Back to Journals » Drug Design, Development and Therapy » Volume 16
Magnesium Lithospermate B Protects Against Cisplatin-Induced Acute Kidney Injury via Alleviating Mitochondrial Dysfunction
Authors Shen D, Guo M, Geng X, Yu J, Zhang Z, Lin J, Lin P, Ding X, Xu X
Received 18 January 2022
Accepted for publication 15 June 2022
Published 15 July 2022 Volume 2022:16 Pages 2293—2304
DOI https://doi.org/10.2147/DDDT.S358830
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Tuo Deng
Daoqi Shen,1 Man Guo,1 Xuemei Geng,1 Jinbo Yu,1– 4 Zhen Zhang,1– 4 Jing Lin,1– 4 Pan Lin,1– 4 Xiaoqiang Ding,1– 4 Xialian Xu1– 4
1Department of Nephrology, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 2Shanghai Institute of Kidney Disease and Dialysis (SIKD), Shanghai, People’s Republic of China; 3Shanghai Key Laboratory of Kidney and Blood Purification, Shanghai, People’s Republic of China; 4Shanghai Medical Center of Kidney Disease, Shanghai, People’s Republic of China
Correspondence: Xialian Xu; Xiaoqiang Ding, Department of Nephrology, Zhongshan Hospital, Fudan University, Shanghai Institute of Kidney Disease and Dialysis (SIKD), Shanghai Key Laboratory of Kidney and Blood Purification, Shanghai Medical Center of Kidney Disease, Shanghai, People’s Republic of China, Email [email protected]; [email protected]
Purpose: Apoptosis plays a critical role in cisplatin-induced acute kidney injury (AKI) and is related to mitochondrial dysfunction. Magnesium lithospermate B (Mlb), one of the most important components of Salvia miltiorrhiza Bunge, is mainly used to treat cardiovascular diseases because of its anti-apoptotic effects. The mechanism underlying the protective effect of Mlb against cisplatin-induced AKI remains unclear. In this study, we investigated the protective effect of Mlb on mitochondrial function against apoptosis caused by cisplatin-induced renal injury.
Methods: Renal injury induced by cisplatin in mouse renal tubular epithelial cells (mTECs) was measured by quantifying serum creatinine levels, mitochondrial morphology, cell viability, apoptosis, Dynamin-related protein 1(Drp1) expression, etc. The cells were then administered Mlb to determine its protective effects against cisplatin-induced AKI.
Results: Mlb treatment significantly reduced serum creatinine levels and pathological injury of renal, inhibited the production of malondialdehyde, and reduced the depletion of superoxide dismutase. In addition, Mlb reduced Bax/Bcl2, cleaved caspase-3/caspase-3, and maintained mitochondrial integrity after AKI. Mlb administration also improved cell viability and reduced the percentage of apoptotic cells in vitro. Furthermore, Mlb reduced mitochondrial reactive oxygen species, improved mitochondrial membrane potential, and ameliorated mitochondrial morphological abnormalities by downregulating Drp1 expression.
Conclusion: These results indicated that Mlb could protect the kidneys against cisplatin-induced apoptosis by alleviating mitochondrial dysfunction.
Keywords: acute renal injury, cisplatin-induced nephrotoxicity, mitochondria homeostasis, apoptosis, Salvia miltiorrhiza Bunge
Introduction
Cisplatin is an extensively used chemotherapeutic drug for various types of human cancer. However, 20–35% of patients are diagnosed with renal dysfunction after cisplatin intervention, even after hydration pretreatment.1 Acute kidney injury (AKI) is the primary manifestation of cisplatin-induced nephrotoxicity. The mechanisms include DNA damage, inflammation, cellular redox imbalance, and mitochondrial dysfunction.2,3
The kidney has the second-highest mitochondrial content and oxygen consumption among all organs, after the heart.4 As the reabsorption of solutes, maintenance of the acid-base balance, and elimination of metabolic waste in tubular epithelial cells are highly ATP-demanding processes, mitochondrial homeostasis is particularly important for the kidney. Mitochondria are dynamic organelles that maintain their shape via fission and fusion.5–7 Dynamin-related protein 1 (Drp1) is one of the most crucial fission proteins that regulates mitochondrial length. Under physiological conditions, Drp1 protein is mainly located in the cytoplasm, while only approximately 3% of Drp1 is located in the mitochondrial outer membrane.6 Upon stimulation or stress, Drp1 can translocate from the cytoplasm to the mitochondria, which induces over-fission of the mitochondria and releases pro-apoptotic factors such as cytochrome C, leading to the activation of caspase-3 and ultimately cell apoptosis.8,9
Salvia miltiorrhiza Bunge, a well-known traditional Chinese medicine, has many pharmacological activities such as promoting blood circulation, reducing oxidative stress damage, and anti-apoptosis.10–12 Magnesium lithospermate B (Mlb) is the major water-soluble active ingredient of Salvia miltiorrhiza Bunge.13 It has been clinically used in the treatment of cardiovascular diseases because of its anti-apoptosis and antioxidative properties.14 Recent studies have indicated that Mlb not only plays a role in cardiovascular diseases but also possesses beneficial effects against renal disease. In renal ablation/infarction model, Mlb significantly reduced renal injury and apoptosis.15 Salvianolate, derived from extracts of Salvia miltiorrhiza and mainly composed of salvia magnesium acetate, has been shown to attenuate contrast-induced AKI and ameliorate oxidative stress in podocyte injury.10,16 However, there is still limited evidence to determine the mechanisms of these protective effects against AKI. Thus, in the present study, we hypothesized that Mlb could improve mitochondrial function by regulating Drp1 expression, which might contribute to alleviating cisplatin-induced renal injury.
Materials and Methods
Establishment of Animal Models and Treatment of Renal Injury
Male C57BL/6 mice (6–8 weeks old) were housed at the Laboratory Animal Center of Zhongshan Hospital, Fudan University (Shanghai, China) with free access to food and water. The environmental temperature was maintained at 22 ± 2 °C with a 12 h light/dark cycle and humidity of 50%. This study was approved by Zhongshan Hospital and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animals were divided into five groups (n = 6): control (Con), Mlb (Mlb), cisplatin (Cis), saline+cisplatin (Sal+Cis), and Mlb+cisplatin (Mlb+Cis). Magnesium lithospermate B (Green Valley, ≥ 85%, Shanghai, China) dissolved in saline was administered intraperitoneally at a dose of 50 mg/kg for 3 days before cisplatin injection, based on previous studies.11,17 Cisplatin (Sigma, St. Louis, MO, USA) was administered at a single dose of 20 mg/kg per mouse. All of the mice were sacrificed 72 h after cisplatin injection and kidney and blood samples were collected for analysis.
Histological Analysis of Renal Injury
Kidney samples were fixed in 4% paraformaldehyde, embedded in paraffin, and stained with Periodic Acid-Schiff. The tubular injury scores were calculated according to the percentage of impaired tubules (0: < 10%, 1:10–25%, 2:26–50%, 3:51–75%; 4: > 75%), as previously described.18
Transmission Electron Microscopy
Fresh kidney tissue fragments were immobilized in 2.5% glutaraldehyde and 0.1 M carboxylic acid buffer for at least 2 h, then immobilized in 1% osmium tetroxide, dehydrated with increasing ethanol concentrations, and embedded into propylene oxide and resin. The tissues were cut into ultrathin sections and inspected using transmission electron microscopy.
Cell Culture and Treatment
Mouse renal tubular epithelial cells (mTECs) were cultured at 37°C in 5% CO2 in Dulbecco’s modified Eagle’s medium (Gibco, California, USA) containing 10% fetal bovine serum. The cells were exposed to cisplatin (50 μM) for 24 h in the presence or absence of Mlb (200 μM).
Western Blot Analysis
The following antibodies were used in the present study: Drp1 (1:1000, ab184247, Abcam), Bax (1:1000, ab32503, Abcam), Bcl-2 (1:1000, 12789-1-AP, Proteintech), caspase-3 (1:1000, 19677-1-AP, Proteintech), and VDAC (1:1000, ab14734, Abcam). The blots were incubated overnight at 4°C with the primary antibodies and then incubated with goat anti‐rabbit/goat anti-mouse immunoglobulin G antibodies. The bands were visualized using ECL Western blotting reagents.
RNA Extraction and Quantitative Real-Time PCR (RT-qPCR)
Total RNA was extracted from mTECs and kidney tissues using TRIzol reagent (Sigma, St Louis, MO, USA). A total of 500 ng of RNA was reverse transcribed to cDNA using the RT Master Mix (Takara, Osaka, Japan), followed by SYBR Green Premix Ex Taq II (Takara, Osaka, Japan) and the 7500 RT-qPCR system. The RT-qPCR reaction was repeated over 40 cycles with the following conditions: 95°C for 30s, 95°C for 5s, 60°C for 34s, and 72°C for 30s. All target genes were normalized to those of β-actin. The following primers were used for RT-qPCR: IL-6 forward, 5’-TGGCTAAGGACCAAGACCATCCAA-3’ and reverse, 5’-AACGCACTAGGTTTGCCGAGTAGA-3’; TNF-α forward, 5’-AGGGTCTGGGCCATAGAACT-3’ and reverse, 5’-CCACCACGCTCTTCTGTCTA-3’; KIM-1 forward, 5’-CTGGAATGGCACTGTGACATCC-3’ and reverse, 5’-GCAGATGCCAACATAGAAGCCC-3’; NGAL forward, 5’-ATGTCACCTCCATCCTGGTCAG-3’ and reverse, 5’-GCCACTTGCACATTGTAGCTCTG-3’.
Malondialdehyde (MDA)
The MDA levels were assessed according to the manufacturer’s instructions (Beyotime, Shanghai, China). Briefly, an appropriate amount of TBA was weighed for use as a working solution. Kidney samples and the working solution were mixed carefully and heated for 15 min. After the mixture cooled to room temperature, it was centrifuged at 1000 rpm. Finally, 200 μL of supernatant was placed into a 96-well plate and the OD value was read at 532 nm to measure the MDA level.
Superoxide Dismutase (SOD)
The SOD activity was assessed according to the manufacturer’s instructions (Beyotime, Shanghai, China). Briefly, the SOD detection buffer and samples were mixed proportionally and incubated at 37 °C for 30 min. The OD value at 450 nm was used to measure the SOD levels.
Cell Counting Kit-8 (CCK-8)
Cell viability was measured using CCK-8 (Dojindo, Kyushu, Japan) as described by a previous study.19 The cells were mixed and placed in 96-well plates, and cell intervention was performed after the cells grew to an appropriate confluency. The cell culture medium was mixed with CCK-8 reagent at a ratio of 9:1 and then incubated at 37°C for approximately 1 h. The OD value at 450 nm was recorded. Cell viability was calculated using the following equation:
Flow Cytometry
Cell apoptosis was measured using the Annexin V-FITC Apoptosis Detection Kit (Keygen Biotech, Nanjing, China), according to the manufacturer’s instructions. Briefly, the cells were collected and washed twice with phosphate buffer saline (PBS). After resuspending the cells in 500 μL binding buffer, the cells were incubated with 5 μL Annexin V-FITC and 5 μL propidium iodide for 10 min at room temperature, and apoptosis rates were analyzed using flow cytometry.
Isolation of Mitochondria
The mitochondria were extracted from the tissue samples according to the manufacturer’s instructions (Beyotime, Shanghai, China). Briefly, 50–100 mg of tissue was cut into pieces and homogenized using a homogenizer. The lysed tissues were then centrifuged at 600 × g for 10 min. The supernatant was then carefully transferred to another tube and centrifuged at 11,000 × g for 10 min at 4°C. The supernatant was removed, and the precipitate contained the isolated mitochondria.
Mitochondria from the mTECs were isolated using a cell mitochondrial extraction kit (Beyotime, Shanghai, China). The cells were digested with trypsin-EDTA solution and centrifuged at room temperature for 5–10 minutes to collect the cells. After resuspending the cells in PBS, the cell suspension was transferred to a suitably sized glass homogenizer and homogenized approximately 10–30 times. The cells were then centrifuged at 600 × g for 10 min. The supernatant was collected after centrifugation at 11,000 × g for 10 min at 4°C. The supernatant was removed, and the mitochondria were collected.
Mitochondrial Morphology
Living mTECs were stained with the 100 nM MitoTracker Red (Invitrogen, California, USA) probes for 30 min at 37 °C and DAPI for 5 min at room temperature to determine mitochondrial morphology. After staining was complete, the staining solution was replaced and the cells were washed with fresh medium. The percentage of mitochondrial fragmentation was estimated according to the method described by Brooks et al.5
Mitochondrial Membrane Potential
The mitochondrial membrane potential of the mTECs was measured according to the manufacturer’s instructions. After collecting cells, mTECs were cultured in 0.5 mL JC‐1 working solution for 20 min at 37°C. After rinsing three times with JC-1 wash buffer, flow cytometry was used to quantitatively analyze the red and green fluorescence signals. Red fluorescence represents JC‐1 aggregates in normal mitochondria, while green fluorescence represents JC‐1 monomer. The relative ratio of green and red fluorescence was calculated to assess mitochondrial damage.20
Statistical Analysis
All data are expressed as mean ± SEM. Statistical analyses were performed using GraphPad Prism software. Comparisons between the groups were made using one-way ANOVA followed by post hoc comparison with Tukey’s post-hoc test. Statistical significance was set at P < 0.05.
Results
Mlb Pretreatment Protected Against Cisplatin-Induced AKI in Mice
Lower viability and greater weight loss were observed in mice treated with the cisplatin. Cisplatin treatment resulted in a significantly enlarged kidney size and increased kidney index (kidney weight/body weight) (Figure 1A and B). Pretreatment with Mlb before cisplatin injection efficiently reduced AKI severity, as shown by decreased serum creatinine (Scr) levels, and histological renal tubular injury, indicated by lowered tubular casts, cellular swelling, and loss of the brush border (Figure 1C–E). The mRNA expression of kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) in the mice kidneys from the Mlb+Cis group were significantly lower than those in the Sal+Cis group (Figure 1F and G). Saline, a vehicle for Mlb, could not alleviate cisplatin-induced kidney injury.
Mlb Could Reduce Kidney Apoptosis and Inflammation Induced by Cisplatin
Pretreatment with Mlb before cisplatin injection significantly reduced the number of TUNEL-positive cells in the kidney, compared to Sal+Cis group (Figure 2A and B). The activation of caspase proteins plays a central role in apoptosis, and the ratio of Bax/Bcl2 is a marker of apoptosis. Cisplatin upregulated the expression of cleaved caspase-3 and the Bax/Bcl-2 ratio, which was subsequently counteracted by Mlb treatment (Figure 2C and D). Similarly, the increased mRNA expression of IL-6 and TNF-α induced by cisplatin administration was rescued by Mlb pretreatment (Figure 2E and F).
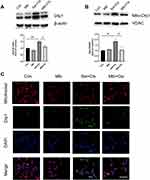
Mlb Decreased Drp1 Expression and Attenuated Cisplatin-Induced Mitochondrial Dysfunction in Mice
As shown in Figure 3A, cisplatin treatment dramatically increased the total Drp1 protein expression in the kidneys. However, pre-treatment with Mlb reversed Drp1 expression. As mentioned, the translocation of Drp1 from the cytoplasm to the mitochondria suggests mitochondrial fission. We also observed that mito-Drp1 levels that were significantly upregulated by cisplatin insult, were decreased by Mlb pretreatment (Figure 3B).
Additionally, we examined mitochondrial morphology by transmission electron microscopy. As shown in Figure 3C, the control group had elongated mitochondria with intact mitochondrial cristae, whereas the mitochondria in renal tubular epithelial cells from mice in the Sal+Cis group were fragmented and swollen. Meanwhile, the majority of mitochondria in the the Mlb+Cis-treated mice showed long filamentous morphology, indicating the possibility of Mlb rescuing cisplatin-induced mitochondrial fission (Figure 3C). Furthermore, the levels of the lipid peroxidation reaction substance MDA and antioxidant protein SOD were measured. The MDA levels in the kidneys of the Mlb+Cis group were significantly lower than those in the kidneys of the Sal+Cis group, but Mlb pretreatment showed an opposite trend in the level of SOD (Figure 4A and B).
Mlb Protected Against Cisplatin-Induced Cell Apoptosis in vitro
Considering that cisplatin dramatically reduced the viability of mTECs, we chose 50 μM cisplatin as the dosage for the following experiments based on the CCK-8 results. Mlb (200 μM) was used for further studies because it effectively reduced cisplatin cytotoxicity without influencing cell viability (Figure 5A–C). A higher apoptotic rate in mTECs was observed in the Sal+Cis group than in the control group. However, Mlb pretreatment attenuated the apoptotic rates (Figure 5D). Consistent with the in vivo results, cisplatin upregulated the Bax/Bcl-2 ratio and the protein level of cleaved caspase-3 in mTECs, and Mlb treatment rescued them (Figure 5E and F). These results indicated that Mlb protects mTECs against cisplatin-induced apoptosis.
Mlb Contributed to Protection Against Cisplatin by Improving Mitochondrial Function in Tubular Epithelial Cells
The protein expression of total Drp1 and mito-Drp1 in mTECs from the Mlb+Cis group was lower than that in the Sal+Cis group (Figure 6A and B). As shown in Figure 6C, cisplatin treatment increased Drp1 levels in the fragmented mitochondria, whereas Mlb clearly decreased these levels in the cisplatin-treated mTECs.
Mitochondrial function was also analyzed after cisplatin treatment with or without Mlb treatment. We measured mitochondria morphology using MitoTracker, mitochondrial reactive oxygen species (mtROS) using MitoSox staining, and mitochondrial membrane potential using the JC-1 assay. Cisplatin treatment caused the fragmentation of mitochondria, overexpression of mtROS, and loss of mitochondrial membrane potential, which suggested that cisplatin caused over-fission of mitochondria. However, treatment with Mlb reversed these changes (Figure 7). This indicated that Mlb could protect against cisplatin-induced oxidative damage by improving mitochondrial dysfunction.
Discussion
Cisplatin can induce abnormal mitochondrial fission and fusion, oxidative stress, and the release of pro-apoptotic substances in the kidney.5 Therefore, maintaining mitochondrial function and inhibiting apoptosis could be potential targets for the amelioration of cisplatin-induced AKI. In this study, it was suggested that Mlb could protect renal against cisplatin induced injury. Further experiments showed that Mlb regulated the Bax/Bcl2/caspase-3 pathway to inhibit apoptosis caused by cisplatin. In addition, mitochondrial over-fission after AKI was alleviated by Mlb treatment, which was characterized by the inhibition of Drp1 activity. Collectively, these results suggested that Mlb has potential therapeutic effects against cisplatin-induced AKI.
Under normal conditions, mitochondria are dynamic organelles that divide and fuse constantly. The balance between fission and fusion contributes to mitochondrial function and morphology. Mitochondrial fusion is regulated by mitofusin 1 (Mfn1) and Mfn2,21,22 whereas mitochondrial fission is regulated mainly by Drp1.9 During cisplatin-induced AKI, the mitochondria broke up into short rods or balls, and cisplatin induced mitochondrial fragmentation in rat proximal tubular cells in a time-dependent manner.5,23,24 Loss of crest structures also occurred under cisplatin treatment, which decreased mitochondrial membrane potential and stopped ATP production.4 Therefore, cisplatin can disrupt mitochondrial homeostasis, which results in over-fission of mitochondria in the kidney and lung.25,26 During fission, Drp1 oligomerizes around the mitochondria to form a ring structure that uses the energy generated by GTP hydrolysis to divide the mitochondria.27 In STZ-induced diabetic mice, the increase in Drp1 expression was accompanied by mitochondrial and cardiac dysfunction.28 Chen et al indicated that mito-Drp1 is recruited from the cytoplasm to the mitochondria in high glucose-induced podocytes.29 In our study, cisplatin-induced nephrotoxicity was also associated with the activation and translocation of Drp1 to the mitochondrial membrane. Mlb effectively attenuated the increase in Drp1 levels, thus maintaining mitochondrial morphology and decreasing mtROS levels. However, the mRNA expression of Mfn1 and Mfn2 was not altered by Mlb treatment (Supplementary 1).
Disorder of mitochondrial dynamics and apoptosis are closely associated, and both promote the development of AKI.30 Drp1 is involved in programmed cell death pathways such as caspase-dependent apoptosis, which is related to mitochondrial damage.5,31 The dissociation of cristae junctions and the activation of Bax penetrates the mitochondrial membrane by forming holes, leading to the activation of caspase-3 and ultimately, cell apoptosis.8,32 Brooks et al suggested that Bcl-2 may regulate mitochondrial dynamics by sequestering BH3-only proteins and/or interacting with mitochondrial fission-fusion proteins.33 Apoptosis and mitochondrial fragmentation are observed in neurons, which usually coincide with mitochondrial outer membrane permeabilization and cytochrome c release, suggesting a crossover between the Bcl-2 family and mitochondrial kinetic regulation.34 Moreover, the depletion of Drp1 alleviated mitochondrial fission during apoptosis, whereas overexpression of Drp1 reversed these changes.34–36 In our study, Mlb effectively inhibited the activation of Drp1; simultaneously, apoptosis was inhibited. Cisplatin increased the expression of Bax/Bcl2 and cleaved caspase-3/caspase-3 in AKI models. Pre-treatment with Mlb attenuated these changes. In addition, the results of Annexin V-PI clearly showed that Mlb decreased the apoptotic level of mTECs to near normal levels.
Previous studies have found that accumulation of cisplatin in renal tubular cells induces excessive production of ROS,26 which contributes to cisplatin-induced AKI. However, antioxidant supplementation is effective for renal protection,4,37,38 and Salvia miltiorrhiza Bunge exerts effective antioxidant effects by donating numerous hydrogen atoms.14 In rats with contrast-induced AKI, salvianolic acid B, another ingredient of Salvia miltiorrhiza Bunge, inhibited the development of oxidative stress by activating Nrf2.10 It also alleviated oxidative stress-induced intestinal cell damage by activating the Akt/GSK3β signalling pathway and maintaining mitochondrial function.39 Magnesium lithospermate B prevented glucose-induced oxidative podocyte injury by modulating NOX4 activity.16 In the present study, we demonstrated increased MDA levels and decreased SOD levels in cisplatin-treated mice. Treatment with Mlb effectively reversed these effects. Moreover, Mlb reduced cisplatin-induced mtROS production in vitro.
Our study has several limitations. We observed Drp1 expression and apoptosis levels at the same time after cisplatin treatment with or without Mlb treatment. Decreased Drp1 expression was associated with lower apoptotic rates. However, the exact mechanism of how Drp1 influences the apoptotic-related proteins remains unclear. In the future, we will perform further experiments where we overexpress Drp1 to confirm the specific role of Mlb in protecting against cisplatin-induced kidney injury and explore the underlying mechanism.
Conclusion
An extract of a traditional Chinese herb, Mlb, could alleviate cisplatin-induced AKI by decreasing Drp1 expression and improving mitochondrial function. Our data suggested that Mlb treatment attenuated cisplatin-induced kidney injury by decreasing ROS production, apoptosis, and mitochondrial damage by regulating Drp1 expression. It is suggested that Salvia miltiorrhiza Bunge could be implemented as a potential medication to rescue renal function for patients undergoing cisplatin treatment in the future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China grants (82104617) and (81903969), Innovation Program of Shanghai Municipal Education Commission (2017-01-07-00-07-E00009), and Shanghai Municipal Hospital Frontier Technology Project supported by Shanghai ShenKang Hospital Development Center (No. SHDC12018127).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Duan Z, Cai G, Li J, Chen X. Cisplatin-induced renal toxicity in elderly people. Ther Adv Med Oncol. 2020;12:1758835920923430. doi:10.1177/1758835920923430
2. McSweeney KR, Gadanec LK, Qaradakhi T, Ali BA, Zulli A, Apostolopoulos V. Mechanisms of cisplatin-induced acute kidney injury: pathological mechanisms, pharmacological interventions, and genetic mitigations. Cancers. 2021;13(7):1572. doi:10.3390/cancers13071572
3. Volarevic V, Djokovic B, Jankovic MG, et al. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J Biomed Sci. 2019;26(1):25. doi:10.1186/s12929-019-0518-9
4. Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol. 2017;13(10):629–646. doi:10.1038/nrneph.2017.107
5. Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119(5):1275–1285. doi:10.1172/JCI37829
6. Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann N Y Acad Sci. 2010;1201:34–39. doi:10.1111/j.1749-6632.2010.05629.x
7. Perry HM, Huang L, Wilson RJ, et al. Dynamin-related protein 1 deficiency promotes recovery from AKI. J Am Soc Nephrol. 2018;29(1):194–206. doi:10.1681/ASN.2017060659
8. Cho SG, Du Q, Huang S, Dong Z. Drp1 dephosphorylation in ATP depletion-induced mitochondrial injury and tubular cell apoptosis. Am J Physiol Renal Physiol. 2010;299(1):F199–206. doi:10.1152/ajprenal.00716.2009
9. Sumida M, Doi K, Ogasawara E, et al. Regulation of mitochondrial dynamics by dynamin-related protein-1 in acute cardiorenal syndrome. J Am Soc Nephrol. 2015;26(10):2378–2387. doi:10.1681/ASN.2014080750
10. Tongqiang L, Shaopeng L, Xiaofang Y, et al. Salvianolic acid B prevents iodinated contrast media-induced acute renal injury in rats via the PI3K/Akt/Nrf2 pathway. Oxid Med Cell Longev. 2016;2016:7079487. doi:10.1155/2016/7079487
11. Gao F, Li JM, Xi C, et al. Magnesium lithospermate B protects the endothelium from inflammation-induced dysfunction through activation of Nrf2 pathway. Acta Pharmacol Sin. 2019;40(7):867–878. doi:10.1038/s41401-018-0189-1
12. Jeong JW, Lee B, Kim DH, et al. Mechanism of action of magnesium lithospermate B against aging and obesity-induced ER stress, insulin resistance, and inflammsome formation in the liver. Molecules. 2018;23(9):2098. doi:10.3390/molecules23092098
13. Luo X, Deng Q, Xue Y, et al. Anti-fibrosis effects of magnesium lithospermate B in experimental pulmonary fibrosis: by inhibiting TGF-betaRI/Smad signaling. Molecules. 2021;26(6):1715. doi:10.3390/molecules26061715
14. Xiao Z, Liu W, Mu YP, et al. Pharmacological effects of salvianolic acid B against oxidative damage. Front Pharmacol. 2020;11:572373. doi:10.3389/fphar.2020.572373
15. Wang M, Yang L, Yang J, Zhou Y, Wang C. Magnesium lithospermate B attenuates renal injury in 5/6 renal ablation/infarction rats by mitochondrial pathway of apoptosis. Biomed Pharmacother. 2019;118:109316. doi:10.1016/j.biopha.2019.109316
16. Liang Y, Liu H, Fang Y, et al. Salvianolate ameliorates oxidative stress and podocyte injury through modulation of NOX4 activity in db/db mice. J Cell Mol Med. 2021;25(2):1012–1023. doi:10.1111/jcmm.16165
17. Li T, Peng JJ, Wang EL, et al. Magnesium lithospermate B derived from salvia miltiorrhiza ameliorates right ventricle remodeling in pulmonary hypertensive rats via inhibition of NOX/VPO1 pathway. Planta Med. 2019;85(9–10):708–718. doi:10.1055/a-0863-4741
18. Guo M, Xu J, Zhao S, et al. Suppressing syndecan-1 shedding to protect against renal ischemia/reperfusion injury by maintaining polarity of tubular epithelial cells. Shock. 2021. doi:10.1097/shk.0000000000001838
19. Han B, Li S, Lv Y, et al. Dietary melatonin attenuates chromium-induced lung injury via activating the Sirt1/Pgc-1α/Nrf2 pathway. Food Funct. 2019;10(9):5555–5565. doi:10.1039/c9fo01152h
20. Song N, Lu Z, Zhang J, et al. Acid-sensing ion channel 1a is involved in ischaemia/reperfusion induced kidney injury by increasing renal epithelia cell apoptosis. J Cell Mol Med. 2019;23(5):3429–3440. doi:10.1111/jcmm.14238
21. Yang D, Yang Q, Fu N, et al. Hexavalent chromium induced heart dysfunction via Sesn2-mediated impairment of mitochondrial function and energy supply. Chemosphere. 2021;264(Pt2):128547. doi:10.1016/j.chemosphere.2020.128547
22. Han B, Lv Z, Han X, et al. Harmful effects of inorganic mercury exposure on kidney cells: mitochondrial dynamics disorder and excessive oxidative stress. Biol Trace Elem Res. 2022;200(4):1591–1597. doi:10.1007/s12011-021-02766-3
23. Yuan Y, Zhu L, Li L, et al. S-sulfhydration of SIRT3 by hydrogen sulfide attenuates mitochondrial dysfunction in cisplatin-induced acute kidney injury. Antioxid Redox Signal. 2019;31(17):1302–1319. doi:10.1089/ars.2019.7728
24. Hu X, Ma Z, Wen L, Li S, Dong Z. Autophagy in cisplatin nephrotoxicity during cancer therapy. Cancers. 2021;13(22):5618. doi:10.3390/cancers13225618
25. Han Y, Kim J, Jang G, Park K. Cisplatin induces lung cell cilia disruption and lung damage via oxidative stress. Free Radic Biol Med. 2021;177:270–277. doi:10.1016/j.freeradbiomed.2021.10.032
26. Mapuskar KA, Steinbach EJ, Zaher A, et al. Mitochondrial superoxide dismutase in cisplatin-induced kidney injury. Antioxidants. 2021;10(9):1329. doi:10.3390/antiox10091329
27. Li Z, Liu Z, Luo M, et al. The pathological role of damaged organelles in renal tubular epithelial cells in the progression of acute kidney injury. Cell Death Discov. 2022;8(1):239. doi:10.1038/s41420-022-01034-0
28. Ding M, Feng N, Tang D, et al. Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1alpha pathway. J Pineal Res. 2018;65(2):e12491. doi:10.1111/jpi.12491
29. Chen Z, Ma Y, Yang Q, et al. AKAP1 mediates high glucose-induced mitochondrial fission through the phosphorylation of Drp1 in podocytes. J Cell Physiol. 2020;235(10):7433–7448. doi:10.1002/jcp.29646
30. Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z. Regulated cell death in AKI. J Am Soc Nephrol. 2014;25(12):2689–2701. doi:10.1681/asn.2014030262
31. Huang Z, Li Q, Yuan Y, et al. Renalase attenuates mitochondrial fission in cisplatin-induced acute kidney injury via modulating sirtuin-3. Life Sci. 2019;222:78–87. doi:10.1016/j.lfs.2019.02.042
32. Wang J, Zhu P, Li R, Ren J, Zhou H. Fundc1-dependent mitophagy is obligatory to ischemic preconditioning-conferred renoprotection in ischemic AKI via suppression of Drp1-mediated mitochondrial fission. Redox Biol. 2020;30:101415. doi:10.1016/j.redox.2019.101415
33. Brooks C, Wei Q, Feng L, et al. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci USA. 2007;104(28):11649–11654. doi:10.1073/pnas.0703976104
34. D’Orsi B, Mateyka J, Prehn JHM. Control of mitochondrial physiology and cell death by the Bcl-2 family proteins Bax and Bok. Neurochem Int. 2017;109:162–170. doi:10.1016/j.neuint.2017.03.010
35. Rasmussen ML, Gama V. A connection in life and death: the BCL-2 family coordinates mitochondrial network dynamics and stem cell fate. Int Rev Cell Mol Biol. 2020;353:255–284. doi:10.1016/bs.ircmb.2019.12.005
36. Cho SG, Xiao X, Wang S, et al. Bif-1 interacts with prohibitin-2 to regulate mitochondrial inner membrane during cell stress and apoptosis. J Am Soc Nephrol. 2019;30(7):1174–1191. doi:10.1681/ASN.2018111117
37. Cao X, Nie X, Xiong S, et al. Renal protective effect of polysulfide in cisplatin-induced nephrotoxicity. Redox Biol. 2018;15:513–521. doi:10.1016/j.redox.2018.01.012
38. Wang XL, Wang L, Lin FL, Li SS, Lin TX, Jiang RW. Protective effect of penetratin analogue-tagged SOD1 on cisplatin-induced nephrotoxicity through inhibiting oxidative stress and JNK/p38 MAPK signaling pathway. Oxid Med Cell Longev. 2021;2021:5526053. doi:10.1155/2021/5526053
39. Wang D, Lu X, Wang E, Shi L, Ma C, Tan X. Salvianolic acid B attenuates oxidative stress-induced injuries in enterocytes by activating Akt/GSK3beta signaling and preserving mitochondrial function. Eur J Pharmacol. 2021;909:174408. doi:10.1016/j.ejphar.2021.174408
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.