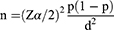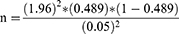Back to Journals » Journal of Blood Medicine » Volume 15
Magnitude, Associated Factors and Morphological Types of Anemia Among Hospitalized 6–59 Months Age Children at Jimma Medical Center, Southwest Ethiopia – A Hospital-Based Cross-Sectional Study
Authors Kebede RA, Yemane T, Berihun GA, Lamesa TA , Regasa DA
Received 27 October 2023
Accepted for publication 15 February 2024
Published 26 February 2024 Volume 2024:15 Pages 87—99
DOI https://doi.org/10.2147/JBM.S442240
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Regassa Alemu Kebede,1 Tilahun Yemane,1 Gebeyaw Arega Berihun,1 Tolera Ambisa Lamesa,1 Dereje Abebe Regasa2
1Department of Medical Laboratory, Institute of Health Science, Jimma University, Jimma, South West, Ethiopia; 2Department of Medical Laboratory, College of Health Science, Wolkite University, Wolkite, Central Part of Ethiopia, Ethiopia
Correspondence: Regassa Alemu Kebede, Email [email protected]
Background: Anemia is among the major public health problems that cause significant morbidity and mortality among children around the world. Anemia in children of age 6 months to 5 years is a major health problem in most developing world countries with estimated prevalence of about 43%.
Objective: To determine the magnitude, associated factors and morphological types of anemia among hospitalized 6– 59 months age children from June 15 to October 15, 2022 at Jimma Medical Center, southwest Ethiopia.
Methodology: Hospital-based cross- sectional study design was conducted from June 15 to October 15, 2022 at Jimma Medical Center, involving 383 hospitalized children aged 6– 59 months by employing convenient sampling technique. Data of sociodemographic characteristics and other associated factors of the study individuals waere collected using a pre-structured questionnaire. Clinical data were collected by physical examination and from history of client by medical interns and nurses. Then 3 mL venous blood was collected and analyzed for complete blood count. Data were coded, cleared and entered into EpiData version 4.6 and exported to SPSS version 25 for analysis. Bivariable and multivariable binary logistic regression was used to identify associated factors.
Results: The overall prevalence of anemia among hospitalized 6– 59 months age children was 57.2%; out of them 30.82% were moderate. In the present study children with malaria infection, AOR = 1.15 (95% CI: 0.017, 0.781), Cchildren with severe malnutrition, AOR = 2.046 (95% CI: 0.306, 1.366), and children with low family income, AOR = 2.6 (95% CI 0.475, 0.894) were independent variables associated with anemia.
Conclusion and Recommendation: Anemia among study participants is found to be a severe public health problem. Based on this finding, more intervention is needed with health education on nutrition and child feeding.
Keywords: anemia, magnitude, 6– 59 months, morphological types, southwest Ethiopia
Background
Anemia is defined as a decrease below the reference range for healthy people of roughly same age, sex, and race, under the same environmental conditions, in hemoglobin (Hgb) concentration, hematocrit, or the number of red blood cells per liter.1
Anemia is most prevalent micronutrient deficient disarray, which can affect a person at all stages of life at any time, particularly children of 6–59 months and pregnant women due to their increased need for iron, a micronutrient.2 The World Health Organization (WHO) defines anemia depending on age and sex based on the typical amounts of hemoglobin: children 6 month to 5 years old = 11g/dl, children aged 6–14 years = 12g/dl, adult men >15 years = 13g/dl, expectant mothers = 11g/dl and women who are not pregnant = 12g/dl.3
The size of red blood cells (RBCs), as determined by the mean corpuscular volume (MCV), is typically used to categorize anemia or classified morphologically through peripheral blood smear examination based on hemoglobinization of red blood cells (RBC) as normochromic, characterizing red blood cell coloring normally, and as observed when 2/3rds of a red cell is hemoglobin, or hypochromic, characterized by light red cell coloring and an increase in the central pale region.4,5
The etiology of anemia is complex and multifactorial; it is correlated with biological, socioeconomic, and environmental factors such as intestinal parasite infestations, malaria, HIV infection, wasting, low dietary diversity, food scarcity, timely initiation of supplemental feeding, hematological malignancies, persistent diseases such as sickle cell disease (SCD), maternal weight, and prenatal care visits.6–8
One of the main public health issues affecting children worldwide is anemia, which significantly increases morbidity and mortality rates.9 One of the biggest global public health concerns, it has a significant impact on social and economic growth as well as human health in both developed and developing nations.9,10 Although its prevalence has decreased, it is remains a major global public health concern.11 According to new demographic and health survey (DHS) report cycles between 2005 and 2018, the prevalence of anemia among children aged 6 to 59 months was more than 40% in the majority of developing nations with low and intermediate incomes, and it is categorized as a serious public health issue.12 Sub-Saharan African nations such as Kenya 48.9%,13 Mali 55.8%,14 Tanzania 79.6%,15 and Ethiopia 57%16 have terrible problems.
Methods
Study Area
The study was conducted at JMC which is found in Jimma town. The town is located in the southwestern part of Ethiopia, 345 km from Addis Ababa, the capital city of Ethiopia. The geographic coordinates of the area are approximately 7° 40’N latitude and 36° 50’E longitude with an altitude of 1,780 meters above sea level. Based on the 2007 census conducted by CSA, the total population of the zone is 2,486,155 of whom 1,250,527 are men and 1,235,628 are women. Jimma Medical Center (JMC) is one of the oldest hospitals in Ethiopia and it is the only teaching and referral hospital in southwest Ethiopia, with 800 bed capacity and a catchment population of over 15 million people.
Jimma Medical Center laboratory provides a laboratory service for both inpatients and outpatients. The laboratory is now departmentalized into seven major sections known as Central Processing Unit, Parasitology, Hematology and Parasitology, Microbiology, Clinical Chemistry and Urine Analysis, Serology (Immunology) units, general bacteriology and molecular biology. Moreover it has two site labs namely, CD4 testing site lab and emergency test site lab. Jimma Medical Center provides a service for around 2,544 children annually.
Study Design and Period
A hospital-based cross-sectional study was conducted to determine the magnitude and associated factors of anemia among hospitalized children aged 6–59 months at JMC, southwest Ethiopia from June 15 to October 15, 2022.
Population
Source population
All 6–59 months old hospitalized children at Jimma Medical Centre.
Study population
All 6–59 months old hospitalized children whose parents or guardians consented to participate in the study at JMC during the study period and who fulfilled the selection criteria were the study population.
Inclusion and Exclusion Criteria
Inclusion Criteria
All children whose age was between 6–59 months and whose parent or guardian was willing to give information during the study period was included into the study.
Sample Size
The minimum sample size required for analysis was determined by using single population proportion formula,
Where n= sample size, z = statistic for a level of confidence (z =1.96 at 95% CI), p = expected prevalence or proportion by taking 48.9%,17 d = margin of error (if 5%, d= 0.05).
n = 383
The final minimum sample size was 383.
Sampling Technique
The convenient sampling technique was used until the required sample size was achieved.
Variables
Dependent variables
- Anemia
Independent variables
- Sex and age of child (months)
- Residence of family
- Annual family income
- Mean Upper Arm Circumference (MUAC)
- Diagnosis at admission
- Intestinal parasites infection
- Household food insecurity
- Malaria infection
- Children <5 years in the house
- History of chronic disease
- Habit of tea drinking
Materials Required
- 70% alcohol
- Cotton
- Gauze
- Glove
- Gown
- Marker
- Microscope
- Oil immersion
- Slide
- Syringe
- Test tube
- Wright stain
Data Collection Techniques and Instrument
Based on a signed consent form by guardians, the children who met the requirements for inclusion were added to the study. Sociodemographic details include the children’s age, sex, place of residence, and history of chronic disease. Additional information was asked on family monthly income, and clinical conditions and additional study-related factors. Data were gathered by six nurses and medical interns working in the children’s department using a pre-structured questionnaire, direct interviews with the guardian, and information from their medical history. Nine questions were used to gather information on household food insecurity, and based on the answers, the situation was classified as either light, moderate, or severe (Annex-1 section-5).
MUAC of children was measured by measuring the circumference of the left upper arm at the mid-point between the tip of the shoulder and the tip of the elbow, using a measuring or MUAC tape having spring tension attachment at the mid-upper arm in millimeters (mm) or in cm. The cut-off points for classification of nutritional status was according to Nutrition Assessment, Counseling, and Support (NACS).18
Sample Collection
Three milliliters of venous blood was collected aseptically by the nurses and it was then transported to hematology unit for hemoglobin, other red blood indices determination (Annex-2), thick and thin blood film was made for hemoparasite (malaria) investigation and species identification, respectively, and for anemic clients thin smears were made for morphological study. Stool sample was collected by giving collection cup and direction to guardians than transported to parasitology unit for parasite examination.
Laboratory Analysis
Hemoglobin and other red blood cell indices was measured by 800 DHX Beckman Coulter (Danaher Corporation, United States) machine by photometric and electrical impedance method,19 (Annex-2) then the child’s hemoglobin level was classified as serious anemic if it was less than 7 g/dl and as anemic if it was less than 11 g/dl. The morphological types of anemia were done by making a thin blood smear then staining the smear by Wright staining and thick and thin blood film was made and stained with Giemsa for malaria investigation and identification of malaria, respectively (Annex-3 and 4). Parasite examination was done by laboratory technology working at JMC parasitology unit by making stool smear by wet mount method and identification of parasite was made by using 10x or 40x objective (Annex-4).
Data Quality Assurance and Control
Before data collection and the actual study was conducted, colleagues reviewed the questionnaire to ensure the quality of the data. They determined whether or not the questionnaire was acceptable and whether or not it had the essential information. If not, they made any necessary modifications. Throughout the data collection period, data collectors received training and regular monitoring.
Standard operating protocols were followed throughout specimen collection and CBC analysis to ensure the quality of the laboratory results (Annex-2). Therefore, blood was distributed to the test tube wall and well mixed by gently inverting the tube 8–10 times in order to prevent hemolysis following collection. Samples were examined to make sure they meet the requirements, which include sufficient volume, proper clotting, hemolysis, and collecting time. Labeling was done on the sample and the request paper with the same identifying number to prevent confusion following collection. The quality of blood film made was checked to whether it met acceptable criteria i.e. not too thick, not too long, free from lines and holes, has a smooth tail, whether the slide is labelled with code and well stained. The quality of stool smear was checked for acceptable criteria.
To reduce background error, a daily background run was conducted. Prior to analyzing the patient’s sample, the reagent’s expiration date was verified, and the necessary internal quality controls were performed before sample assaying. Daily cross-checking of gathered data with records and on-site supervision of the data collector throughout the data collection period served to preserve the quality of the sociodemographic and clinical data. The test findings were not shared with anyone. Every lab test outcome was documented, reported, and specimens were handled carefully.
Data Analysis
After being cleaned, updated, and carefully verified for completeness, the data from the laboratory and the questioner was put into EpiData 4.6. After that, it was moved into a version 25 statistical program for social sciences so that it could be examined.
Prior to any analysis, the Shapiro–Wilk and Kolmogorov–Smirnov tests were used to confirm that the data were normally distributed. Standard deviation, mean, frequency, and percentage were used to characterize a descriptive result. Tables and charts were also used to present the results. A bivariate and multivariate logistic regression analysis was conducted to evaluate the contributing components. When the P-value in the bivariable binary logistic regression was less than or equal to 0.25, the multivariate logistic regression was examined using the backward stepwise likelihood ratio. Through the computation of the odds ratio with a 95% confidence interval, the relationship between the independent variable and the categorical outcome variable was assessed. The Hosmer and Lemeshow test was used to assess the model fitness of the final logistic regression model at a p-value of more than 0.05. Ultimately, a P-value of less than 0.05 was deemed statistically significant.
Results
Sociodemographic Characteristics of Study Participants
Altogether, 383 participant data were included in the study. From this 55.4% (n = 212) of studied children were males. The mean age was 28.44 ±1.48 months. Children below one year old constituted 98 (25.6%) of the study population. About 57.7% (n = 221) were from urban area in residence. About 46.4% (n = 178) of study participant families were low income level (less than 3000 ETB) (Table 1).
 |
Table 1 Socio-Demographic Characteristics of Hospitalized Children of 6–59 Months Age and Their Parents at Jimma Medical Center, Southwest Ethiopia from June 15 to October 15, 2022 |
Health Status and Anthropometry Measurement of Child
Regarding the primary reason for admission, about 30.3% (n = 116) and 29.5% (n = 113) participants were admitted to hospital because of SAM and pneumonia, respectively. From the total, only 9.66% (n = 37) participants were positive for malaria and among the infected participants 75.67% (n = 28) were due to Plasmodium vivax. Additionally only 9.9% of study participants were positive for intestinal parasites. Near to half of the study participants were in normal range for MUAC measurement which were 44.5% (n = 172) (Table 2).
 |
Table 2 Health Status and Anthropometric Measurement of Hospitalized Children of 6–59 Months Age at Jimma Medical Center, Southwest Ethiopia from June 15 to October 15, 2022 |
Knowledge Towards Child Feeding and Household Food Insecurity Access Scale Data
The assessment of knowledge towards child feeding showed that about 71.3% (n = 273) study participant had habit of tea drinking, and out of them, 89.4% (n = 244) were drinking tea along with their meal. A little over 36.3% (n = 139) of children were born and raised in food-secure homes, while 35.5% (n = 136) were born and raised in food-insecure households, according to the assessment of household food insecurity access scale (Table- 3).
Prevalence and Severity Pattern of Anemia Among Study Participants
Male and female prevalence of anemia varied, with the total prevalence of anemia among children aged 6–59 months being 57.2% (219/383) (Table- 4).
 |
Table 4 Bivariate and Multivariate Logistic Regression for Magnitude of Anemia and Associated Factor Among Hospitalized Children of 6–59 Months at Jimma Medical Center |
Among the anemic study participants 18.53% (n = 71), 31.1% (n = 119) and 7.57% (n = 29) had mild, moderate and severe anemia, respectively (Figure 1).
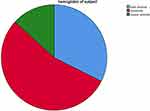 |
Figure 1 Magnitude and severity pattern of anemia among hospitalized children of 6–59 months age at Jimma Medical Center, Southwest Ethiopia from June 15 to October 15, 2022. |
Morphological Classification of Anemia and Severity Pattern of Anemia Among Study Participants
Based on blood film and MCV results, the majority of our participants with anemia had normocytic normochromic blood picture 37.1% (n = 142), microcytic hypochromic were 15.7% (n = 60), and macrocytic normochromic blood picture 4.4% (n = 17) (Figure 2).
 |
Figure 2 Morphological classification of anemia among hospitalized children of 6–59 months age at Jimma Medical Center, Southwest Ethiopia from June 15 to October 15, 2022. |
Factors Associated with Anemia Among Study Participants
A significant incidence of anemia was noted in children who drank tea regularly (36.2%) and those from low-income families (31.9%). Based on the analysis residence, malaria, MUAC, tea drinking and family income were identified as factors to be tested for association with anemia in multivariate analysis by considering P-value less than or equal to 0.25 in bivariable logistic regression. However, in multivariate binary logistic regression analysis, malaria, MUAC, and family income were only still substantially linked with anemia in children (ages 6–59 months) (Table- 4).
Discussion
One of the biggest issues with public health in poor nations is anemia. According to estimates, there are 293.1 million anemic children under the age of 5 years worldwide, with 28.5% of these children residing in sub-Saharan Africa (SSA). It is widely acknowledged as a major public health issue with a prevalence of up to 67%, or 83.5 million children in sub-Saharan Africa.20
The present study showed that the overall prevalence of anemia among hospitalized 6–59 months old children was 57.2%. Our findings indicated a serious public health issue in the research area, in accordance with the WHO’s recommendation.21 Numerous studies with comparable results have been published from many nations, including Brazil (56.6%)22, India (60.55%),23 Ghana (55.0%), and Gondar, Ethiopia (54.1%).21
Nevertheless, the present study’s findings were less than those of a study in India, 72.79%,24 in Tanzania 77.2%,25 and in Southern Tanzania 83.17%.26 The disparity may result from variations in the study participants’ geographic locations, sociodemographic traits, or the socioeconomic standing of the parents in the communities. This may also be due to having vegetarian diets and presence of different types of anemia in those countries.
The study area’s outcome was greater than a study done in Brazil 32.8%,27 in South Lebanon 33.2%,28 in Uganda 46.6%,29 in Sudan 49.4%,38 in Ethiopia at Debra Markos 11.9%,6 at Assela 36.7%,30 at Hawassa Ethiopia 41.7%30 and Shanan Gibe Hospital 48.9%.17 The seasonal variance in research, the unpredictability of an automated analyzer, regional variations, and societal disparities in lifestyle could all be contributing factors to the disparity in the prevalence of anemia.
According to the study’s anemia severity pattern, 18.81%, 30.82%, and 7.57% of participants had mild, moderate, or severe anemia, respectively. The extent of severe anemia was in line with research conducted in Uganda (11.9%) and in Assela (6.2%),31 and research from Shanan Gibe Hospital in Ethiopia 8.2%.17
However it is lower than a study conducted in Ghana 24%,32 in Tanzania 27.7%,25 in Southern Tanzania 46.03%33 in Ethiopia at Gondar 20.9%,34 at Hawassa, Ethiopia 16.1%,30 The reduction in severity observed in this study could be attributed to various reasons such as the implementation of current dietary therapies, public health initiatives, and the ease of access to health information provided by health extension workers.6
The present study result was higher than a study done in South Lebanon with 2 cases of severe anemia,35 the variability of this result may be because of variation of knowledge among different regions’ society in a country or due to sample size variation.
According to the study, the majority of our participants with anemia had a normocytic normochromic blood picture 36.5%, this could be brought on by medication use, long-term illnesses, or inflammation-related anemia, which results in a slightly reduced erythrocyte survival rate (increased destruction). Hyperemia, erythropoiesis inhibited by iron due to cytokine-stimulated hepcidin elevation, Hepcidin excretion from the kidneys and the direct actions of cytokines on the bone marrow can both suppress erythropoiesis. Inflammation can also have varying effects on the production of erythropoietin.36
But the second kind of anemia in this investigation was microcytic-hypochromic anemia. The high percentage of microcytic-hypochromic anemia is thought to be caused by iron depletion (hemoglobin levels are normal, but the body has a small amount of stored iron that will soon run out), decreased iron in diet, poor gut absorption of iron, acute and chronic blood loss, and increased demand for iron in children for rapid growth.35 Our findings disagreed with studies done in Uganda in which microcytic hypochromic anemia was 65.4%,29 in Ghana microcytic hypochromic anemia was 52%,32 in Tanzania, microcytic hypochromic anemia accounted for 37.5% of the anemia cases in children25, and in Kenya on morphological patterns of anemia the microcytic pattern was the most common, representing 42.3%.37 This variation may be closely related to socioeconomic differences and dietary variation.
In the present study anemia was high among children whose age is < 1 year (6–11 months), with prevalence of 15.6% among anemic children. This could be caused by diets deficient in bioavailable iron, low maternal iron reserve during pregnancy, and high iron demands linked to erythropoiesis and rapid growth rate.35 Moreover, the younger children are more susceptible to infections and diseases which inhibit their iron absorption.
Furthermore, the prevalence of anemia was higher in male children (33.43%). This could be attributed to the fact that preschool-aged males develop quicker than females, which raises their iron needs beyond what can be satisfied by diet.38 However, additional research is needed to fully comprehend this aspect. If the body does not receive the proper compensation for this physiological situation, iron deficiency leads to IDA anemia. This finding also shown that children with urban residence have high chance of being anemic with 30.03%, but this might be because many study participants was from an urban area.
The finding additionally shows high magnitude of anemia among children who had habit of tea drinking 36.26%, this might be because tea interferes with iron absorption and can lead to iron deficiency when consumed in large quantities. The process involves the naturally occurring tea chemicals tannins and oxalate, which bind iron—specifically, non-heme iron—found in plant foods such as beans, peas, leafy green vegetables, and nuts.20
In this study, factors associated with children (aged 6–59 months) having anemia were malaria infection (AOR = 1.15, 95% CI: 0.017, 0.781, P = 0.001), MUAC (AOR = 2.046, 95% CI: 0.306, 1.366, P = 0.045) and family income (AOR = 2.6, 95% CI: 0.475, 0.894, P = 0.043). Malaria infection was significantly associated with children (aged 6–59 months) having anemia which is supported by a study reported in Uganda,29 Tanzania and Sudan. Children with malaria infection were ½ times more likely to be anemic as compared with children without malaria infection. Malaria is known to cause anemia through different mechanisms that include a decrease in erythrocytes production or an increase in erythrocytes loss or both. (Malaria is caused by an intra-erythrocytic parasite so there is obligatory destruction of red cells containing parasites at schizont-rupture. But the more important contributor is the accelerated destruction of non-parasitized red cells that parallels disease severity.)23
MUAC was also significantly associated with study subject (6–59 month children) anemia which was supported by a study reported in south Lebanon.35 It was indicated that children with severe malnutrition were 2 times more likely anemic than children with normal nutrition. This might be due to their rapid body growth and their high RBCs expansion, children below 5 years of age have increased iron needs, as a result they are more susceptible to develop anemia.35
Additionally, family income was also significantly associated with study subject (aged 6–59 months children) anemia which is supported by a study reported in Uganda,29 Tanzania25 and Sudan.39 It shown that children with a low family income are two and a half times more likely to be anemic then high family income children. A possible reason for the association might be due to families with low income being less likely to buy nutrient-rich foods (like iron, vitamins etc.), secure food availability, and not being able to afford health-care service during illness for their children. Therefore, it is necessary to engage women in income-generating activities so that their children have better health care and supplementary food.
Conclusion
In general, anemia among 6–59 months old children was a major public health problem in the study area and these results indicate that anemia is still an important public health problem even though interventions have been made. The severity of anemia among anemic clients shows moderate anemia was the highest and morphological classification of anemia indicates that most of anemic blood picture was normocytic-normochromic. Malaria parasitemia, nutritional status of child (using MUAC) and family annual income were the factors significantly associated with anemia.
Abbreviations
ANC, Antenatal care; AOR, adjusted odds ratio; CBC, complete blood count; CD, chronic disease; CHr, reticulocyte hemoglobin content; CI, confidence interval; COR, crude odds ratio; CSA, central statistics agency; EDHS, Ethiopia demographic health survey; EDTA, Ethylenediaminetetra acetic acid; HFIAS*, Household food insecurity access scale; Hgb, hemoglobin; IRB, institutional review board; JMC, Jimma Medical Center; MCV, mean corpuscular volume; MUAC, mid-upper arm circumference; NACS, Nutrition Assessment; Counseling, and Support; RBC, red blood cell; SAM, severe acute malnutrition; SCD, sickle cell disease; SOP, standard operation procedure; SSA, sub-Saharan Africa; UTI, urinary tract infection; WHO, World Health Organization.
Data and Materials
The necessary data analyzed during the current study are available from the corresponding author when requested.
Ethical Considerations
The study was conducted following the Declaration of Helsinki and an approved ethical clearance was obtained from the Institutional Review Board of the Jimma University Institute of Health under Ref. No. IHPPGJ/837, permission to conduct the study was obtained from the Head of School of Medical Laboratory Science and chief clinical director of the JMC. A support letter from Jimma University Health Science Research Coordinating Office was written to JMC. After discussing the research aims to each participant’s mother, parents, or guardians, they was asked to sign an informed written consent and assent form, and those who were willing to participate were included in the study. Participation was fully voluntarily, refusal at any time during data collection was permitted. Confidentiality was kept. Any abnormal test results of participants were communicated to their attending physician immediately to make proper management and treatment.
Acknowledgments
The authors would like to extend their gratitude to Jimma Medical Center, pediatrics department staff, intern students (Dr.), laboratory staff and all study participants for their willingness to participate in this study.
This manuscript was prepared from thesis entitled, “Magnitude, associated factors and morphological types of anemia among hospitalized 6–59 months age children at Jimma medical center”, southwest Ethiopia, (authors Regassa Alemu (candidate), Dr. Tilahun Yemane (advisor) and Mr. Gebeyaw Arega (advisor). All of the data, results, and information in this manuscript are taken from the mentioned research. https://repository.ju.edu.et//handle/123456789/7907.
Funding
No funding was received for this study.
Disclosure
The authors have declared that they have no existing competing interests in this work.
References
1. Lanzkowsky P, Lipton J, Fish JD. Lanzkowsky’s Manual of Pediatric Hematology and Oncology. Elsevier; 2016.
2. Pasricha SR, Black J, Muthayya S, et al. Determinants of anemia among young children in rural India. Pediatrics. 2010;126(1):e140–e149. doi:10.1542/peds.2009-3108
3. Breiman RF, Olack B, Shultz A, et al. Healthcare-use for major infectious disease syndromes in an informal settlement in Nairobi, Kenya. J Heal Popul Nutr. 2011;29(2):123–133.
4. Ferry H, Virginia W. Evaluation of Anemia in Children. 2016.
5. Cheesbrough M. District Laboratory Practice in Tropical Countries Part 2.
6. Alamneh YM, Akalu TY, Shiferaw AA, Atnaf A. Magnitude of anemia and associated factors among children aged 6–59 months at Debre Markos referral hospital, Northwest Ethiopia: a hospital-based cross-sectional study. Ital J Pediatr. 2021;47(1). doi:10.1186/s13052-021-01123-3
7. Clements ACA, Magalha RJS. Mapping the Risk of Anaemia in Preschool-Age Children: the Contribution of Malnutrition, Malaria, and Helminth Infections in West Africa. PLoS Med. 2011;8(6). doi:10.1371/journal.pmed.1000438
8. Muoneke UV. Pediatrics & Therapeutics Prevalence and Aetiology of Severe Anaemia; 2011.
9. World Health Organization. The global prevalence of anaemia in 2011; 2011:1–48. Available from: https://apps.who.int/iris/handle/10665/177094.
10. Chatterjee A, Bosch RJ, Kupka R, Hunter DJ, Msamanga GI, Fawzi WW. Predictors and consequences of anaemia among antiretroviral-naive HIV-infected and HIV-uninfected children in Tanzania. Public Health Nutr. 2010;13(2):289–296. doi:10.1017/S1368980009990802
11. Brabin BJ, Premji Z, Verhoeff F. An analysis of anemia and child mortality. J Nutr. 2001;131(2 SUPPL):2.
12. Hasan M, Magalhaes RJS, Ahmed S, Pervin S, Fatima Y, Mamun AA. Geographical variation and temporal trend in anemia among children aged 6 – 59 months in low- and middle-income countries during 2000 – 2018: forecasting the 2030 SDG target. Pub Health Nutr. 2021;24(18):6236–6246. doi:10.1017/S1368980021002482
13. Neumann CG, Bwibo NO, Murphy SP, et al. Animal source foods to improve micronutrient nutrition and human function in developing countries animal source foods improve dietary quality, micronutrient status, growth and cognitive function in Kenyan school children. J Nutr. 2003;133:3941–3949
14. Maiga F, Hall A, Roschnik N, et al. A randomised trial in Mali of the effectiveness of weekly iron supplements given by teachers on the haemoglobin concentrations of schoolchildren. Pub Health Nutr. 2002;5(3):413–418. doi:10.1079/phn2001327
15. Tatala SR, Kihamia CM. Risk factors for anemia in schoolchildren in Tanga region. Tanzania J Health Res. 2008;10:4.
16. Survey H. Ethiopia Demographic and Health survey 2016. Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF; 2016.
17. Kebede D, Getaneh F, Endalamaw K, Belay T, Fenta A. Prevalence of anemia and its associated factors among under-five age children in Shanan gibe hospital, Southwest Ethiopia. BMC Pediatr. 2021;21(1):1–9. doi:10.1186/s12887-021-03011-5
18. NACS. Nutrition Assessment, Counseling, and Support (NACS): a User’s Guide—Module 2: nutrition Assessment and Classification, Version 2. Nutr Assessment Couns Support. 2016;2:1–12.
19. Miers SL. Rodaks hematology 5th edition clinical and laboratory automated blood cell analysis; 2019.
20. Paramastri R, Hsu CY, Lee HA, Lin LY, Kurniawan AL, Chao JCJ. Association between dietary pattern, lifestyle, anthropometric status, and anemia-related biomarkers among adults: a population-based study from 2001 to 2015. Int J Environ Res Public Health. 2021;18(7):1–15. doi:10.3390/ijerph18073438
21. World Health Organization, Chan M. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva, Switz: World Health Organization; 2011:1–6. Available from: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Haemoglobin+concentrations+for+the+diagnosis+of+anaemia+and+assessment+of+severity#1.
22. Dos Santos RF, Gonzalez ESC, De albuquerque EC, et al. Prevalence of anemia in under five-year-old children in a children’s hospital in Recife, Brazil. Rev Bras Hematol Hemoter. 2011;33(2):100–104. doi:10.5581/1516-8484.20110028
23. Narayan R, Singh S. Severity and frequency of anemia in different age group in 6 months to 5 years children: a prospective study at a teaching hospital in rural Haryana, India. Highlights Med Med Res. 2021;05(09):26–31.
24. Poornima S, Balaji PR, Varne SR, Jayashree K, Saba F. Anemia among hospitalized children at a multispecialty hospital, Bangalore (Karnataka. India J Fam Med Prim Care. 2014;3(1):48. doi:10.4103/2249-4863.130275
25. Simbauranga RH, Kamugisha E, Hokororo A, Kidenya BR, Makani J. Prevalence and factors associated with severe anaemia amongst under-five children hospitalized at Bugando Medical Centre, Mwanza, Tanzania. BMC Hematol. 2015;15(1):1–9. doi:10.1186/s12878-015-0033-5
26. Mghanga FP, Genge CM, Yeyeye L, et al. Magnitude, severity, and morphological types of anemia in hospitalized children under the age of five in Southern Tanzania. Cureus. 2017;9(7). doi:10.7759/cureus.1499
27. Leal LP, Filho MB, de Lira PIC, Figueiroa JN, Osório MM. Prevalence of anemia and associated factors in children aged 6–59 months in Pernambuco, Northeastern Brazil. Rev Saude Publica. 2011;45(3):457–466. doi:10.1590/S0034-89102011000300003
28. Djokic D, Radojicic Z, Rakic L, et al. Risk factors associated with anemia among Serbian school-age children 7–14 years old: results of the first national health survey. Hippokratia. 2010;14:252–260.
29. Ocan A, Oyet C, Webbo F, Mwambi B, Taremwa IM. Prevalence, morphological characterization, and associated factors of anemia among children below 5 years of age attending St. Mary’s hospital Lacor, Gulu district, northern Uganda. J Blood Med. 2018;9:195–201. doi:10.2147/JBM.S184126
30. Gebereselassie Y, Birhanselassie M, Menjetta T, Alemu J, Tsegaye A. Magnitude, severity, and associated factors of anemia among under-five children attending hawassa university teaching and referral hospital, Hawassa, Southern Ethiopia, 2016. Anemia. 2020;2020:1. doi:10.1155/2020/7580104
31. Alemu GM, Ayalneh ST, Waye BG. Prevalence of anemia and associated risk factor among under- five children in ASELLA TEACHING AND REFERRAL HOSPITAL, ARSI UNIversity, Asella, Ethiopia. Res Sq. 2020;10(21):1–8.
32. Adu-Amankwaah J, Allotey EA, Kwasie DA, et al. Prevalence and morphological types of anaemia among children under-five years in the volta regional hospital of Ghana. OALib. 2018;05(02):1–10. doi:10.4236/oalib.1104351
33. Assefa S, Mossie A, Hamza L. Prevalence and severity of anemia among school children in Jimma Town, Southwest Ethiopia. BMC Hematol. 2014;14(1). doi:10.1186/2052-1839-14-3
34. Enawgaw B, Workineh Y, Tadesse S, Mekuria E, Addisu A, Genetu M. Prevalence of anemia and associated factors among hospitalized children attending the University of Gondar Hospital, Northwest Ethiopia. Electron J Int Fed Clin Chem Lab Med. 2019;30(1):35–47.
35. Salami A, Bahmad HF, Ghssein G, Salloum L, Fakih H, De Re V. Prevalence of anemia among Lebanese hospitalized children: risk and protective factors. PLoS One. 2018;13(8):1–11. doi:10.1371/journal.pone.0201806
36. Batra J, Sood A. Iron deficiency anemia effect cognitive development of children: a review Jyoti Batra and Archana Sood Iron deficiency evolves slowly through several stages. Early iron deficiency caused a depletion in Author f. Indian J Clin Biochem. 2005;20(2):119–125. doi:10.1007/BF02867410
37. Awuor SO, Eric OO, Musyoki S, et al. Morphological patterns of anemia among under five children on Prevention of Mother-To-Child Transmission (PMTCT) programmes in Masogo sub-county hospital, Kisumu county, Kenya. J Clin Images Med Case Rep. 2021;2(2). doi:10.52768/2766-7820/1048
38. Alemu Y, Atomsa A. Hematology. 2006.
39. Elmardi KA, Adam I, Malik EM, et al. Anaemia prevalence and determinants in under 5 years children: findings of a cross-sectional population-based study in Sudan. BMC Pediatr. 2020;20(1):1–14. doi:10.1186/s12887-020-02434-w
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.