Back to Journals » Pathology and Laboratory Medicine International » Volume 15
Microbial Threshold Guidelines for UTI Diagnosis: A Scoping Systematic Review
Authors Hilt EE , Parnell LK , Wang D , Stapleton AE , Lukacz ES
Received 14 June 2023
Accepted for publication 3 August 2023
Published 16 August 2023 Volume 2023:15 Pages 43—63
DOI https://doi.org/10.2147/PLMI.S409488
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Paul Zhang
Video abstract of "Microbial threshold guidelines for UTI diagnosis" [ID 409488].
Views: 134
Evann E Hilt,1 Laura KS Parnell,2 Dakun Wang,3 Ann E Stapleton,4 Emily S Lukacz5
1Author affiliations Department of Laboratory Medicine and Pathology, M Health Fairview University of Minnesota Medical Center, Minneapolis, MN, USA; 2Author affiliations Department of Scientific Writing, Precision Consulting, Missouri City, TX, USA; 3Author affiliations Department of Writing, Stat4Ward, Pittsburgh, PA, USA; 4Author Affiliations Department of Medicine, University of Washington, Seattle, WA, USA; 5Author affiliations Department of Obstetrics, Gynecology & Reproductive Sciences, University of California San Diego, La Jolla, CA, USA
Correspondence: Laura KS Parnell, Precision Consulting, 6522 Harbor Mist, Missouri City, TX, 77459, USA, Tel +1 281-208-3037, Email [email protected]
Abstract: Given the growing impact of antimicrobial resistance, improvements in diagnosis and treatment of the most common outpatient infection, urinary tract infection (UTI), are of great interest to stakeholders. Regulatory authorities have long accepted a microbial threshold of 105 CFU/mL as the standard for diagnosing UTI based on standard urine cultures. However, microbial thresholds considered clinically relevant remain in dispute. The aim of this systematic scoping review is to assess the evidence supporting a threshold of 105 CFU/mL, to review microbial threshold guidelines, and highlight knowledge gaps in the diagnosis of UTI. A total of 36 guidelines containing 144 recommendations were identified with 64% of guidelines (n = 23) and 58% of recommendations (n = 83) published in the last six years (2016– 2023). Recommendations have changed over time and across variables including the geographical location of the guideline, urine specimen collection method, patient sex, and category of UTI. Guidelines uniformly agreed with suprapubic needle specimen collection; however, there was no consensus for midstream collected urine samples. Guideline microbial thresholds for clinical UTI diagnosis were higher for women at average risk (105 CFU/mL) than for men (102 to 105 CFU/mL) and high-risk patients (102 to 104 CFU/mL). Guidelines relied heavily on 48 research articles from 20 author teams published between 1956 and 2019 and recommendations frequently cited 23 research articles by 15 author teams published between 1956 and 2013. Evidence supporting 105 CFU/mL threshold originated in the mid-1950s from 4 research articles, whereas 18 frequently cited peer-reviewed publications focused their research on the clinical relevance of lower thresholds (101 to < 105 CFU/mL). This review demonstrates a lack of consensus for urine culture microbial threshold recommendations for the clinical diagnosis of UTI. Guidelines are primarily based upon sparse and dated evidence. Additional research is needed to inform clinically meaningful diagnostic microbial thresholds in the diagnosis of UTI.
Keywords: urinary tract infection, guideline, microbial, diagnostic threshold, uropathogen, scoping systematic review
Introduction
Urinary tract infections (UTIs) are one of the most common infections affecting persons of all ages.1 Approximately 20–30% of these UTIs are nosocomial, with the remainder being community-acquired.1 In 2019, prior to the global pandemic, nearly 30.9 million UTI events were reported from US households, resulting in $11.45 billion in expenditures.2 Considering inpatient UTI treatment and worldwide data, the actual cost of UTI, including healthcare costs, lost wages, and morbidity and mortality, are substantial.2–8 Given the prevalence and high costs of UTIs, and the growing impact of antimicrobial resistance worldwide, improvements in diagnosis and treatment are of great interest to stakeholders, including clinicians and patients, reimbursement organizations, and policymakers. Many US regulatory agencies believe there are clinically accepted microbial thresholds, based on standard urine culture (SUC) results, for diagnosing or surveying UTIs.9,10 However, clinically accepted microbial thresholds for diagnosing UTI from SUC results are subject to vigorous debate.11–13
UTIs are classified clinically as either uncomplicated (uUTI) or complicated (cUTI). By traditional definitions, uUTIs occur in individuals who do not have any additional risk factors predisposing them to complications.8 In contrast, cUTIs are associated with factors that predispose the individual to a higher likelihood of treatment failure and poor outcomes, such as persistence, recurrence, or increasing severity of the UTI such as sepsis requiring hospitalization and/or resulting morbidity and mortality.6,14 These predisposing factors can include structural and functional abnormalities of the urinary tract (eg, obstruction or retention, catheter or foreign body, renal tract calculi, pelvic radiation and surgical alterations), metabolic (eg, diabetes, renal impairment), or immunologic (eg, medical or inherited immune suppression). Likewise, certain patient populations, including the elderly and pregnant patients, are at a higher risk for complications related to UTIs, including sepsis and preterm delivery, respectively. Those with multidrug resistance and recurrent UTI (two symptomatic episodes within six months or at least three symptomatic episodes within 12 months) are also more complicated to diagnose and treat. Additionally, in men, all UTIs are considered cUTIs because of the inherent protective factors normally found in the male urinary tract anatomy.
Newer definitions of uUTI and cUTI, termed “acute simple cystitis” and “acute complicated UTI”, are based on the presumed extent of infection and the severity of illness.15 Infections confined to the bladder define simple cystitis, whereas signs and symptoms indicating upper urinary tract involvement thus spread beyond the bladder define acute complicated UTI. This schema was developed because it more accurately reflects clinical practice and antimicrobial stewardship principles in infectious disease. This is especially relevant for patients with UTI in the presence of traditional complicating factors who may not show signs of systemic infection but are considered to represent cases of cUTI using the traditional classification system and thus automatically receive longer therapy with more broad spectrum agents. At the same time, patients with traditional complicating factors are recognized as having a higher risk of upper tract and/or systemic infection and are thus monitored more closely, with a low threshold for escalating to management as cases of acute cUTI. For the purpose of this review, guideline descriptions of UTI recommendations satisfying either the traditional or the newer definition were considered a cUTI (Table 1).
 |
Table 1 cUTI Definition Used for Classification in This Review |
We developed a scoping systematic review protocol, a priori, to review the available guideline information and the evidence referenced to justify the recommendations. This review aimed to identify a “generally accepted threshold” level for positive SUC categorized by UTI classification (eg, uUTI or cUTI), region, year, specimen collection method, diagnosis, and patient sex. Since the clinical diagnosis of simple cystitis is frequently based on a positive chemical urine dipstick result, without requiring microscopy or species identification, the importance of standardizing thresholds is most important for cUTI, where bacterial identification and antibiotic sensitivity reporting are important in the treatment algorithm.11,17 Thus, we also aimed to determine if consensus recommendations exist, to highlight knowledge gaps in the laboratory diagnosis of UTI, and evaluate for consistency between current clinical guidelines and practice for adult UTI diagnosis, especially for cUTI.
Materials and Methods
Protocol
A microbiologist/immunologist (LKSP) with experience in literature searches and systematic reviews developed an a priori protocol using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) and PRISMA extension guides.18,19 The research team discussed, revised, and finalized the protocol before conducting the literature search for clinical guidance and practice documents for microbial thresholds for UTI diagnosis based on SUC. The final review protocol was not registered with PROSPERO (https://www.crd.york.ac.uk/PROSPERO) because this register does not currently include scoping reviews. The authors will share the final protocol upon request.
Eligibility Criteria
Clinical guidance documents were defined as publications that inform clinicians of suggested or required microbial thresholds, with or without other conditions, used to determine if a human patient has a UTI. Clinical guidance documents, clinical practice guidelines, consensus statements, or similar records regarding UTI diagnosis with microbial thresholds for positive SUC were considered eligible. For this scoping review, both peer-reviewed published documents and “gray” guidance documents not published through the peer-review process were eligible for inclusion if they were considered final at the time of publication or posting.18,20 Examples of non-peer-reviewed guidelines include guidelines published on professional society websites and government-issued guidance documents. A thirty-year time period was selected to provide adequate internet access availability and determine what if any changes occurred over time. Files were collected from any geographical region but were required to be in English, published from January 1992 through January 2023, and focused on adults. Records that focused on asymptomatic bacteriuria were excluded. Only symptomatic UTI recommendations were collected if a publication included symptomatic and asymptomatic UTI microbial threshold guidance.
Information Sources
Multiple information sources for clinical guidance documents were searched to find as many eligible records as possible. Published and “gray” guidance documents were searched for in electronic databases and on key stakeholder websites in areas of 1) urology, urogynecology, infectious disease, epidemiology, and microbiology societies, 2) regulatory or governing agencies, 3) clinical diagnostic laboratory societies, and 4) other potential stakeholders, such as certifying clinical or laboratory agencies. All potentially relevant supplementary documents cited in the retrieved documents were also acquired. The PROSPERO and Cochrane Reviews registry searches were conducted on January 24, 2023, and the PubMed search on January 25, 2023. The supplemental citation and organizational website searches were completed on February 9, 2023. Three authors were contacted for out-of-print records but did not respond.
Search Strategy
The search strategy developed for this review used the population, intervention, control, and outcome (PICO) and peer review of electronic search strategies (PRESS) checklists to ensure the strategy was appropriate for this systematic scoping review.21 The draft Boolean search strategy employed to search peer review published journal databases included medical subject headings (MeSH) terms and parameters for ((UTI OR “urinary tract infection” OR bacteriuria) AND (guidelines OR consensus OR evaluation OR surveillance) NOT (pediatric OR child)) to determine the type of literature returned. Additional refined searches ran a variety of additional term items such as (diagnostic OR diagnosis OR definition), (threshold OR cutoff), (“colony forming unit” OR colony OR CFU OR “urine culture”), (microbial OR microbe OR bacteria), etc., to calibrate the search parameters. This calibration process was tailored using five documents, one of which was not a guidance document, from different sources, years, regions, and abstract styles. Once the search parameters retrieved the test guidance documents, the calibration was complete. The final calibrated parameter search is in Table 2. Applied filters for the English language, human species, and years (1992–2023) defined limits on the search. Internet searches for guidance documents included a similar keyword strategy and met the same inclusion and exclusion criteria as the database searches.
 |
Table 2 Final Calibrated Parameter Search for This Review |
Study Records
Final search results were exported to Microsoft Excel (Redmond, WA), a searchable electronic database. Each search was stored as an individual file to maintain its integrity as a source document.
Selection of Source Evidence
Removal of duplicates using digital object identifier (DOI), publication dates, and author list occurred prior to screening. Two reviewers (LKSP and DW) independently screened each record using identical screening forms containing the reference data and a link to the abstract. The reviewers screened for uniqueness and for eligibility based on the abstract. Using the screening form, reviewers documented any relevant comments, if the record was eligible for further review, the reason for exclusion, or if additional information was required. The screening files were combined and sorted for examination. The reviewers discussed all preliminary eligibility screening discrepancies, and the final decision was documented in the combined reviewer screening file. Papers with preliminary screening discrepancies were ordered and read in full by both reviewers.
Data Charting
Prior to drafting the systematic review protocol, researchers developed and refined a charting form based on previously identified guidelines. Once finalized, the charting form could capture all potentially pertinent information.
The two reviewers shared each record and the Excel charting form electronically via Zoom screen (San Jose, CA). The reviewers worked in tandem and agreed upon data extraction from the record prior to charting the data and saving. Any discrepancies were discussed and the guideline in question was re-read by the reviewers, and data were cited and discussed prior to inclusion or exclusion in the charting file. The charting file format was not revised during the review, and once complete, the data set was locked and dated.
Data Items
Data variables extracted verbatim included type of UTI, microbial threshold, first author, publication year, information source, and publication URL. Other data variables summarized included additional conditions used with the microbial threshold to validate a UTI diagnosis, specimen collection method, patient sex, type of patient (ie, spinal cord injury, institutionalized, etc.), and geographical region. Patient sex was categorized as “both” when not explicitly mentioned in the record. The current definition of uncomplicated versus complicated UTI enabled the sorting of UTI as a separate classification system for data simplification.6,14,15 For this study, a UTI was considered complicated when the individual had one or more risk factors that predispose the individual to a higher treatment failure or poor outcomes or possibly extended beyond the bladder, see Table 1.3,6,14–16 In addition, for this review, catheter associated UTI (CAUTI), a subset of cUTI, was determined by either guideline author designation or by guideline descriptors indicating catheterization and the presence of symptoms or signs compatible with UTI with no other identified source of infection along with positive microbial identification. Data for cUTI and uUTI were included; however, this review focused primarily on the diagnostic microbial thresholds for non-CAUTI cUTI.
Critical Appraisal of Individual Sources of Evidence
Since all clinical guidance documents were presumed to be based on the best evidence, the reviewers did not perform a critical appraisal of the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) Scale as part of the eligibility criteria.22 A review of the evidence cited by guidelines was conducted to determine which references were most frequently used to justify microbial threshold recommendations.
Synthesis of Results
Key variable data were grouped and summarized by text, table, and figures. The level of the microbial threshold recommended for UTI diagnosis was the priority outcome. Secondary priorities included the number of guidelines, geographic location, changes in recommendations over time, and changes in other key variables.
Results
Summary of Quantity and Basic Characteristics of the Guidelines Included in the Review
Of the 10,312 initial results, 833 met inclusion criteria for screening. Two reviewers independently conducted abstract screenings resulting in 229 records selected for retrieval, 129 from databases and 100 from elsewhere. From these, one from the databases and ten from elsewhere, a total of 11 could not be retrieved due to lack of public accessibility or citation not available for purchase or review. Thirty papers could not be assessed by the reviewers using only abstracts and were included in the retrieval total for full review. Reviewers completed protocol eligibility assessments and excluded another 182 (112 from the databases set and 70 from elsewhere). The reasons for exclusion were that the document was not a guideline (n = 36), the document was a duplicate (n = 24), the document contained no microbial thresholds for UTI diagnosis (n = 109), the microbial thresholds were for surveillance rather than diagnosis (n = 6), or the document was in a language other than English (n = 7). Only six guidelines required additional scrutiny by the reviewers to determine recommendation eligibility. The eligibility assessment resulted in the final inclusion of 16 records from databases and registries and 20 records from organizational websites and citation searches for uUTI and cUTI guidelines (Figure 1).18
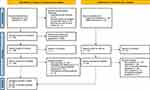 |
Figure 1 PRISMA flow diagram for this UTI guideline scoping review. Notes: Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. Creative Commons. For more information, visit: http://www.prisma-statement.org/. |
A total of 36 eligible guidelines and 144 microbial threshold clinical recommendations from over 30 years were collected.1,9,12,13,23–54 All microbial threshold recommendations specifically mentioned patient symptoms as part of the condition being diagnosed. The signs and symptoms collected for each recommendation resulted in a large variety of both broad and vague sets of conditions accompanying each threshold. There were 21 guidelines with 47 specific uUTI microbial threshold recommendations, whereas cUTIs had 29 guidelines and 97 recommendations. The cUTI recommendations were subdivided into 22 CAUTI recommendations and 75 other non-CAUTI recommendations. Only 14 guidelines had recommendations for both uUTI and cUTIs and 2 guidelines were dedicated to CAUTI. Three guidelines discussed candiduria thresholds. Details of the 75 recommendations for cUTI diagnostic microbial thresholds, excluding CAUTI recommendations, can be found in Supplemental Table 1. Recommendation details for uUTI and CAUTI can be found in Supplemental Information pages, Supplemental Figures 1 and 2, Supplemental Tables 2 and 3.
Lack of Microbial Threshold Consensus Between and Within Geographical Regions
Microbial threshold consensus was lacking for cUTIs within and between geographical regions, see Figure 2. No guidelines with microbial thresholds in English originated from the Middle East region. A single guideline from South America and two guidelines from Australia could not provide a consensus microbial threshold recommendation. Three guidelines containing 11 recommendations came from the Asian region. North America had 12 guidance documents with 40 cUTI recommendations, whereas Europe had 9 with 21 recommendations. Both regions recommended at least five different microbial thresholds (range: None to 105 CFU/mL) for a variety of cUTI conditions and collection methods. See Supplemental Table 1 for more guideline specific details.
 |
Figure 2 Complicated UTI (cUTI) microbial threshold recommendations by geographical region; CAUTI not included. The vertical axes indicate the region and number of guidelines. The horizontal axes indicate the number of recommendations. Guidelines often included more than one recommendation. Microbial thresholds in CFU/mL are indicated by color (none = navy blue, any count = yellow, 101 = pink, 102 = orange, 103 = light blue, 104 = green, 105 = Oxford blue, and no consensus = khaki). Definition of cUTI used is outlined in Table 1. |
Bacterial Threshold for cUTI Changes Over the 30-Year Period
Guidelines and recommendations were evaluated by quartiles to thoroughly view potential changes over time (Figure 3). Each quartile period showed a range of multiple threshold levels. More guidelines for cUTI were published in the most recent period, from 2016 to 2023 (n = 17), than in the prior 24 years combined, from 1992 to 2015 (n = 10). Guidelines from 2016 to 2023 contained 44 recommendations ranging from any count to 105 CFU/mL to no consensus. The largest change in recommendations to 102 and 103 was observed during this period. The 1992–1999 quartile had the second most published guidelines (n = 5) and contained 19 recommendations with the same range of thresholds as more recent publications. The fewest publications occurred from 2008 to 2015, two guidelines with three recommendations, and from 2000 to 2007, three guidelines with nine recommendations. During the 30-year period, there were five cUTI recommendations that were unable to provide a consensus recommendation.
Differences in Bacterial Thresholds for Different cUTI Diagnoses
The analysis focused on unspecified cUTI (other) diagnosis as well as two specific diagnoses within the cUTI category, pyelonephritis and recurrent UTI (rUTI). Recommended microbial thresholds for cUTI diagnoses have become more varied over time (Figure 4). Recurrent UTIs (rUTI) and pyelonephritis had large shifts in recommendations in the last 15 years including no threshold, 102, 103, 105 CFU/mL, and unable to provide a recommendation. The diagnosis thresholds for pyelonephritis cUTI for the first 15 years recommended 104 CFU/mL (n = 3). More recent guidelines, 2008–2023, published 11 recommendations; five (45.5%) used 104 and three (27.3%) used 105 CFU/mL. Three others suggested no threshold, 102 CFU/mL, or were unable to provide a recommendation for pyelonephritis. Eight guidelines on “other” cUTI diagnoses published 23 recommendations from 1992 to 2007. Similarly, ten guidelines from 2008 to 2023 provided 23 “other” cUTI recommendations. The recommendations from these two time periods lacked consensus but were most similar across time. The biggest shift between the two 15-year periods was the increased recommendations of None (1992–2007 n = 0 versus 2008–2023 n = 5, 18.5%). Both the first and the last 15-year periods each had one guideline unable to provide a consensus threshold recommendation.
Bacterial Thresholds Differ Based on Urine Specimen Collection Method
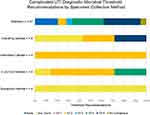
Many guidance documents specified distinct diagnostic thresholds for different specimen collection methods (Figure 5). All four guidelines with recommendations for the suprapubic needle aspirate collection method agreed that any microbial count was clinically relevant for cUTI diagnosis. The four guidelines with recommendations for the intermittent catheterization method agreed on a CFU/mL threshold of 102 CFU/mL. Of the seven guideline recommendations for the indwelling catheter collection method, three (43%) were for any count, two (28.5%) were 102, and two (28.5%) were 105 CFU/mL. For the in-and-out catheterization method, five guidelines contained seven recommendations, four for 102, two for 104 CFU/mL, and one for any count. For this review, the midstream category included guidelines for clean catch, voided, and those that did not specify collection details since this method is the most commonly used. Midstream collection had 27 guidelines with 39 recommendations. The majority of these recommended 104 (n = 11, 28%) and 105 CFU/mL (n = 13, 33%).
Bacterial Thresholds for cUTI Differ by Biological Sex
To better understand how patient sex influenced recommendations, we focused on the midstream collection method because it had the largest number of available guideline data points. Recommendations for cUTI and cystitis were stratified by sex and risk (Figure 6). Diagnosis of cUTI cystitis specific to women resulted in one guideline recommendation for a threshold of 105 CFU/mL. No guideline recommendations specific to women at additional risk (ie, spinal cord injury (SCI)) were found. Six guideline recommendations specific to men not at additional risk lacked consensus but tended to have lower threshold recommendations with the majority suggesting 103 (n = 3, 50%) and 104 CFU/mL (n = 2, 33.3%). Three guidelines for men at high risk for SCI (n = 2) and for solid organ transplant (n = 1) had 2 recommendations for 104 and one each for 102 and 105 CFU/mL. Of the seven guidelines for diagnosis of average risk patients that did not specify sex, 6 (86%) recommended 105 CFU/mL. High-risk patients without specified sex had seven guidelines covering solid organ transplant (n = 3), SCI (n = 2), intensive care unit (ICU, n = 1), and long-term care (LTC, n = 1) analyzed. The recommended thresholds lacked consensus but leaned toward using no or low thresholds as diagnostically important with the exception of the LTC recommendation of 105 CFU/mL.
References Cited by the Guidelines as Supporting Evidence for Thresholds
A review of the evidence cited in the guidelines as justification for the microbial recommendations relied heavily on 20 author teams with 48 research articles published between 1956 and 2019.26,51,54–99 Of these, 23 papers written by 15 author teams between 1956 and 2013 were the most frequently cited references in the reviewed guidelines.26,51,54,55,57,58,60–62,64–67,76–78,81,84–86,88–91 Table 3 details the most frequently cited author teams and their publications. The two most frequently cited references (Hooton et al and Stamm et al)58,62 indicated that low bacterial quantification thresholds of 102 CFU/mL were highly predictive of UTI in symptomatic patients. In fact, of the 23 references most frequently cited by guidelines, 17 focused on the clinical impact of lower bacterial quantification thresholds (101 to <105 CFU/mL) in symptomatic patients. The four most frequently cited references (Kass and Savage et al)64–66,90 justifying the use of 105 CFU/mL as a threshold are based on research results in pregnant and pyelonephritis patients published 1956–1967. Guidelines cited other literature as justification for microbial threshold recommendations; however, none of these citations were used by guidelines analyzed in this study.
 |
Table 3 Quality of Evidence – References Most Frequently Cited as Justification in the Guidelines |
Discussion
This systematic scoping review demonstrates a lack of consensus across globally reported guidelines relating to clinically relevant thresholds for SUC-based UTI diagnosis. This lack of consensus for both uUTI and cUTI has persisted over 30 years despite the increased number of published guidelines in the last decade. While the most common microbial threshold for symptomatic cUTI recommended in guidelines was 105 CFU/mL for voided urine specimens, roughly 67% of guidelines recommended lower thresholds. Guidelines were universally in agreement that a threshold of any bacterial growth should be considered a UTI and treated when considering suprapubic aspirate and ≥102 CFU/mL for intermittent catheterization; however, guidelines varied widely when considering indwelling or in-and-out catheterization, voided midstream collections and based on sex or patient risk stratification. In this review, we identified twice as many overall cUTI recommendations for <105 CFU/mL as those advocating for ≥105 CFU/mL. When considering the feasibility of recommendations for the use of lower diagnostic microbial thresholds (<105 CFU/mL), it is important to consider that not all laboratory techniques can grow low threshold cultures and most do not quantify or report these lower threshold results.13,16,23,100–102 While all guidelines reviewed included patient symptoms in the diagnostic criteria, many also included “positive” urinalysis results, such as white blood cell count in the urine, as part of the diagnostic criteria, further increasing variability. The variability in these reports highlights an urgent need for future investigation and standardization of urine testing methods, diagnostic thresholds and clinical definitions of UTI.
When examining the primary evidence used to justify these guidelines, we found that almost two-thirds (64%) of the 36 guidelines and 144 recommendations were published between 2016 and 2023, yet the evidence cited to justify the recommendations relied heavily on 23 research articles published between 1956 and 2013. This highlights a lack of contemporary research in the area of UTI diagnosis and appropriate treatment thresholds. Although commonly cited, much of the published literature may not be universally generalizable as individual studies include variable populations including asymptomatic, pyelonephritis, or pregnant patients.64–66,90 The urine culture threshold of 105 CFU/mL suggested in these papers was indicative that significant bacteria were present and unlikely to represent any contamination found in low microbial growth. However, at some point, a UTI positive urine culture of 105 CFU/mL became the standard diagnostic threshold, even though the authors specifically stated true bacteriuria could occur at much lower thresholds.58,60,67 Over 80% of the most heavily cited publications focus their results on the clinical relevance of lower thresholds (101 to <105 CFU/mL), which account for the individual characteristics of uropathogens and patients and do not specifically recommend a 105 CFU/mL threshold.
Traditionally, a UTI is considered complicated when the individual has one or more risk factors that predispose the individual to a higher treatment failure or poor outcomes. A new definition classifies cUTIs as those that have possibly extended beyond the bladder as cUTI.15 In this review, UTIs satisfying either definition were defined as cUTI. Sensibly, the recommendations for cUTI diagnosis were influenced by specific diagnosis and trended toward lower threshold recommendations over time. The number and variety of recommended thresholds for cUTI diagnosis also increased over time. Threshold recommendations for diagnosis in men tended to be lower than recommendations for the diagnosis of women or no specified sex, reflecting considerations for the relatively protective arrangement of the male urethral anatomy. High-risk patient groups, such as ICU patients, transplant recipients, and patients with SCI also tended to have lower overall microbial threshold recommendations. This lack of a one-size-fits-all consensus threshold for cUTI diagnosis is unsurprising, given the variety of patient risk factors and prognoses associated with differing cUTI diagnoses. We identified a general trending shift in threshold recommendations for any cUTI diagnosis toward a lower (<105 CFU/mL) threshold since 2008. However, the most recent guidelines, published since 2016, were still highly inconsistent, with recommendations ranging from any count to 105 CFU/mL.
Though the recommended diagnostic microbial thresholds for non-CAUTI catheter-based urine specimens varied, a majority of recommendations for indwelling, in-and-out catheterization, and intermittent catheterization set 102 CFU/mL as a diagnostic threshold. Suprapubic aspirate urine specimen collection method guidelines unanimously agreed that any microbial count was diagnostic for a cUTI. Since this method draws directly from the bladder via a needle puncture in the abdomen, it is the most invasive, but the least prone to contamination thus this consensus finding is in alignment with the low potential for contamination. In contrast, midstream clean catch specimens are non-invasive, but more susceptible to contamination from the genitourinary anatomy and the environment. Importantly, recommendations for midstream clean catch, which is the most common specimen collection method in clinical use, were inconsistent, with no one threshold reaching a majority recommendation. This variability highlights the need for standard terminology and descriptions of techniques for urine collection in future UTI research.
Ultimately, we conclude that it is essential to keep the intended use in mind when both designing and implementing recommendations for diagnostic microbial thresholds. A single one-size-fits-all threshold for SUC-based UTI diagnosis does not exist and would not be practical. Clinically relevant thresholds for a pyelonephritis patient are and should be different than those of a spinal cord injury because of patient risk factors.1,87,91 Likewise, a standard positive SUC-based urine culture diagnostic threshold (ie, ≥105 CFU/mL) is unlikely to equal the clinically relevant threshold which is frequently much lower at 101, 102, or 103 CFU/mL.23,58,62,76 Different bacterial species may have lower clinically relevant thresholds because of their characteristics (ie, virulence, adherence, fastidiousness, etc.).16,103,104 S. saprophyticus is one such example in which the diagnostic threshold may be important at ≥102–103 CFU/mL.43,78,89 Relatively little research has been done verifying if cUTI uropathogens are similar to uUTI ones and at what microbial thresholds these microbes become clinically relevant.
If a clinician is not provided with timely diagnostic test results based on accurate and reliable thresholds, the patient may be undertreated, overtreated, or treated with an inappropriate antimicrobial.45,100,105 As antimicrobial resistance continues to rise, this is a valid and urgent concern.100–102,105,106 In an effort to adhere to antibiotic stewardship recommendations, clinical reports discourage empiric antibiotic therapy prior to final SUC susceptibility results.45,107 Unfortunately, SUC can take up to 72 hours to result and often fail to identify polymicrobial and fastidious microbial infections.101 However, better diagnostic accuracy and prompt treatment can resolve the infection avoiding empiric therapy, inappropriate dosing or repeat courses of antimicrobials.13,85,100–102 The majority of the literature supports E. coli as the primary causative bacterium in acute cystitis.62,65 Use of SUC to semi-quantitate classic uropathogens in a specimen can provide evidence to help diagnose an active UTI; however, SUC has a few disadvantages. Culturing with SUC methods uses specific selective media which results in cultivating easy to grow microbes like E. coli but not non-E. coli pathogens and fastidious microbes.16,85,101,108,109 Improvement in methodologies for the accurate identification and quantification of uropathogens is needed.85,104 Novel techniques with improved accuracy compared to SUC methods may represent an opportunity to refine and modernize the diagnosis and treatment algorithms for UTI.101,109–111 As more information about the urinary microbiome is discovered due to advanced in culture-independent technologies, such as polymerase chain reaction and next generation sequencing, better diagnostic microbial thresholds may be ascertained.112–114
Strengths of this study include its rigorous design, comprehensive review of the literature and focus on cUTI, the most significant populations at risk for serious sequelae related to missed diagnosis. Inherent limitations include that many guidelines included both uUTI and CAUTI or failed to clearly define UTI diagnosis, collection method, and/or populations included. For the purposes of this review, guidelines for CAUTI and uUTI are summarized in Supplemental Tables 2 and 3. While typically included under the umbrella of cUTI, CAUTIs represent a unique situation in which UTIs have a known cause, namely a foreign body within the urinary tract. As such, the diagnosis and management of CAUTI is more uniform with most microbial threshold recommendations being 103 CFU/mL. Additionally, because uUTI is often diagnosed without culture, we elected not to combine threshold guidelines in the primary results. However, data are available in Supplementary Materials. Additionally, we did not include reviews of asymptomatic bacteriuria or screening guidelines. The guidelines and recommendations examined in this review focus on symptomatic patients and that best clinical practices for preventing antimicrobial resistance indicate that asymptomatic bacteriuria should not be treated.75 An additional inherent limitation is that some guidelines cited surveillance thresholds as justification for clinical diagnostic threshold, but these two different thresholds should not be confused and are not interchangeable.115,116 Lastly, most UTI guidelines analyzed originated from the USA and Europe. While this review limited guidelines to those published in the English language, fewer than 50 additional abstracts were identified when the language filter was removed. It is not known how many of these would have been eligible for review if written in English.
Based on the knowledge gaps we identified in this scoping review, we call for future research efforts to:
- Develop a consensus and validate UTI definitions, symptoms, and diagnoses
- Improve clarity and consistency of diagnostic and clinical guidelines
- Determine what clinically relevant uropathogens exist and whether they differ for uUTI, cUTI, and CAUTI
- Develop relevant, evidence-based, and individual microbial quantification thresholds for clinical use
These goals may be difficult or impossible to achieve with SUC-based techniques, due to its limitations in identifying non-classical uropathogens, such as slow-growing, fastidious, or anaerobic microbes.108,117 Culture-independent tests, such as molecular tests, may provide a more comprehensive microbial landscape of the patients’ urine to help physicians make more educated treatment decisions and help establish the thresholds described above. Overall, a more uniform consensus on diagnostic microbial threshold recommendations should lead to better clinical care and reduce inappropriate antimicrobial exposure.
Conclusion
Clinical microbial threshold guidelines for diagnosing UTI impact patient care. Many US regulatory agencies believe there are clinically accepted thresholds for determining whether a UTI exists based on SUC results. However, this scoping review demonstrates that SUC-based microbial threshold recommendations have inconsistencies and that additional evidence is needed to identify a gold standard in the diagnosis of true bacterial infection of the urinary tract.
Abbreviations
UTI, urinary tract infection; cUTI, complicated UTI; uUTI, uncomplicated UTI; rUTI, recurrent UTI; CAUTI, catheter associated UTI; SUC, standard urine culture; CFU, colony forming unit; PRISMA-P, preferred reporting items for systematic reviews and meta-analysis protocols; PICO, population, intervention, control, and outcome; PRESS, peer review of electronic search strategies; MeSH, medical subject headings; GRADE, grading of recommendations, assessment, development, and evaluations scale.
Acknowledgments
The authors thank Brenna C. Li from Dublin Jerome High School in Dublin, OH for volunteering her expertise in creating the video abstract for this manuscript.
Funding
Financial support for this scoping review was partially provided by Pathnostics.
Disclosure
LKSP and DW received monetary support from Pathnostics for this project. AES reports consultation and advisory board for GSK – V and BioMerieux. ESL reports consulting fees from and advisory board for Emmi Solutions; research grants from National Institutes of Health (NICHD & NIDDK) and PCORI; scientific advisor/consultant for Pathnostics, and royalties from UpToDate. The authors report no other conflicts of interest in this work.
References
1. de Cueto M, Aliaga L, Alós JI, et al. Consensus document (Appendix A) executive summary of the diagnosis and treatment of urinary tract infection: guidelines of the spanish society of clinical microbiology and infectious diseases (SEIMC). Enfermedades Infecciosas Y Microbiol Clin Engl Ed. 2017;35(5):314–320. doi:10.1016/j.eimc.2016.11.005
2. Agency for Healthcare Research and Quality. Total expenditures ($) in millions & Number of events in thousands by condition, United States, 2019. Medical Expenditure Panel Survey. Medical Expenditure Panel Survey; 2019. Available from: https://datatools.ahrq.gov/meps-hc?type=tab&;tab=mepshcmc.
3. Medina M, Castillo-Pino E. An introduction to the epidemiology and burden of urinary tract infections. Ther Adv Urology. 2019;11:1756287219832172. doi:10.1177/1756287219832172
4. Liang SY. Sepsis and other infectious disease emergencies in the elderly. Emerg Medicine Clin North Am. 2016;34(3):501–522. doi:10.1016/j.emc.2016.04.005
5. Rothrock SG, Cassidy DD, Guetschow B, et al. Predicting outcome of patients with severe urinary tract infections admitted via the emergency department. J Am Coll Emerg Physicians Open. 2020;1(4):502–511. doi:10.1002/emp2.12133
6. Wagenlehner FME, Johansen TEB, Cai T, et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol. 2020;17(10):586–600. doi:10.1038/s41585-020-0362-4
7. Mody L, Juthani-Mehta M. Urinary tract infections in older women: a clinical review. JAMA. 2014;311(8):844–854. doi:10.1001/jama.2014.303
8. Detweiler K, Mayers D, Fletcher SG. Bacteruria and Urinary Tract Infections in the Elderly. Urol Clin North Am. 2015;42(4):561–568. doi:10.1016/j.ucl.2015.07.002
9. Food and Drug Administration, Center for Drug Evaluation and Research. Uncomplicated urinary tract infections: developing drugs for treatment. Guidance for Industry; 2019. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/uncomplicated-urinary-tract-infections-developing-drugs-treatment-guidance-industry.
10. Rubi H, Mudey G, Kunjalwar R. Catheter-Associated Urinary Tract Infection (CAUTI). Cureus. 2022;14(10):e30385. doi:10.7759/cureus.30385
11. Markowitz MA, Wood LN, Raz S, Miller LG, Haake DA, Kim JH. Lack of uniformity among United States recommendations for diagnosis and management of acute, uncomplicated cystitis. Int Urogynecol J. 2019;30(7):1187–1194. doi:10.1007/s00192-018-3750-z
12. Kwok M, McGeorge S, Mayer‐Coverdale J, et al. Guideline of guidelines: management of recurrent urinary tract infections in women. Bju Int. 2022;130(Suppl 3):11–22. doi:10.1111/bju.15756
13. Harding C, Rantell A, Cardozo L, et al. How can we improve investigation, prevention and treatment for recurrent urinary tract infections – ICI‐RS 2018. Neurourol Urodyn. 2019;38(S5):S90–S97. doi:10.1002/nau.24021
14. Sabih A, Leslie SW. Complicated Urinary Tract Infections. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
15. Gupta K. Acute complicated urinary tract infection (including pyelonephritis) in adults. In: UpToDate. The Netherlands: Wolters Kluwer; 2023.
16. Price TK, Hilt EE, Dune TJ, Mueller ER, Wolfe AJ, Brubaker L. Urine trouble: should we think differently about UTI? Int Urogynecol J. 2018;29(2):205–210. doi:10.1007/s00192-017-3528-8
17. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the infectious diseases society of America and the European society for microbiology and infectious diseases. Clin Infect Dis. 2011;52(5):e103–e120. doi:10.1093/cid/ciq257
18. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
19. Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and Explanation. Ann Intern Med. 2018;169(7):467–473. doi:10.7326/M18-0850
20. Paez A. Grey literature: an important resource in systematic reviews. J Évid Based Medicine. 2020;10(3):233–240.
21. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi:10.1016/j.jclinepi.2016.01.021
22. Granholm A, Alhazzani W, Møller MH. Use of the GRADE approach in systematic reviews and guidelines. Brit J Anaesth. 2019;123(5):554–559. doi:10.1016/j.bja.2019.08.015
23. Kouri T, Fogazzi G, Gant V, Hallander H, Hofmann W, Guder WG. European urinalysis guidelines. Scand J Clin Laboratory Investigation. 2000;60(sup231):1–96. doi:10.1080/00365513.2000.12056993
24. Naber KG, Bergman B, Bishop MC, et al. EAU guidelines for the management of urinary and male genital tract infections1. Eur Urol. 2001;40(5):576–588. doi:10.1159/000049840
25. Nicolle LE; Committee* ACG. Complicated Urinary Tract Infection in Adults. Can J Infect Dis Medical Microbiol. 2005;16(6):349–360. doi:10.1155/2005/385768
26. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the infectious diseases society of America. Clin Infect Dis. 2010;50(5):625–663. doi:10.1086/650482
27. Colombo AL, Guimarães T, Camargo LFA, et al. Brazilian guidelines for the management of candidiasis – a joint meeting report of three medical societies: sociedade brasileira de infectologia, sociedade paulista de infectologia and sociedade brasileira de medicina tropical. Braz J Infect Dis. 2013;17(3):283–312. doi:10.1016/j.bjid.2013.02.001
28. Compton S, Trease L, Cunningham C, Hughes D. Australian institute of sport and the australian paralympic committee position statement: urinary tract infection in spinal cord injured athletes. Br J Sports Med. 2015;49:1236–1240. doi:10.1136/bjsports-2014-094527
29. Vidal E, Cervera C, Cordero E, et al. Management of urinary tract infection in solid organ transplant recipients: consensus statement of the Group for the Study of Infection in Transplant Recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) and the Spanish Network for Research in Infectious Diseases (REIPI). Enfermedades Infecciosas Y Microbiol Clínica. 2015;33(10):679.e1–679.e21.
30. Alfouzan WA, Dhar R. Candiduria: evidence-based approach to management, are we there yet? J De Mycol Médicale J Medical Mycol. 2017;27(3):293–302. doi:10.1016/j.mycmed.2017.04.005
31. Garcia R, Spitzer ED. Promoting appropriate urine culture management to improve health care outcomes and the accuracy of catheter-associated urinary tract infections. Am J Infect Control. 2017;45(10):1143–1153. doi:10.1016/j.ajic.2017.03.006
32. Kim KH, Lee SJ, Cho YH, et al. 2017 guidelines of the Korean association of urogenital tract infection and inflammation: acute uncomplicated cystitis. Urogenit Tract Infect. 2017;12(1):3–6. doi:10.14777/uti.2017.12.1.3
33. Kranz J, Schmidt S, Lebert C, et al. The 2017 update of the German clinical guideline on epidemiology, diagnostics, therapy, prevention, and management of uncomplicated urinary tract infections in adult patients: part 1. Urol Int. 2018;100(3):263–270. doi:10.1159/000486138
34. Kranz J, Schmidt S, Lebert C, Schneidewind L, Schmiemann G, Wagenlehner F. Uncomplicated bacterial community-acquired urinary tract infection in adults. Deutsches Ärzteblatt Int. 2017;114(50):866–873.
35. Caron F, Galperine T, Flateau C, et al. Practice guidelines for the management of adult community-acquired urinary tract infections. Médecine Et Maladies Infect. 2018;48(5):327–358. doi:10.1016/j.medmal.2018.03.005
36. Choe H, Lee S, Yang SS, et al. Summary of the UAA‐AAUS guidelines for urinary tract infections. Int J Urol. 2018;25(3):175–185. doi:10.1111/iju.13493
37. Kang CI, Kim J, Park DW, et al. Clinical practice guidelines for the antibiotic treatment of community-acquired urinary tract infections. Infect Chemoth. 2018;50(1):67–100. doi:10.3947/ic.2018.50.1.67
38. Anger J, Lee U, Ackerman AL, et al. Recurrent uncomplicated urinary tract infections in women: AUA/CUA/SUFU guideline. J Urology. 2019;202(2):282–289. doi:10.1097/JU.0000000000000296
39. Bhargava B. Treatment Guidelines for Antimicrobial Use in Common Syndromes 2019.
40. Goldman JD, Julian K. Urinary tract infections in solid organ transplant recipients: guidelines from the american society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33(9):e13507. doi:10.1111/ctr.13507
41. Ashraf MS, Gaur S, Bushen OY, et al. Diagnosis, treatment, and prevention of urinary tract infections in post-acute and long-term care settings: a consensus statement from AMDA’s infection advisory subcommittee. J Am Med Dir Assoc. 2020;21(1):12–24.e2. doi:10.1016/j.jamda.2019.11.004
42. Betschart C, Albrich WC, Brandner S, et al. Guideline of the Swiss Society of Gynaecology and Obstetrics (SSGO) on acute and recurrent urinary tract infections in women, including pregnancy. Swiss Med Wkly. 2020;150(1920):w20236. doi:10.4414/smw.2020.20236
43. McNulty C. PHE/NHS Diagnosis of Urinary tract infections; 2010. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/927195/UTI_diagnostic_flowchart_NICE-October_2020-FINAL.pdfov.uk.
44. Venkatesan AM, Oto A, Allen BC, et al.; Expert Panel on Urological Imaging. ACR appropriateness criteria® recurrent lower urinary tract infections in females. J Am Coll Radiol. 2020;17(11):S487–S496. doi:10.1016/j.jacr.2020.09.003
45. Claeys KC, Trautner BW, Leekha S, et al. Optimal urine culture diagnostic stewardship practice—results from an expert modified-delphi procedure. Clin Infect Dis. 2021;75(3):ciab987.
46. SOGC 2010 Guidelines. Society of obstetricians and gynaecologists of Canada; 2010. Available from: https://sogc.org/en/en/content/guidelines-jogc/guidelines-and-jogc-new.aspx.
47. Wagenlehner F, Nicolle L, Bartoletti R, et al. A global perspective on improving patient care in uncomplicated urinary tract infection: expert consensus and practical guidance. J Glob Antimicrob Re. 2022;28:18–29. doi:10.1016/j.jgar.2021.11.008
48. American Society for Microbiology. Urine Cultures. In: Burnham AL, Burnham CAD, editors. ClinMicroNow. Washington DC: ASM Press; 2022.
49. Bonkat G, Baertoletti R, Bruyère F, Cai T, Geerlings SE. EAU Guidelines on Urological Infections. Available from: https://uroweb.org/guidelines/urological-infections.
50. Rubin RH, Beam TR, Stamm WE. An approach to evaluating antibacterial agents in the treatment of urinary tract infection. Clin Infect Dis. 1992;14(Supplement_2):S246–S251. doi:10.1093/clinids/14.Supplement_2.S246
51. Rubin RH, Shapiro ED, Andriole VT, Davis RJ, Stamm WE. Evaluation of new anti-infective drugs for the treatment of urinary tract infection. Clin Infect Dis. 1992;15(Supplement_1):S216–S227. doi:10.1093/clind/15.Supplement_1.S216
52. Cardenas DD, Hooton TM. Urinary tract infection in persons with spinal cord injury. Arch Phys Med Rehab. 1995;76(3):272–280. doi:10.1016/S0003-9993(95)80615-6
53. O’Grady NP, Barie PS, Bartlett JG, et al. Practice guidelines for evaluating new fever in critically ill adult patients. Clin Infect Dis. 1998;26(5):1042–1059. doi:10.1086/520308
54. Doherty JE. NIDRR consensus statement: the prevention and management of urinary tract infection among people with spinal cord injuries 1992. Available from: https://eric.ed.gov/?id=ED414662.
55. Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin N Am. 1997;11(3):551–581. doi:10.1016/S0891-5520(05)70373-1
56. Hooton TM. The epidemiology of urinary tract infection and the concept of significant bacteriuria. Infection. 1990;18(Suppl 2):S40–S43. doi:10.1007/BF01643424
57. Hooton TM. Uncomplicated Urinary Tract Infection. New Engl J Medicine. 2012;366(11):1028–1037. doi:10.1056/NEJMcp1104429
58. Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. New Engl J Med. 2013;369(20):1883–1891. doi:10.1056/NEJMoa1302186
59. Stamm WE. Urinary tract infections in young men. In: Bergen T, editor. Urinary Tract Infections. Infectiology. Vol. 1. Basel: Karger; 1997:46–47.
60. Stamm WE, Wagner KF, Amsel R, et al. Causes of the acute urethral syndrome in women. New Engl J Medicine. 1980;303(8):409–415. doi:10.1056/NEJM198008213030801
61. Stamm WE, Hooton TM, Hooton TM. Management of urinary tract infections in adults. New Engl J Medicine. 1993;329(18):1328–1334. doi:10.1056/NEJM199310283291808
62. Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. New Engl J Medicine. 1982;307(8):463–468. doi:10.1056/NEJM198208193070802
63. Kass EH. The role of asymptomatic bacteriuria in the pathogenesis of pyelonephritis. In: Quinn ELQ, Kass EH, editors. Biology of Pyelonephritis. Boston: Little & Brown; 1960:399–412.
64. Kass EH. Bacteriuria and the diagnosis of infections of the urinary tract: with observations on the use of methionine as a urinary antiseptic. M Arch Int Med. 1957;100(5):709–714. doi:10.1001/archinte.1957.00260110025004
65. Kass EH. Bacteriuria and pyelonephritis of pregnancy. M Arch Int Med. 1960;105(2):194–198. doi:10.1001/archinte.1960.00270140016003
66. Kass EH. Asymptomatic infections of the urinary tract. Trans Assoc Am Phys. 1956;69:56–63.
67. Kass EH, Finland M. Asymptomatic infections of the urinary tract. J Urol. 2002;168(2):420–424. doi:10.1016/S0022-5347(05)64650-2
68. Nicolle LE. Pivmecillinam in the treatment of urinary tract infections. J Antimicrob Chemother. 2000;46:35–39. doi:10.1093/jac/46.suppl_1.35
69. Nicolle LE. A practical guide to the management of complicated urinary tract infection. Drugs. 1997;53(4):583–592. doi:10.2165/00003495-199753040-00004
70. Nicolle LE. Urinary tract infections in special populations diabetes, renal transplant, HIV infection, and spinal cord injury. Infect Dis Clin N Am. 2014;28(1):91–104. doi:10.1016/j.idc.2013.09.006
71. Nicolle LE. Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol Clin N Am. 2008;35(1):1–12. doi:10.1016/j.ucl.2007.09.004
72. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3(1):23. doi:10.1186/2047-2994-3-23
73. Nicolle LE, Ronald AR. Recurrent urinary tract infection in adult women: diagnosis and treatment. Infect Dis Clin North Am. 1987;1(4):793–806. doi:10.1016/S0891-5520(20)30150-1
74. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the infectious diseases society of America. Clin Infect Dis. 2019;68(10):1611–1615. doi:10.1093/cid/ciz021
75. Nicolle LE, Bradley S, Colgan R, et al. Infectious diseases society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654. doi:10.1086/427507
76. Stark RP, Maki DG. Bacteriuria in the catheterized patient — what quantitative level of bacteriuria is relevant? New Engl J Medicine. 1984;311(9):560–564. doi:10.1056/NEJM198408303110903
77. Kunin CM. Urinary Tract Infections: Detection, Prevention, and Management. Kunin M, ed. Philadelphia: Lea & Febiger; 1997.
78. Kunin CM, White LV, Hua TH. A reassessment of the importance of low-count bacteriuria in young women with acute urinary symptoms. Ann Intern Med. 1993;119(6):454. doi:10.7326/0003-4819-119-6-199309150-00002
79. Kunin CM. Bacteriuria, pyuria, proteinuria, hematuria y pneumaturia. In: Kunin M, editor. Urinary Tract Infection. Detection, Prevention and Management.
80. Rubin RH, Shapiro ED, Andriole VT, Davis RJ, Stamm WE. With modifications by a European Working Party (Norrby SR). General guidelines for the evaluation of new anti-infective drugs for the treatment of urinary tract infection. Taufkirchen, Germany. Europ Soc Clin Microbiol Infect Dis. 1993;1993:240–310.
81. Sobel J. Controversies in the diagnosis of candiduria: what is the critical colony count? Curr Treatment Opinions Infect Dis. 2002;2002:81–83.
82. Sobel JD. Urinary tract infections. In: Principles and Practice of Infectious Diseases.
83. Sobel JD, Kauffman CA, McKinsey D, et al. Candiduria: a randomized, double-blind study of treatment with fluconazole and placebo. Clin Infect Dis. 2000;30(1):19–24. doi:10.1086/313580
84. Lipsky BA, Ireton RC, Fiho SD, Hackett R, Berger RE. Diagnosis of bacteriuria in men: specimen collection and culture interpretation. J Infect Dis. 1987;155(5):847–854. doi:10.1093/infdis/155.5.847
85. Giesen LG, Cousins G, Dimitrov BD, van de Laar FA, Fahey T. Predicting acute uncomplicated urinary tract infection in women: a systematic review of the diagnostic accuracy of symptoms and signs. Bmc Fam Pract. 2010;11(1):78. doi:10.1186/1471-2296-11-78
86. Epp A, Larochelle A, Lovatsis D, et al. Recurrent Urinary Tract Infection. J Obstet Gynaecol Can. 2010;32(11):1082–1090. doi:10.1016/S1701-2163(16)34717-X
87. Roberts KB. Management subcommittee on UTI steering committee on quality improvement. urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595–610. doi:10.1542/peds.2011-1330
88. Roberts FJ. Quantitative urine culture in patients with urinary tract infection and bacteremia. Am J Clin Pathol. 1986;85(5):616–618.
89. Hovelius B, Mårdh PA, Bygren P. Urinary tract infections caused by staphylococcus saprophyticus: recurrences and complications. J Urology. 1979;122(5):645–647. doi:10.1016/S0022-5347(17)56541-6
90. Savage WE, Hajj SN, Kass EH. Demographic and prognostic characteristics of bacteriuria in pregnancy. Medicine. 1967;46(5):385–407. doi:10.1097/00005792-196709000-00002
91. Gribble MJ, McCallum NM, Schechter MT. Evaluation of diagnostic criteria for bacteriuria in acutely spinal cord injured patients undergoing intermittent catheterization. Diagn Micr Infec Dis. 1988;9(4):197–206. doi:10.1016/0732-8893(88)90109-5
92. Platt R. Quantitative definition of bacteriuria. Am J Med. 1983;75(1):44–52. doi:10.1016/0002-9343(83)90072-4
93. Platt R, Polk BF, Murdock B, Rosner B. Risk factors for nosocomial urinary tract infection. Am J Epidemiol. 1986;124(6):977–985. doi:10.1093/oxfordjournals.aje.a114487
94. Johnson JR, Stamm WE. Urinary tract infections in women: diagnosis and treatment. Ann Intern Medicine. 1989;111(11):906. doi:10.7326/0003-4819-111-11-906
95. Johnson JR, Stamm WE. Diagnosis and treatment of acute urinary tract infections. Infect Dis Clin North Am. 1987;1(4):773–791. doi:10.1016/S0891-5520(20)30149-5
96. Naber KG, Schito G, Botto H, Palou J, Mazzei T. Surveillance study in europe and brazil on clinical aspects and antimicrobial resistance epidemiology in females with cystitis (ARESC): implications for empiric therapy. Eur Urol. 2008;54(5):1164–1178. doi:10.1016/j.eururo.2008.05.010
97. Naber KG. Empfehlungen zur antimikrobiellen Therapie von Infektionen der Nieren und des Urogenitaltraktes bei Erwachsenen. Chemother J. 2000;9:193–199. German.
98. Fihn SD. Acute uncomplicated urinary tract infection in women. New Engl J Medicine. 2003;349(3):259–266. doi:10.1056/NEJMcp030027
99. Fihn SD, Johnson C, Stamm WE. Escherichia coli urethritis in women with symptoms of acute urinary tract infection. J Infect Dis. 1988;157(1):196–199. doi:10.1093/infdis/157.1.196
100. Sfeir MM, Hooton TM. Practices of clinical microbiology laboratories in reporting voided urine culture results. Clin Microbiol Infect. 2018;24(6):669–670. doi:10.1016/j.cmi.2017.12.023
101. Price TK, Dune T, Hilt EE, et al. The clinical urine culture: enhanced techniques improve detection of clinically relevant microorganisms. J Clin Microbiol. 2016;54(5):1216–1222. doi:10.1128/JCM.00044-16
102. Brecher SM. Commentary: complicated urinary tract infections: what’s a lab to do? J Clin Microbiol. 2016;54(5):1189–1190. doi:10.1128/JCM.00370-16
103. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–284. doi:10.1038/nrmicro3432
104. Stamm WE. Quantitative urine cultures revisited. European J Clin Microbiol. 1984;3(4):279–281. doi:10.1007/BF01977472
105. Caterino JM, Leininger R, Kline DM, et al. Accuracy of current diagnostic criteria for acute bacterial infection in older adults in the emergency department. J Am Geriatr Soc. 2017;65(8):1802–1809. doi:10.1111/jgs.14912
106. Samimi P, Ackerman AL, Handler S, Eilber KS, Anger J. Recurrent urinary tract infection in women: primary care referral patterns in a tertiary care center. Female Pelvic Medicine Reconstr Surg. 2021;27(2):118–120. doi:10.1097/SPV.0000000000000752
107. Langford BJ, Leung E, Haj R, et al. Nudging in microbiology laboratory evaluation (NIMBLE): a scoping review. Infect Control Hosp Epidemiol. 2019;40(12):1400–1406. doi:10.1017/ice.2019.293
108. Kline KA, Lewis AL. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.UTI-0012-2012
109. Sathiananthamoorthy S, Malone-Lee J, Gill K, et al. Reassessment of routine midstream culture in diagnosis of urinary tract infection. J Clin Microbiol. 2018;57(3):e01452–18.
110. Vollstedt Baunoch D, Wojno KJ, Wolfe A, et al. Multisite prospective comparison of multiplex polymerase chain reaction testing with urine culture for diagnosis of urinary tract infections in symptomatic patients. J Surg Urol. 2020;2020:1.
111. Wojno KJ, Baunoch D, Luke N, et al. Multiplex PCR Based Urinary Tract Infection (UTI) analysis compared to traditional urine culture in identifying significant pathogens in symptomatic patients. Urology. 2020;136:119–126. doi:10.1016/j.urology.2019.10.018
112. Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. Mbio. 2014;5(4):e01283–14. doi:10.1128/mBio.01283-14
113. Wolfe AJ, Toh E, Shibata N, et al. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol. 2012;50(4):1376–1383. doi:10.1128/JCM.05852-11
114. Chen C, Hao L, Wei W, et al. The female urinary microbiota in relation to the reproductive tract microbiota. Gigabyte. 2020;2020:1–9. doi:10.46471/gigabyte.9
115. Loeb M, Bentley DW, Bradley S, et al. Development of minimum criteria for the initiation of antibiotics in residents of long-term–care facilities: results of a consensus conference. Infect Control Hosp Epidemiol. 2001;22(2):120–124. doi:10.1086/501875
116. Stone ND, Ashraf MS, Calder J, et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol. 2012;33(10):965–977. doi:10.1086/667743
117. Lainhart W, Gonzalez MD. Aerococcus urinae, alloscardovia omnicolens, and actinotignum schaalii: the AAA minor league team of urinary tract infection pathogens. Clinical Microbiology Newsletter. 2018;40(10):77–82. doi:10.1016/j.clinmicnews.2018.05.001
118. Albert X, Huertas I, Pereiro I, Sanfélix J, Gosalbes V, Perrotta C. Antibiotics for preventing recurrent urinary tract infection in non‐pregnant women. Cochrane Db Syst Rev. 2004;2004:CD001209.
119. Parasuraman R, Julian K. The AIDC of Practice. urinary tract infections in solid organ transplantation. Am J Transplant. 2013;13(s4):327–336. doi:10.1111/ajt.12124
120. Tenney JH, Warren JW. Bacteriuria in women with long-term catheters: paired comparison of indwelling and replacement catheters. J Infect Dis. 1988;157(1):199–202. doi:10.1093/infdis/157.1.199
121. Backer DD, Christiaens T, Heytens S, Sutter AD, Stobberingh EE, Verschraegen G. Evolution of bacterial susceptibility pattern of Escherichia coli in uncomplicated urinary tract infections in a country with high antibiotic consumption: a comparison of two surveys with a 10 year interval. J Antimicrob Chemoth. 2008;62(2):364–368. doi:10.1093/jac/dkn197
122. Heytens S, Sutter AD, Coorevits L, et al. Women with symptoms of a urinary tract infection but a negative urine culture: PCR-based quantification of Escherichia coli suggests infection in most cases. Clin Microbiol Infec. 2017;23(9):647–652. doi:10.1016/j.cmi.2017.04.004
123. Centers for Disease Control, National Healthcare Safety Network. LTC Surveillance for UTIs 2018. Available from: https://www.cdc.gov/nhsn/ltc/uti/index.html.
124. Public Health England. Public Health England Investigation of urine, UK Standards for Microbiology Investigations. London: PHE; 2018. Available from: www.gov.uk/government/publications/smi-b-41-investigation-of-urine.
125. Mabeck CE. Studies in urinary tract infections. I. The diagnosis of bacteriuria in women. Acta Med Scand. 1969;186(1–2):35–38. doi:10.1111/j.0954-6820.1969.tb01435.x
126. Papapetropoulou M, Pappas A. The acute urethral syndrome in routine practice. J Infection. 1987;14(2):113–118. doi:10.1016/S0163-4453(87)91852-4
127. Österberg E, Hallander HO, Kallner A, Lundin A, Svensson SB, Åberg H. Female urinary tract infection in primary health care: bacteriological and clinical characteristics. Scand J Infect Dis. 1990;22(4):477–484. doi:10.3109/00365549009027080
128. Wilson ML, Gaido L. Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis. 2004;38(8):1150–1158. doi:10.1086/383029
129. Grabe M. Guidelines on urological infection. European Association of Urology; 2015. Available from: http://uroweb.org/wp-content/uploads/19-Urological-infections_LR2.pdf.
130. Säemann M, Hörl WH. Urinary tract infection in renal transplant recipients. Eur J Clin Invest. 2008;38:58–65. doi:10.1111/j.1365-2362.2008.02014.x
131. Scottish Intercollegiate Guidelines Network (SIGN). Management of suspected bacterial urinary tract infection in adults (SIGN88); 2012. Available from: http://www.sign.ac.uk.
132. Deresinski SC, Perkash I. Urinary tract infections in male spinal cord injured patients: part one: bacteriologic diagnosis. J Am Paraplegia Soc. 1985;8(1):4–6. doi:10.1080/01952307.1985.11719610
133. Wie SH. Urinary tract infections. In: Korean Society for Infectious Diseases Ed. Infectious Diseases.
134. Linares LA, Thornton DJ, Strymish J, Baker E, Gupta K. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. infection Control & Hospital Epidemiology. 2011;32(7):644–648. doi:10.1086/660764
135. Daley P, Garcia D, Inayatullah R, Penney C, Boyd S. Modified reporting of positive urine cultures to reduce inappropriate treatment of asymptomatic bacteriuria among nonpregnant, noncatheterized inpatients: a randomized controlled trial. Infect Control Hosp Epidemiol. 2018;39(7):814–819. doi:10.1017/ice.2018.100
136. Kwon JH, Fausone MK, Du H, Robicsek A, Peterson LR. Impact of laboratory-reported urine culture colony counts on the diagnosis and treatment of urinary tract infection for hospitalized patients. Am J Clin Pathol. 2012;137(5):778–784. doi:10.1309/AJCP4KVGQZEG1YDM
137. Krieger JN, Kaiser DL, Wenzel RP. Urinary tract etiology of bloodstream infections in hospitalized patients. J Infect Dis. 1983;148(1):57–62. doi:10.1093/infdis/148.1.57
138. Garibaldi RA. Hospital-acquired urinary tract infections. In: Wenzel RP, editor. Prevention and Control of Nosocomial Infections. Williams & Wilkins; 1993:600–613.
139. Fisher JF, Newman CL, Sobel JD. Yeast in the urine: solutions for a budding problem. Clin Infect Dis. 1995;20(1):183–189. doi:10.1093/clinids/20.1.183
140. Ang BSP, Telenti A, King B, Steckelberg JM, Wilson WR. Candidemia from a urinary tract source: microbiological aspects and clinical significance. Clin Infect Dis. 1993;17(4):662–666. doi:10.1093/clinids/17.4.662
141. D’Hondt F, Everaert K. Urinary tract infections in patients with spinal cord injuries. Curr Infect Dis Rep. 2011;13(6):544. doi:10.1007/s11908-011-0208-6
142. Kennedy K, Collignon P. Colonisation with Escherichia coli resistant to “critically important” antibiotics: a high risk for international travellers. Eur J Clin Microbiol Infect Dis. 2010;29(12):1501–1506. doi:10.1007/s10096-010-1031-y
143. Swamy S, Barcella W, Iorio MD, et al. Recalcitrant chronic bladder pain and recurrent cystitis but negative urinalysis: what should we do? Int Urogynecol J. 2018;29(7):1035–1043. doi:10.1007/s00192-018-3569-7
144. Khasriya R, Barcella W, Iorio MD, et al. Lower urinary tract symptoms that predict microscopic pyuria. Int Urogynecol J. 2018;29(7):1019–1028. doi:10.1007/s00192-017-3472-7
145. Arnold JJ, Hehn LE, Klein DA. Common questions about recurrent urinary tract infections in women. Am Fam Physician. 2016;93(7):560–569.
146. de Cueto M, Aliaga L, Alós JI, et al. Executive summary of the diagnosis and treatment of urinary tract infection: guidelines of the Spanish society of clinical microbiology and infectious diseases (SEIMC). Enfermedades Infecciosas Y Microbiol Clínica. 2017;35(5):314–320.
147. Talan DA, Stamm WE, Hooton TM, et al. Comparison of ciprofloxacin (7 Days) and trimethoprim-sulfamethoxazole (14 Days) for acute uncomplicated pyelonephritis in women: a randomized trial. JAMA. 2000;283(12):1583–1590. doi:10.1001/jama.283.12.1583
148. Christiaens T, Callewaert L. Aanbeveling voor goede medische praktijkvoering: cystitis bij de vrouw. Huisarts Nu. 2000;29:7. Dutch.
149. Drutz D. Fungal infections of the kidney and urinary tract. In: Schrier R, Gottschalk C, editors. Diseases of the Kidney.
150. Bradbury SM. Collection of urine specimens in general practice: to clean or not to clean? J Royal Coll Gen Pract. 1988;38(313):363–365.
151. Lifshitz E, Kramer L. Outpatient urine culture: does collection technique matter? Arch Intern Med. 2000;160(16):2537–2540. doi:10.1001/archinte.160.16.2537
152. Kodner CM, Gupton EKT. Recurrent urinary tract infections in women: diagnosis and management. Am Fam Physician. 2010;82(6):638–643.
153. Glover M, Moreira CG, Sperandio V, Zimmern P. Recurrent urinary tract infections in healthy and nonpregnant women. Urol Sci. 2014;25(1):1–8. doi:10.1016/j.urols.2013.11.007
154. Mishra M, Agrawal S, Raut S, Kurhade AM, Powar RM. Profile of yeasts isolated from urinary tracts of catheterized patients. J Clin Diagn Res. 2014;8(2):44–46. doi:10.7860/JCDR/2014/6614.4003
155. Kauffman CA, Fisher JF, Sobel JD, Newman CA. Candida urinary tract infections—diagnosis. Clin Infect Dis. 2011;52(suppl_6):S452–S456. doi:10.1093/cid/cir111
156. Wise GJ, Goldberg P, Kozinn PJ. Genitourinary Candidiasis: diagnosis and Treatment. The J Urol. 1976;116(6):778–780. doi:10.1016/S0022-5347(17)59009-6
157. Rishpana MS, Kabbin JS. Candiduria in catheter associated urinary tract infection with special reference to biofilm production. J Clin Diagn Res. 2015;9(10):DC11–3. doi:10.7860/JCDR/2015/13910.6690
158. Chabasse D. Intérêt de la numération des levures dans les urines.Revue de la littérature et résultats préliminaires dˈune enquête multicentrique réalisée dans 15 centres hospitaliers universitaires. Ann Françaises d’Anesthésie et de Réanimat. 2001;20(4):400–406. French. doi:10.1016/S0750-7658(01)00376-8
159. Rathor N, Khillan V, Sarin SK. Nosocomial candiduria in chronic liver disease patients at a hepatobilliary center. Indian J Crit Care Medicine Peer-Reviewed off Publ Indian Soc Crit Care Medicine. 2014;18(4):234–237.
160. Dolan VJ, Cornish NE. Urine specimen collection: how a multidisciplinary team improved patient outcomes using best practices. Urol Nurs. 2013;33(5):249. doi:10.7257/1053-816X.2013.33.5.249
161. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464–479. doi:10.1086/675718
162. Gould C, Umscheid C; Committee HICPA. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319–326. doi:10.1086/651091
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.