Back to Journals » Open Access Journal of Contraception » Volume 15
Migration of Intra-Uterine Devices
Authors Verstraeten V , Vossaert K, Van den Bosch T
Received 22 January 2024
Accepted for publication 29 February 2024
Published 12 March 2024 Volume 2024:15 Pages 41—47
DOI https://doi.org/10.2147/OAJC.S458156
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Igal Wolman
Victoria Verstraeten,1,2 Karlien Vossaert,2 Thierry Van den Bosch1
1Obstetrics & Gynaecology - UZ Leuven Gasthuisberg, Leuven, Belgium; 2Obstetrics & Gynaecology – AZ Sint- Blasius Dendermonde, Dendermonde, Belgium
Correspondence: Victoria Verstraeten, Email [email protected]
Abstract: Intrauterine devices (IUDs) are a widely used contraceptive. Possible complications from IUDs include failed insertion, pain, vasovagal reaction, infection, abnormal bleeding, and expulsion. Uterine perforation and migration of the IUD are rare complications occurring in approximately 1– 2 per 1000 insertions. We executed a systematic review by reviewing all case reports and case series on IUD migration, published between December 2002 and December 2022. Our review indicates that about half of these patients present with pain and that a third are completely asymptomatic. The most common sites of migration are the intestine, bladder, and omentum. We found that the preferred method for removing the migrated IUD is laparoscopy. Generally, there are no lasting injuries after the removal of the migrated IUD, but occasionally, severe complications have been reported. Healthcare providers should be vigilant about this rare complication, especially in cases of painful insertion or the presence of other risk factors for perforation. When uterine perforation is diagnosed, it is advisable to remove the IUD to prevent severe complications.
Keywords: uterine perforation, missing IUD, long-acting reversible contraceptive, complication
Introduction
The intrauterine device (IUD) is a widely used contraceptive method. This type of contraception belongs to the family of long-acting reversible contraceptives (LARC) and is among the methods with the highest contraceptive effectiveness. The two most used types are the copper intrauterine device (Cu-IUD) and the levonorgestrel intrauterine device (LNG-IUD). These methods offer 99.2% and 99.8% effectiveness, respectively, in preventing pregnancy.1 Both types are generally well-tolerated. The Cu-IUD is the most used reversible contraceptive method worldwide.2 Besides contraception, the 52-mg LNG-IUD is also indicated for the treatment of heavy menstrual bleeding.3
Possible complications of the IUD include failed insertion, pain during and after insertion, vasovagal reaction during insertion, infection, abnormal bleeding, expulsion, and uterine perforation.4 Uterine perforation is a rare complication with an incidence ranging from 0.3 to 2.6 per 1000 insertions for LNG-IUD and from 0.3 to 2.2 per 1000 insertions for Cu-IUD.5 Uterine perforation may be asymptomatic or cause pain, abnormal bleeding, bowel or bladder perforation or fistula formation.6 The number of published case reports on uterine perforation has increased significantly in the last two decades, presumably due to the increasing use of the IUD. We present a systematic review on the incidence of perforation and migration of the IUD, its complications, and approaches to diagnosing and treating a perforated IUD.
Materials and Methods
The purpose of our review was to identify randomized clinical trials, case reports and systematic reviews regarding migrated IUDs. The search was conducted by one author (VV). The reference lists of all primary articles were also examined by the same author (VV). Through PubMed, the “related articles” feature was also used, as well as reference lists of the reviewed articles.
A systematic search was performed on several electronic databases (Medline/Pubmed, Cochrane and Sciencedirect). The search terms and MeSH terms used were “Intrauterine device migration”, “Intrauterine device perforation”, “Uterine perforation” and “Intrauterine device”. All articles in Dutch, French, English, or Spanish between 2002 and 2022 that described complete uterine perforation or migration of an IUD were included. The selection of articles was performed by one author (VV).
The study was completed using the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.7 The following data were collected from each case: demographic data, including age and parity, clinical symptoms at presentation, the specific type of IUD, length of time between IUD placement and diagnosis of perforation, location of the perforated IUD, method of IUD removal, and reported complications.
Results
We screened 936 articles, of which 119 articles were included in the review (Figure 1). All included articles were case reports or case series. Within these 119 articles, 165 cases of IUD perforation were identified. The average age of the patients was 39 years, ranging from 19 to 74 years (data by case in Appendix). Parity was mentioned in only 60% of the cases, of which 68% were multiparous (median: 2). The time between IUD placement and diagnosis of perforation ranged from one week to 42 years (median: 3.75 years). Patients presented with various clinical symptoms, with pain and urological complaints being the most common (Table 1). Approximately 31% of the patients were completely asymptomatic at the time of diagnosis. The type of IUD was specified in 59% of the cases, with two-thirds being Cu-IUDs and one-third LNG-IUDs. Table 2 provides an overview of the intra-abdominal locations of the migrated IUDs, with the bladder, intestine, and omentum being the most common. In half of the cases, the IUD could be removed laparoscopically. Other methods for removal included cystoscopy, laparotomy, and colonoscopy (Table 3). In some cases, multiple techniques were combined during a single procedure. Most migrations did not result in serious complications. Four cases of abscess formation, seven cases of complex adhesions, eighteen cases of bladder lithiasis, and four cases requiring partial bowel resection were described. Occasionally, other serious complications were reported, such as small bowel obstruction, urosepsis, hydronephrosis, the need for partial gastrectomy, the need for partial cystectomy, and even nephrectomy.
 |
Table 1 Symptoms |
 |
Table 2 Location of the IUD |
 |
Table 3 Technique Used for the Removal of the IUD |
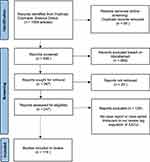 |
Figure 1 Prisma flowchart systematic review. |
Discussion
IUDs are a widely used contraceptive method worldwide due to their high effectiveness and reliability. IUDs require only a single insertion procedure for long-term use. The contraceptive effect of IUDs is not dependent on user compliance, gastrointestinal function, or the user’s weight. Therefore, the Pearl index is the same for perfect use and normal use. The number of women who become pregnant with an IUD is low, with a Pearl index of 0.06 for the LNG-IUD and 0.52 for the Cu-IUD.8
Contraindications for IUD placement include the presence of cervical or endometrial carcinoma, active pelvic inflammatory disease (PID), and pregnancy or suspicion thereof (except for emergency contraception using the IUD). Abnormalities of the uterine cavity, such as certain uterine anomalies (eg, uterus subseptus, uterus bicornis, uterus didelphys), and other structural abnormalities (eg, distortion of the cavity due to fibroids or uterine synechiae), increase the risk of expulsion and dislocation of the IUD.8
Complications of the IUD include failed insertion, pain during and after insertion, vasovagal reaction during insertion, infection, abnormal bleeding, expulsion, and uterine perforation.4 The risk of IUD expulsion within five years of placement is approximately five percent and is highest in the first year of use, immediately after abortion, and in the postpartum period. Expulsions are more common in women under 25 years of age, with the use of copper IUDs compared to LNG-IUDs, and in women who have given birth fewer than two times.9,10 Expulsion can result from inadequate depth of placement, discrepancy between the dimensions of the uterus and the IUD, or heavy menstrual bleeding. Expulsion may sometimes cause symptoms of spotting, dyspareunia, or lower abdominal pain, but can also be completely asymptomatic.11
The incidence of uterine perforation varies from 0.3 to 2.6 per 1000 insertions for LNG-IUDs and from 0.3 to 2.2 per 1000 insertions for Cu-IUDs.5 Risk factors for this include: first time users, nulliparity, placement during breastfeeding, placement within less than 6 months postpartum, a higher number of abortions, poor healing of a uterine scar due to a hematoma or postoperative infection, the type of IUD (higher risk of perforation with LNG-IUDs), and insufficient experience of the healthcare provider.5,12–16 IUD placement under anesthesia, cervical dilation (during placement or in the medical history), or a history of cesarean section are not significantly associated with an increased risk of uterine perforation.5,13,17 Other factors such as cervical stenosis (in primigravida or after a previous procedure) and the position of the uterus (hyperflexion, retroversion, deformation of the cavity after a procedure, or intracavitary pathology) have been identified as risk factors for uterine perforation in hysteroscopic procedures and dilation and curettage, but could not be significantly associated with an increased risk of perforation during IUD placement.12–14,16 Our review illustrates that the bowel is the most affected organ in cases of perforated and migrated IUDs.18 The second most affected organ is the bladder, often associated with lithiasis formation and urinary complaints.
Most uterine perforations occur during IUD placement.5 Routinely performing an ultrasound to check the length and position of the uterus before placement can lead to a smoother and more accurate procedure and higher patient satisfaction.19 Research on the use of transabdominal ultrasound during IUD placement could only demonstrate that the procedure is less painful compared to the “blind” technique.20 This is presumably due to a more targeted approach with less friction against the uterine wall and fundus. Patients with a uterine perforation from an IUD often do not show symptoms but may experience mild discomfort in the lower abdomen or brief abnormal vaginal bleeding immediately after placement. Absent or nonspecific symptoms may lead to a delayed diagnosis. Once there are suspicions of a uterine perforation, either immediately after placement or later on, it is advisable to conduct medical imaging.
Gynecological ultrasound is recommended as the initial examination due to its widespread availability, cost-effectiveness, and absence of radiation exposure. Ultrasound can easily confirm whether the IUD is correctly positioned and often provides the ability to identify complications related to the IUD. The stem of the Cu-IUD can be easily identified as a linear, echogenic structure. The arms of Cu-IUDs are moderately echogenic.21 The LNG-IUD is not echogenic, but the stem creates an acoustic shadow in a sagittal section of the uterus between the proximal and distal parts of the stem, which facilitates visualization. The arms of the LNG-IUD are lightly echogenic and are usually visible on transverse sections.22 Three-dimensional (3D) ultrasound is useful for mapping the IUD and is increasingly used in routine IUD examinations.23 If the IUD cannot be localized via ultrasound, abdominal radiography should be performed. This involves taking anteroposterior and lateral images, paying extra attention to completely visualizing the diaphragmatic domes and the Douglas pouch. Conventional radiography results in minimal radiation exposure for the patient, and the radiopaque IUD can be easily detected.23 Only when the IUD is not visualized on X-ray can the diagnosis of “IUD expulsion” be made. Computed tomography (CT) is the most suitable modality for evaluating complications associated with intra-abdominal IUDs, such as visceral perforation, abscess formation, and intestinal obstruction. The use of 3D-CT can additionally be useful for preoperatively determining the exact location of the IUD. However, CT use entails higher radiation exposure. Magnetic resonance imaging (MRI) is generally not used for evaluating migrated IUDs, but modern IUDs can be safely imaged using both 1.5-T and 3.0-T magnets and will then show a signal void.23
Although some authors suggest leaving the IUD in situ if patients are asymptomatic, it is generally recommended to remove the IUD in every case of uterine perforation.24 None the less, adhesions are often observed in both symptomatic and asymptomatic patients.25 A wait-and-see approach is generally discouraged due to the risk of intestinal obstruction, chronic lower abdominal pain, bladder or intestinal perforations, and the development of pelvic abscesses.26–30 In our literature review the IUD was not removed in only two cases. Minimally invasive techniques such as hysteroscopy, cystoscopy, colonoscopy, or laparoscopy are preferred for removal, depending on the location of the IUD.27 In asymptomatic, stable patients, immediate admission after diagnosis is not necessary, and IUD removal can be scheduled electively. Intraoperative radiography or ultrasound can be useful to locate the IUD. They are particularly helpful if the IUD is not visible during the procedure due to embedding in the omentum, caudal displacement, presence amidst adhesions, or within an organ.27,31–33 Only in exceptional cases, such as significant visceral damage due to the migrated IUD, laparotomy is necessary.
Most IUD manufacturers recommend clinical follow-up after 4 to 12 weeks, which is also preferred by patients.34 If the IUD threads are not visible during clinical examination or in cases of atypical symptoms such as pain, abdominal cramps, or bleeding, an ultrasound examination should be performed.15,35 If the threads are not visible, it should not be hastily concluded that the IUD has been expelled. Several cases have been reported where two IUDs were found in one patient.36–39 After confirming a uterine perforation, a new placement should be postponed for at least 2–6 weeks.8
Conclusion
Uterine perforation and migration of an intrauterine device (IUD) are rare and often late-detected complications. It is essential for clinicians to be aware of the risk factors and possible symptoms of this condition. A routine follow-up after IUD placement is recommended. If an IUD is not visible on ultrasound, it should not be automatically interpreted as “expelled” but further imaging should be conducted. Although the chance of permanent damage to the patient after an IUD perforation is small, it is not non-existent. Gynaecologists must remain vigilant, especially shortly after placement, to prevent later serious damage to visceral organs due to IUD migration.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397–404. doi:10.1016/J.CONTRACEPTION.2011.01.021
2. World Contraceptive Use. Population division; 2023. Available from: https://www.un.org/development/desa/pd/data/world-contraceptive-use.
3. Adeyemi-Fowode OA, Bercaw-Pratt JL. Intrauterine devices: effective contraception with noncontraceptive benefits for adolescents. J Pediatr Adolesc Gynecol. 2019;32(5S):S2–S6. doi:10.1016/J.JPAG.2019.07.001
4. Mestad R, Secura G, Allsworth JE, Madden T, Zhao Q, Peipert JF. Acceptance of long-acting reversible contraceptive methods by adolescent participants in the contraceptive CHOICE project. Contraception. 2011;84(5):493–498. doi:10.1016/J.CONTRACEPTION.2011.03.001
5. Heinemann K, Reed S, Moehner S, Do Minh T. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European active surveillance study on intrauterine devices. Contraception. 2015;91(4):274–279. doi:10.1016/j.contraception.2015.01.007
6. Kho KA, Dina MPH. Perforated intraperitoneal intrauterine contraceptive devices: diagnosis, management and clinical outcomes HHS public access. J Minim Invasive Gynecol. 2014;21(4):596–601. doi:10.1016/j.jmig.2013.12.123
7. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi:10.1016/J.JCLINEPI.2009.06.006
8. Baker CC, Creinin MD. Long-acting reversible contraception. Obstetrics Gynecol. 2022;140(5):883–897. doi:10.1097/AOG.0000000000004967
9. Keenahan L, Bercaw-Pratt JL, Adeyemi O, Hakim J, Sangi-Haghpeykar H, Dietrich JE. Rates of intrauterine device expulsion among adolescents and young women. J Pediatr Adolesc Gynecol. 2021;34(3):362–365. doi:10.1016/J.JPAG.2020.11.003
10. Simonatto P, Bahamondes MV, Fernandes A, Silveira C, Bahamondes L. Comparison of two cohorts of women who expulsed either a copper-intrauterine device or a levonorgestrel-releasing intrauterine system. J Obstetrics Gynaecol Res. 2016;42(5):554–559. doi:10.1111/JOG.12939
11. Van Schoubroeck D, T VDB, Ameye L, et al. Pain and bleeding pattern related to levonorgestrel intrauterine system (LNG-IUS) insertion. Eur J Obstetrics Gynecol Reprod Biol. 2013;171(1):154–156. doi:10.1016/J.EJOGRB.2013.08.029
12. Caliskan E, Öztürk N, Bö D, Dilbaz S. Analysis of risk factors associated with uterine perforation by intrauterine devices. Eur J Contracept Reprod Health Care. 2003;8(3):150–155. doi:10.1080/EJC.8.3.150.155
13. Barnett C, Moehner S, Do Minh T, Heinemann K. Perforation risk and intra-uterine devices: results of the EURAS-IUD 5-year extension study. Eur J Contracept Reprod Health Care. 2017;22(6):424–428. doi:10.1080/13625187.2017.1412427
14. Harrison-Woolrych M, Ashton J, Coulter D. Uterine perforation on intrauterine device insertion: is the incidence higher than previously reported? Contraception. 2003;67(1):53–56. doi:10.1016/S0010-7824(02)00417-1
15. Agacayak E, Tunc SY, Icen MS, et al. Evaluation of predisposing factors, diagnostic and treatment methods in patients with translocation of intrauterine devices. J Obstetrics Gynaecol Res. 2015;41(5):735–741. doi:10.1111/JOG.12620
16. Gatz JL, Armstrong MA, Postlethwaite D, et al. Association between intrauterine device type and risk of perforation and device expulsion: results from the association of perforation and expulsion of intrauterine device study. Am J Obstet Gynecol. 2022;227(1):57.e1–57.e13. doi:10.1016/j.ajog.2022.03.062
17. Rowlands S, Oloto E, Horwell DH. Intrauterine devices and risk of uterine perforation: current perspectives. Open Access J Contracept. 2016;7:19. doi:10.2147/OAJC.S85546
18. Zakin D, Stern WZ, Rosenblatt R. Complete and partial uterine perforation and embedding following insertion of intrauterine devices. I. Classification, complications, mechanism, incidence, and missing string. Obstet Gynecol Surv. 1981;36(7):335–353. doi:10.1097/00006254-198107000-00001
19. Abbas A, Ali MK, Abdalmageed OS, Yosef AH, Abdelkader AM, Shaaban OM. Evaluation of a novel uterine sound sparing approach for copper intrauterine device insertion. Fertil Steril. 2017;108(3):e123. doi:10.1016/J.FERTNSTERT.2017.07.376
20. Maged AM, Nada AM, Abdelwahab H, et al. The value of ultrasound guidance during IUD insertion in women with RVF uterus: a randomized controlled trial. J Gynecol Obstet Hum Reprod. 2021;50(4):101875. doi:10.1016/J.JOGOH.2020.101875
21. Nowitzki KM, Hoimes ML, Chen B, Zheng LZ, Kim YH. Ultrasonography of intrauterine devices. Ultrasonography. 2015;34(3):183. doi:10.14366/USG.15010
22. Van Schoubroeck D, Van Den Bosch T, Mortelman P, Timmerman D. Picture of the Month: sonographic determination of the position of a levonorgestrel intrauterine device. Ultrasound Obstet Gynecol. 2009;33(1):121–124. doi:10.1002/UOG.6288
23. Boortz HE, Margolis DJA, Ragavendra N, Patel MK, Kadell BM. Migration of intrauterine devices: radiologic findings and implications for patient care. Radiographics. 2012;32(2):335–352. doi:10.1148/RG.322115068
24. Adoni A, Ben Chetrit A. The management of intrauterine devices following uterine perforation. Contraception. 1991;43(1):77–81. doi:10.1016/0010-7824(91)90128-3
25. Tabatabaei F, Masoumzadeh M. Dislocated intrauterine devices: clinical presentations, diagnosis and management. Eur J Contra. 2021;26(2):160–166. doi:10.1080/13625187.2021.1874337
26. Nelson A, Massoudi MPHN. New developments in intrauterine device use: focus on the US. Open Access J Contracept. 2016;7:127. doi:10.2147/OAJC.S85755
27. Mechanism of action, safety and efficacy of intrauterine devices: report of a WHO Scientific Group; 1986. Available from: https://apps.who.int/iris/handle/10665/38182.
28. Gill RS, Mok D, Hudson M, Shi X, Birch DW, Karmali S. Laparoscopic removal of an intra-abdominal intrauterine device: case and systematic review. Contraception. 2012;85(1):15–18. doi:10.1016/J.CONTRACEPTION.2011.04.015
29. Ozgun MT, Batukan C, Serin IS, Ozcelik B, Basbug M, Dolanbay M. Surgical management of intra-abdominal mislocated intrauterine devices. Contraception. 2007;75(2):96–100. doi:10.1016/J.CONTRACEPTION.2006.09.011
30. World Health Organization. Reproductive Health and Research, World Health Organization. Medical Eligibility Criteria for Contraceptive Use. MDPI; 2009. 268.
31. Goldstuck ND, Wildemeersch D. Role of uterine forces in intrauterine device embedment, perforation, and expulsion. Int J Womens Health. 2014;6(1):735–744. doi:10.2147/IJWH.S63167
32. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recommendations Rep. 2020;65(3):1–104. doi:10.15585/MMWR.RR6503A1
33. Mascilini F, Moro F, De Leo R, Scambia G, Fagotti A, Testa AC. Intraoperative ultrasound assistance for surgical removal of lost intrauterine device. Ultrasound Obstet Gynecol. 2019;53(5):705–706. doi:10.1002/UOG.19167
34. Wright JD. NewYork-presbyterian hospital New York, NY patient preference for intrauterine device follow-up. Am J Obstet Gynecol. 2022;69:1109–1125. doi:10.1016/j.ajog
35. Ansari AS, Tullius TG, Ross JR. The role of ultrasound in the assessment of intrauterine device complications. Donald School J Ultrasound Obstet Gynecol. 2012;6(3):318–326. doi:10.5005/jp-journals-10009-1255
36. Bozkurt M, Ender Yumru A, Coskun EI, Ondes B. Ebru inci coskun, banu öndes. laparoscopic management of a translocated intrauterine device embedded in the gastric serosa. J Pak Med Assoc. 2011;61(10):1020–1022.
37. Tsafrir A, Plotkin V. One intrauterine device lost, two found. Fertil Steril. 2008;90(1):185. doi:10.1016/j.fertnstert.2007.09.065
38. Toumi O, Ammar H, Ghdira A, et al. Pelvic abscess complicating sigmoid colon perforation by migrating intrauterine device: a case report and review of the literature. Int J Surg Case Rep. 2018;42:60–63. doi:10.1016/J.IJSCR.2017.10.038
39. Istanbulluoglu MO, Ozcimen EE, Ozturk B, Uckuyu A, Cicek T, Gonen M. Bladder perforation related to intrauterine device. J Chin Med Assoc. 2008;71(4):207–209. doi:10.1016/S1726-4901(08)70105-9
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
