. 2021;190(4):1649–1652. doi:10.1007/s11845-020-02485-z
45. McGrath BA, Brenner MJ, Warrillow SJ, et al. Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. Lancet Respir Med. 2020;8(7):717–725. doi:10.1016/S2213-2600(20)30230-7
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.