Back to Journals » Journal of Pain Research » Volume 17
Mast Cells and Their Related Gene HK-1 are Closely Associated with Discogenic Low Back Pain: A Bioinformatics and Clinical Sample Study
Authors He S, Liu X, Luo S, Li H, Min J, Shi Q
Received 18 December 2023
Accepted for publication 2 April 2024
Published 8 April 2024 Volume 2024:17 Pages 1401—1412
DOI https://doi.org/10.2147/JPR.S454785
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Alaa Abd-Elsayed
Shouyu He,1,* Xiaowen Liu,2,* Shenchang Luo,1 Haidong Li,1 Jikang Min,1 Qian Shi3
1Department of Spine Surgery, First Affiliated Hospital, The First People’s Hospital of Huzhou, Huzhou University, Huzhou, 313000, People’s Republic of China; 2Department of Orthopedic Surgery, Changzheng Hospital, Second Military Medical University, Shanghai, 200003, People’s Republic of China; 3Key Laboratory for Translational Medicine, First Affiliated Hospital, The First People’s Hospital of Huzhou, Huzhou University, Huzhou, 313000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qian Shi, Key Laboratory for Translational Medicine, First Affiliated Hospital, The First People’s Hospital of Huzhou, Huzhou University, Huzhou, 313000, People’s Republic of China, Email [email protected]
Background: Low back pain (LBP) is primarily caused by intervertebral disc degeneration (IVDD). Immune cells penetrating nucleus pulposus (NP) tissues may play an important role in generating IVDD and LBP.
Methods: The clinical data from 100 cases of IVDD patients was initially analyzed retrospectively. Subsequently, peripheral blood and NP tissues from 41 IVDD patients were gathered for a validated investigation. Among them, ribosome-removed-RNA sequencing (RNA-seq) was performed on 10 cases of NP tissues of specific classifications (VAS 3 and Pfirrmann 3 were used as the controls, while patients with VAS 6 and Pfirrmann 5 were used as the experimental group). Differentially expressed genes (DEGs) were identified for the subsequent bioinformatics analysis. Further methods to confirm the underlying cause of discogenic LBP included mast cell immunohistochemistry (IHC), 12 cytokine detection, Western blot (WB), and real-time polymerase chain reaction (RT-PCR).
Results: Discogenic LBP and IVDD severity are strongly associated, and immunological cell infiltration has been demonstrated to be a significant factor in LBP by bioanalytical research. Tryptase-positive mast cells were found to be significantly more abundant in the VAS 6 NP tissues of IVDD patients than in the VAS 3 NP tissues. It was initially demonstrated that IVDD and LBP were significantly impacted by hemokinin-1 (HK-1), the mast cell-related gene. Furthermore, blood levels of interleukin 12 p70 (IL-12P70) are noticeably elevated and strongly correlated with HK-1, indicating that HK-1 may be involved in the regulation of mast cell activity and IL-12P70 production.
Conclusion: The severity of LBP was observed to be positively correlated with the IVDD Pfirrmann grading. Further research indicates that patients with IVDD may experience persistent low back pain due to HK-1 activation of mast cells and the release of the cytokine IL12P70. This work will offer new insights into the diagnosis and treatment of discogenic LBP.
Keywords: IVDD, LBP, immune cell infiltration, mast cell, HK-1
Introduction
Low back pain (LBP) is a widespread public health issue that can cause severe lifetime disability and place a significant financial burden on patients and society.LBP has a complex etiology that involves genetic variables, lifestyle factors (such as occupational exposure, alcohol use, and smoking), and aging.Intervertebral disc degeneration (IVDD), which manifests as noticeable morphological changes brought on by aging or mechanical stress, is the most prevalent etiology of LBP.3
Numerous lumbar degenerative conditions, including lumbar disc herniation, degenerative scoliosis, and lumbar instability, have been linked to discogenic LBP.4 A sustained examination of 1037 individuals with LBP by Arnbak et al5 revealed a correlation between higher occurrence of LBP and the degree of disc deterioration. The expression of molecules linked to inflammation and pain, such as tumor necrosis factor-α (TNF-α), interleukins (IL-1β, IL-4, IL-6, IL-8, IL-12), prostaglandin E2 (PGE2), and nitric oxide (NO), has been found to be considerably upregulated in 87% of patients with persistent IVDD.6 Extracellular matrix (ECM) biosynthesis, particularly proteoglycan synthesis, water content, anabolic factors, and catabolism, all decrease as a result of IVDD.7 Both animal models and human clinical specimens have been used to demonstrate that disc degeneration and herniation promote the growth of sensory nerves and blood vessels into the inner layer of the disc, which is an essential cause of pain.8 Therefore, nerve growth factor (NGF), and vascular growth factor (VEGF) also are key cytokines for LBP.
Moreover, immune cells may be crucial in promoting IVDD ECM catabolic remodeling. Recent studies have shown that T cells and especially macrophages are essential to the emergence of IVDD.9 Proteolytic enzymes are produced by macrophages to breakdown the ECM in the inflammatory milieu of IVDD, and proinflammatory factors such as IL-1β, IL-6, and TNF-β are secreted, exacerbating the development of IVDD and pain.10 However, the precise functions and mechanisms of other immune cells like mast cells on LBP are unknown.
In this study, we first verified that LBP is closely correlated with IVDD using 100 cases patient clinical information. Next, we gathered NP tissues from 10 patients with specific levels of pain (VAS scores 3 and 6) and IVDD (Pfirrmann grade 3 and 5) and then performed ribosome-removed RNA-seq to investigate the relationship between discogenic LBP and immune cell infiltration. Based on bioinformatics analysis, we found that differentially expressed genes (DEGs) were strongly linked to the inflammatory response and immune cell infiltration. Additionally, immune cell co-expression analysis revealed a substantial negative correlation between DEGs and resting mast cells and a positive correlation between DEGs and mast cell activation. In conclusion, the abundance of mast cells in increased in severe painful IVDD, and upon activation, they may maintain a painful chronic inflammatory IVDD milieu. This study reveals new directions for understanding discogenic LBP, leading to novel therapeutic opportunities.
Materials and Methods
Clinical Sample Collection and Ethical Approval
All experiments were conducted with the approval of the Ethics Committee of the First Affiliated Hospital, Huzhou University (approval number: 2019088). All patients signed informed consent forms, and the Ethics Committee gave their approval for all investigations.
In total, the clinicopathological data from 100 IVDD patients in 2021 was initially gathered to determine if the pain score (VAS) and the severity of IVDD are correlated or not. Subsequently, peripheral blood and diseased NP tissues from the remaining 41 individuals were gathered in 2021 and 2022. Blood samples were taken the day before surgery and medication, and NP tissues were retrieved during IVDD surgery. The clinicopathological data pertaining to these 41 patients will also be documented concurrently. Sixteen samples of blood from healthy volunteers who underwent a medical examination at our facility, all of whom had no surgery or medication within the previous six months, served as the control group. Clinicopathological information including age, sex, pfirrmann degree, LBP VAS-Score, diseased segment, height of the anterior and posterior edge of the disc. The Inclusion/Exclusion criteria are as follows:
Inclusion Criteria
- The patient was diagnosed as IVDD by examination and required surgical treatment, informed and consented to participate in the study;
- The duration of disease is more than 3 months;
- Patients who had not received local injection, immune regulation, previous surgical intervention and other relevant clinical treatment before surgery.
Exclusion Criteria
- Patients with severe cardiopulmonary, liver and kidney dysfunction, poor condition, and difficulty in tolerating general anesthesia and surgical resection;
- Patients with spinal tumor, infection, or explosive vertebral fracture due to trauma and serious injury of disc shape and structure;
- Patients with unconsciousness or mental system disorders;
Ribosome Removed RNA- Seq and DEGs Identification
Among 41 cases of diseased NP tissues, 10 were selected and subjected to ribosome removed RNA-seq (4 cases of VAS 3, grade 3 act as control and 6 cases of VAS 6, grade 5 act as experimental group), The “limma-voom” package of R software was used to process the RNA-seq original data. |log2FC| > 1 and P < 0.05 were considered differentially expressed genes (DEGs) according to the statistical procedures using p value and false discovery rate (FDR). The analysis results are presented as volcano plots and heatmaps. Then these 41 NP tissues were used for next experimental verification.
Functional and Pathway Enrichment Analysis
Gene Ontology (GO) term analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were used for functional pathway analysis. 1613 upregulated DEGs between the VAS 3 and VAS 6-painful NP tissue groups performed using online DAVID analysis (https://david.ncifcrf.gov). Then column and point charts were drawn by Lian Chuan biological platform (https://www.omicstudio.cn).
Weighted Gene Co-Expression Network Construction and Analysis (WGCNA)
The WGCNA is a common method for constructing gene co-expression networks, aiming to find co-expressed gene sets (Modules).11 In our study, we used it to determine the relationship between co-expression gene modules and differentially infiltrating immune cells. The WGCNA package in R3.5.1 was used to create the co-expression network for 1613 upregulated DEGs. Mean connectivity analysis was performed, a β value of 8 was chosen as the appropriate soft-thresholding value, and a cluster dendrogram was displayed. Then, their correlations with differentially infiltrating immune cells were analyzed. Values of |correlation index| > 0.7 and p < 0.01 were considered significant.
Metascape Analysis
Metascape is an online tool which provides a biologist-oriented resource for the analysis of systems-level datasets.12 We defined the brown module as an immune-related module, and 33 genes in this module were extracted for Metascape analysis (http://Metascape.org/gp/index).
Mast Cell Immunohistochemistry (IHC)
Ten cases of VAS 3, grade 3 and VAS 6, grade 5 NP tissues were harvested after surgery. NP tissues were fixed in 4% paraformaldehyde for 2 h before paraffin embedding. All paraffin-embedded tissues were cut into 5-µm sections for subsequent immunohistochemical analysis. Immunohistochemical staining was performed to examine mast cell activation in NP tissues. After blocking with goat serum for 30 min at room temperature, the primary anti-Mast Cell Tryptase (TPSB2) antibody (#A19801, ABclonal, Wuhan, China) was used to stain mast cells. Finally, slices were photographed under a light microscope.
HK-1 Immunofluorescence
After fixation with 4% paraformaldehyde for 15 min, 10 cases of NP paraffin-embedded tissues were permeabilized and blocked with 5% bovine serum albumin/0.3% Triton™ X-100 PBS for 1 h at room temperature. Then, the cells were incubated with primary antibodies against HK-1 (#C35C4, CST, Beverly, MA) and fluorescence-labeled secondary antibodies (#A31572; Invitrogen, Carlsbad, CA, USA). DAPI was used to label the cells after they were washed, and images were acquired using a confocal microscope (Zeiss, Cambridge, UK).
Western Blotting Analysis
Eight cases tissue proteins were separated by SDS-PAGE, transferred to a PVDF membrane, and probed with primary antibodies against HK-1 (C35C4, CST, Beverly, MA), TPSB2 (#A19801, ABclonal, Wuhan, China). Following incubation with primary antibodies overnight at 4°C, incubation with HRP-conjugated secondary antibodies (Beyotime, Cat# A0208, 1:1000, Shanghai, China) was performed for 1 h, and then the immunoblots were visualized using a BeyoECL Plus Kit (Beyotime, China) on a Tanon Gel Imaging System (Tanon 4600, China).
RT-PCR Analysis
RNA from 41 cases NP tissues and peripheral blood mononuclear cells was extracted by TRIzol reagent (#15596018; Invitrogen, Life Technologies, USA) and subjected to reverse transcription using a HiFiScript cDNA Synthesis Kit (CoWin Biosciences, Beijing, China). qRT-PCR was performed for cDNA using the StepOneTM Real-Time PCR System (Life Technologies, Gaithersburg, MD, USA) and the TB Green® premix Ex TaqTM assay (TAKARA). The PCR program was as follows: pre-denaturation at 95°C for 10 min, 40 cycles of denaturation at 95°C for 15s, and annealing and elongation at 60°C for 1 min. GAPDH was used as the internal control. The primers used in the study are shown in Supplementary Table 1.
Flow Cytometry
Twelve cytokines (IL-5, IFN-α, IL-2, IL-6, IL-1B, IL-10, IFN-γ, IL-8, IL-17, IL-4, IL-12P70, TNF-α) were detected by a human inflammatory cytokine CBA kit (#551811, BD biosciences, Boston, MA). Single-cell suspensions were prepared from the peripheral blood of above 41 IVDD patients and 16 health. Following red blood cell lysis, Fc receptors were blocked with fluorescent light-emitting microsphere-conjugated cytokine Abs, and cells were incubated with biotin-labeled cytokine Abs and then incubated with streptavidin (SA-PE) before fluorescence intensity detection using flow cytometry (FACS Calibur, BD, Boston, MA).
Statistical Analysis
GraphPad Prism 8 was employed. Pearson correlation analysis was used to determine correlations between pain level and pfirrmann degree, diseased segment, height of the anterior and posterior edge of the disc, HK-1 and so on. Independent Student’s t-test was utilized for comparisons between two groups. Data are presented as the mean ± SD. P value < 0.05 was considered statistically significant (*P < 0.05; **P < 0.01; ***P < 0.001).
Results
The LBP-VAS Score is Closely Correlated with IVDD Clinical Data
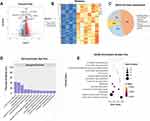
To verify that LBP is closely related to IVDD, we first collected the image data of 100 IVDD patients who were diagnosed and classified with different levels of pain (VAS score) and disc degeneration (Pfirrmann scale) by imaging and clinical diagnostic experience. The results of the retrospective analysis showed that the LBP-VAS score was closely correlated with disc degeneration, age, leading-edge height and trailing-edge height (Figure 1A–D).
DEGs Identification and Biological Function Analysis
To uncover the key causes of LBP that originate from IVDD, we used ribosome-removed RNA-seq data from NP tissues of IVDD patients with specific classifications. Four patients with Pfirrmann 3 and VAS 3 NP tissues were used as controls, and 6 patients with Pfirrmann 5 and VAS 6 NP tissues were used as the experimental group. After standardization analysis, 1812 DEGs were identified, including 1613 upregulated DEGs and 199 downregulated DEGs (Figure 2A and B). A manual check of the 1613 upregulated genes revealed enrichment in inflammatory or immune response (45%), cellular homeostasis (24%), and matrix metalloenzyme (10%) (Figure 2C). We then annotated the GO functions of the 1613 upregulated DEGs. The results showed that these DEGs were mainly enriched in “innate immune response”, “inflammatory response”, and “cell surface receptor signaling pathway” (Figure 2D). These 1613 DEGs were also enriched in KEGG pathways related to “natural killer cell-mediated cytotoxicity”, “cytokine‒cytokine receptor interaction”, “T-cell receptor signaling pathway”, “B-cell receptor signaling pathway”, and others (Figure 2E).
WGCNA and Identification of the Key Module Associated with Immune Infiltration in Discogenic LBP
WGCNA was used to screen the 1613 upregulated DEGs and identify the key gene module associated with immune infiltration.
A β value of 8 was chosen as the appropriate soft-thresholding value (Figure 3A). The WGCNA detected 4 coexpression modules, and a cluster dendrogram was established (Figure 3B). Then, their correlations with differentially infiltrating immune cells were analyzed. The MEbrown module was mainly associated with immune cells, including activated mast cells, resting mast cells, and activated gamma T-cells (|Cor| > 0.7, p value < 0.01, Figure 3C). We defined the MEbrown module as an immune-related module, and a total of 33 genes were subjected to functional analysis using Metascape online software. As shown in Figure 3D, genes were enriched in “innate immune response”, “positive regulation of cytokine production”, and “inflammatory response”. Protein‒protein interaction (PPI) enrichment analysis of the MCODE networks was performed by online Metascape tool, As shown in Figure 3E. The genes were enriched in “natural killer cell mediated cytotoxicity” and the “NF-κB pathway” (Figure 3E).
Mast Cells and Inflammatory Factors Were Significantly Higher in NP Tissues from Patients with More Serious Painful IVDD
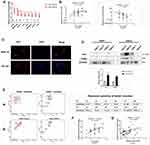
The Pfirrmann grading system is an internationally recognized standard for evaluating the degree of IVDD and is the most widely used scale in the clinical evaluation of disc degeneration. Figure 4A shows typically X-ray and MRI images of the 5–grades of disc degeneration of above 41 IVDD patients. The number and color depth of mast cell tryptase (TPSB2) positive cells was used to observe mast cell activation in IVDD patients using IHC. When comparing VAS 3 to VAS 6 - painful NP tissue, there was a significantly higher percentage of tryptase-positive cells in the VAS 6 painful NP tissues (Figure 4B). Next, we analyzed the expression levels of inflammation- and pain-related factors IL-1β, IL-6 and TNF-α in the above RNA-seq results. The expression levels of IL-1β, IL-6 and TNF-α were significantly higher in VAS 6 than in VAS 3 - painful NP tissue (Figure 4C). Furthermore, NGF and VEGF expression were also increased in VAS 6 compared with VAS 3 painful NP tissue (Figure 4D), indicating that the activation of mast cells plays a major role in IVDD and LBP.
HK-1, Which is Highly Expressed, is Closely Related to Discogenic LBP
Mast cell-related key genes were further validated using above 10 typically NP tissues. The results showed that 8 genes were highly expressed in VAS 6 - painful NP tissue (Figure 5A). Then, those 8 genes were verified in the other 41 cases of NP tissues with different VAS scores. Only HK-1 showed a positive correlation with pain, while TNSF13B showed a negative correlation (Figure 5B). Immunofluorescence staining revealed that HK-1 was mainly located in cytoplasm of NP and mast cell (Figure 5C). WB analysis further showed that HK-1 and TPSB2 expression levels were significantly higher in VAS 6- than in VAS 3-painful NP tissues (Figure 5D), suggesting that the mast cell-related key gene HK-1 may an activating factor of mast cell and inducible factor of LBP.
In additionally, we used flow cytometry to investigate 12 cytokines in the peripheral venous blood of these 41 cases IVDD patients and 16 cases heath. As shown in Figure 5E, levels of IL-1, IL-6 and IL-12P70 were higher in the peripheral venous blood of IVDD patients than in that of healthy physical examination volunteers (Figure 5E). Linear regression analysis showed that only IL-12P70 expression was closely related to HK-1 and pain (Figure 5F and G), suggesting that IL-12P70 plays a vital role in discogenic LBP induced by mast cells.
Discussion
The Novel Grouping Approach of NP Tissues for RNA-Seq
IVDD is a common disease that affects the morphology and normal physiological function of the intervertebral discs. One key mechanism of IVDD is the degeneration of the NP tissues, the core, gel-like portion of the intervertebral disc.13 Most studies have used rat or lumbar vertebral fracture patient NP tissue as a control because normal disc tissue is challenging to obtain.14,15 Moreover, score that is below VAS 3 represents mild pain, these patients might decide against visiting the hospital, score above VAS 7 is severe pain, usually in fracture or spinal tumor patients. To optimize the experiment of RNA-seq, we gathered NP tissues of particular classifications using a novel grouping approach. Patients with low visual analog scale (VAS 3) and Pfirrmann scale (Pfirrmann 3) scores were used as the control, while patients with VAS 6 and Pfirrmann 5 scores were used as the experimental group. We discovered that DEGs between VAS3 and VAS 6 painful NP tissues were significantly enriched in inflammatory or immunological responses following standardization analysis. Furthermore, HK-1 act as mast cell essential related gene may participate in discogenic - LBP.
Mast Cells are Higher Enrichment and Activation in More Serious NP Tissues
Similar to macrophages, mast cells (MCs) release preformed granules with enzymatic (tryptase and a disintegrin and metalloproteinase with a thrombospondin motif 5, ADAMTS5) and inflammatory/pain-associated factors (IL-1β, TNF-α, VEGFA and others) in response to microenvironmental stimuli or allergens.16 Since MCs are active and significantly upregulated in chronic pain conditions, such as migraines, irritable bowel syndrome, rheumatoid arthritis and osteoarthritis (OA),17–21 we primarily hypothesized that mast cells also play an essential role in IVDD and LBP. Subsequently, immune cell co-expression analysis was performed. As expected, we found that DEGs were negatively correlated with resting mast cells and positively associated with mast cell activation. Next, IHC and WB analysis further confirmed that mast cells with higher enrichment and activation in VAS6 than in VAS 3 NP tissues.
Mast Cell Essential Related Gene HK-1 Associated with Discogenic LBP
In 2000, researchers discovered HK-1, the newest tachykinin encoded by the Tac4 gene. Due to its extensive distribution throughout the entire body, HK-1 plays a variety of physiological and pathological roles, including modulating the immune response, respiratory and endocrine systems, tumor development, and inflammation-related pain.22 T.L. Sumpter. etc. discovered that HK-1 transcripts and protein synthesis were upregulated by antigen-IgE complexes of mast cells (Fc RI-MCs). Mast cells degranulation and protein synthesis are both facilitated by HK-1 in a positive signaling loop, with the latter occurring via the PI3K/Akt/nuclear factor-kappaB (NF-κB) pathways.23 Numerous studies have been performed on the function of HK-1 in arthritis,24,25 but none have been performed in IVDD. In our work, we initially found that HK-1 related to mast cells had a strong link with discogenic LBP, which was supported by bioinformatics analysis and clinical sample tests. HK-1 expression is mainly in the cytoplasm of NP and mast cells and may cause LBP though activate the NF-κB pathway.
Interleukin 12 p70 Correlated with HK-1 May Released by Mast Cells
Additionally, it was discovered that interleukin 12 p70 (IL-12P70, commonly designated IL-12) in peripheral venous blood of IVDD is high and closely linked with both the level of HK-1 protein and the VAS score. IL-12p70 is an important immunoregulatory cytokine that is produced mainly by antigen-presenting cells. The expression of IL-12p70 during infection regulates innate responses and determines the type of adaptive immune responses.26 As early as 1994, Tracey J. Smith etc. have been demonstrated that murine bone marrow cells cultured in mast cell growth factor (MGF) take on a connective tissue mast cell-like phenotype and possess transcripts for both of the subunits of the IL-12p70 cytokine. Although there is rare in-depth research on the role of IL-12p70 in pain, most reports emphasize that IL-12p70 being associated with pain of axial disease in ankylosing spondylitis or osteoarthritis knee.27,28
Based on the latest research reports and our research, IL-12P70 may serve as a marker of LBP severity. HK-1 and IL-12P70 were upregulated when mast cell activation in tissues. While the relationship between HK-1 and IL-12P70 and their mechanism on LBP still unknown. The present study has some limitations, including only use of clinical samples for our tests and the need for more animal IVDD models to clarify the precise mechanism of HK-1 and IL-12P70 in IVDD-LBP pain.
Conclusions
In conclusion, we established a positive correlation between IVDD Pfirrmann grading and LBP severity. The primary factor causing discogenic LBP was an increase in abundance and activation of mast cells. Using clinical NP samples, HK-1, the mast cell-associated gene, was first shown to have a significant role in IVDD and LBP. These results provide more evidence that HK-1 may represent a novel target for the management of LBP in IVDD patients, which has important practical implications.
Abbreviations
LBP, Low back pain; IVDD, Intervertebral disc degeneration; NP, Nucleus pulposus; IHC, Immunohistochemistry; WB, Western blot; RT-PCR, Real-time polymerase chain reaction; TNF-α, Tumor necrosis factor-α; PGE2, Prostaglandin E2; NO, Nitric oxide; ECM, Extracellular matrix; NGF, Nerve growth factor; VEGF, Vascular growth factor; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; WGCNA, Weighted gene co-expression network construction and analysis; TPSB2, Mast cell tryptase; MCs, Mast cells; OA, Osteoarthritis; NF-κB, Nuclear factor-kappaB; IL-12P70, Interleukin 12 p70; MGF, Mast cell growth factor.
Ethics Approval and Informed Consent
The study design was approved by the First Affiliated Hospital, Huzhou University’s ethics committee (approval number: 2019088). All participants in the study provided informed consent before specimens were collected.
Author Contributions
Qian Shi wrote the main manuscript text and prepared all figures. Shouyu He, and Xiaowen Liu contribution to the study design, execution, acquisition of data, analysis and interpretation. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Medicine and Health Project of Zhejiang Province (Grant No. 2020KY941).
Disclosure
There are no conflicts of interest to declare.
References
1. Wang L, He T, Liu J, et al. Revealing the immune infiltration landscape and identifying diagnostic biomarkers for lumbar disc herniation. Front Immunol. 2021;12:666355. doi:10.3389/fimmu.2021.666355
2. Ou-Yang DC, Kleck CJ, Ackert-Bicknell CL. Genetics of intervertebral disc degeneration. Curr Osteoporosis Rep. 2023;21(1):56–64. doi:10.1007/s11914-022-00769-0
3. Torgerson WR, Dotter WE. Comparative roentgenographic study of the asymptomatic and symptomatic lumbar spine. J Bone Joint Surg Am. 1976;58(6):850–853.
4. Isa ILM, Teoh SL, Nor NHM, Mokhtar SA. Discogenic low back pain: anatomy, pathophysiology and treatments of intervertebral disc degeneration. Int J Mol Sci. 2023;24(1):208. doi:10.3390/ijms24010208
5. Arnbak B, Jensen RK, Manniche C, et al. Identification of subgroups of inflammatory and degenerative MRI findings in the spine and sacroiliac joints: a latent class analysis of 1037 patients with persistent low back pain. Arthritis Res Therapy. 2016;18(1). doi:10.1186/s13075-016-1131-x
6. Wang Y, Che M, Xin J, Zheng Z, Li J, Zhang S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed Pharmacother. 2020;131:110660. doi:10.1016/j.biopha
7. Lyu F-J, Cui H, Pan H, et al. Painful intervertebral disc degeneration and inflammation: from laboratory evidence to clinical interventions. Bone Res. 2021;9(1):7. doi:10.1038/s41413-020-00125-x
8. Johnson ZI, Schoepflin ZR, Choi H, Shapiro IM, Risbud MV. Disc in flames: roles of TNF-alpha and IL-1beta in intervertebral disc degeneration. Eur Cell Mater. 2015;30:104–116; discussion 116–107. doi:10.22203/eCM.v030a08
9. Yan M, Song Z, Kou H, et al. New progress in basic research of macrophages in the pathogenesis and treatment of low back pain. Front Cell Dev Biol. 2022;10:866857. doi:10.3389/fcell.2022.866857
10. Abdel Fattah IO, Nasr El-Din WA. Granulocyte-colony stimulating factor improves intervertebral disc degeneration in experimental adult male rats: a microscopic and radiological study. Anat Rec. 2021;304(4):787–802. doi:10.1002/ar.24519
11. Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinf. 2008;9(1):559. doi:10.1186/1471-2105-9-559
12. Zhou Y, Zhou B, Pache L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523. doi:10.1038/s41467-019-09234-6
13. Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004;29(23):2691–2699. doi:10.1097/01.brs.0000146101.53784.b1
14. Novais EJ, Tran VA, Johnston SN, et al. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat Commun. 2021;12(1):5213. doi:10.1038/s41467-021-25453-2
15. Ou X, Ying J, Bai X, Wang C, Ruan D. Activation of SIRT1 promotes cartilage differentiation and reduces apoptosis of nucleus pulposus mesenchymal stem cells via the MCP1/CCR2 axis in subjects with intervertebral disc degeneration. Int J Mol Med. 2020;46(3):1074–1084. doi:10.3892/ijmm.2020.4668
16. de Schepper EI, Damen J, van Meurs JB, et al. The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine. 2010;35(5):531–536. doi:10.1097/BRS.0b013e3181aa5b33
17. Zhu Z, He Z, Tang T, et al. Integrative bioinformatics analysis revealed mitochondrial dysfunction-related genes underlying intervertebral disc degeneration. Oxid Med Cell Longev. 2022;2022:1372483. doi:10.1155/2022/1372483
18. Ramachandran R. Neurogenic inflammation and its role in migraine. Semin Immunopathol. 2018;40(3):301–314. doi:10.1007/s00281-018-0676-y
19. Gao J, Xiong T, Grabauskas G, Owyang C. Mucosal serotonin reuptake transporter expression in irritable bowel syndrome is modulated by gut microbiota via mast cell-prostaglandin E2. Gastroenterology. 2022;162(7):1962–1974 e1966. doi:10.1053/j.gastro.2022.02.016
20. Zhou S, Lu H, Xiong M. Identifying immune cell infiltration and effective diagnostic biomarkers in rheumatoid arthritis by bioinformatics analysis. Front Immunol. 2021;12:726747. doi:10.3389/fimmu.2021.726747
21. Zhao X, Younis S, Shi H, et al. RNA-seq characterization of histamine-releasing mast cells as potential therapeutic target of osteoarthritis. Clin Immunol. 2022;244:109117. doi:10.1016/j.clim.2022.109117
22. Borbely E, Helyes Z. Role of hemokinin-1 in health and disease. Neuropeptides. 2017;64:9–17. doi:10.1016/j.npep.2016.12.003
23. Sumpter TL, Ho CH, Pleet AR, et al. Autocrine hemokinin-1 functions as an endogenous adjuvant for IgE-mediated mast cell inflammatory responses. J Allergy Clin Immunol. 2015;135(4):1019–1030 e1018. doi:10.1016/j.jaci.2014.07.036
24. Borbely E, Hunyady A, Pohoczky K, et al. Hemokinin-1 as a mediator of arthritis-related pain via direct activation of primary sensory neurons. Front Pharmacol. 2020;11:594479. doi:10.3389/fphar.2020.594479
25. Tsilioni I, Russell IJ, Stewart JM, Gleason RM, Theoharides TC, Neuropeptides CRH. SP, HK-1, and inflammatory cytokines IL-6 and TNF are increased in serum of patients with fibromyalgia syndrome, implicating mast cells. J Pharmacol Exp Ther. 2016;356(3):664–672. doi:10.1124/jpet.115.230060
26. Hamza T, Barnett JB, Li B. Interleukin 12 a key immunoregulatory cytokine in infection applications. Int J Mol Sci. 2010;11(3):789–806. doi:10.3390/ijms11030789
27. Anandarajah A, Ritchlin CT. Treatment update on spondyloarthropathy. Curr Opin Rheumatol. 2005;17(3):247–256. doi:10.1097/01.bor.0000159926.42761.dd
28. Nees TA, Rosshirt N, Zhang JA, et al. Synovial cytokines significantly correlate with osteoarthritis-related knee pain and disability: inflammatory mediators of potential clinical relevance. J Clin Med. 2019;8(9):1343. doi:10.3390/jcm8091343
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.